Abstract
Emphysema has been associated with loss of aerobic muscle fibers and decreased blood supply. However, when these changes begin and whether exercise can prevent these changes is unknown. The purpose of this study was to examine peripheral muscle at different time points during the development of emphysema and to determine the additional effects of muscle activity. In a series of 3 experiments, emphysema was induced in hamsters. Exercise was simulated through surgical overload (OV) of the plantaris muscle of one leg. Animals were sacrificed at 1, 3, and 5 months following emphysema induction. Fiber type composition and capillary-to-fiber ratio (CFR) were determined. There were no significant changes in fiber type composition in the 1-month group. A significant increase in type IIA fiber composition (mean 72.0 vs. 54.5%) and decrease in type IIB fiber (mean 13.3 vs. 28.1%) was seen in the non-overloaded muscles following 3 months. In the 5-month group, there was a significant decrease in percentage of type I fibers (mean 14.7 vs. 28.0%). There were no significant differences in fiber type composition in the OV limb, regardless of duration. The CFR was significantly lower in the OV limb after 5-months of emphysema (mean 0.92 vs. 1.55 cap/fiber). Muscle overload prevented emphysema-associated changes in fiber type composition, but not in CFR. Peripheral muscle is affected early in the course of emphysema and chronic overload may play an important role in preserving normal muscle composition.
INTRODUCTION
Emphysema, a Chronic Obstructive Pulmonary Disease (COPD), is recognized as a systemic disease and is known to affect skeletal muscle in ways that probably contribute to decreased exercise capacity [Citation[1], Citation[2], Citation[3]]. People with COPD report progressively worsening fatigue and weakness that limit their ability to perform important daily activities and often result in poor quality of life [Citation[2]]. Leg muscle biopsies from subjects with COPD have demonstrated muscle atrophy, loss of fatigue-resistant type I fibers and decreased aerobic enzyme content [Citation[1], Citation[4], Citation[5], Citation[6]]; all of which could explain decreased exercise capacity independent of the degree of lung disease.
Although these muscle changes have been seen in patients with COPD, the time course of their development is unknown. The disease is often asymptomatic for many years and not diagnosed in humans until there is significant impairment in ability to perform daily activities, which corresponds to moderate to severe lung damage [Citation[7]]. Current hypotheses regarding the causes of muscle changes include inactivity, medication effects and malnutrition [Citation[1], Citation[8], Citation[9], Citation[10]]. All of these factors are difficult or impossible to control in humans. Therefore, in order to control for effects of variables that can affect muscle independent of disease, as well as to control disease duration, an animal model is needed.
Instillation of elastase into the lungs of Syrian golden hamsters has been shown to produce lung damage that closely mimics human emphysema [Citation[11], Citation[12]]. This model has been used extensively to study disease effects on lungs and respiratory muscles [Citation[13], Citation[14], Citation[15]]. Recently, changes in hindlimb muscles have been seen in this model that match those seen in human subjects with severe COPD [Citation[16], Citation[17]]. However, animals at the time point studied by these investigators have severe lung damage and low body weight [Citation[12]]. Extraneous factors resulting from these conditions such as malnutrition may independently influence skeletal muscle structure, potentially confounding the investigation of disease effects on peripheral muscle. In addition, the question of whether changes occur in peripheral muscle earlier in the course of the disease remains.
Exercise is encouraged to help alleviate the decreased exercise capacity in patients with COPD [Citation[7]]. However, since these patients usually have moderate to severe lung disease by the time exercise training is recommended [Citation[7]], their muscles may not be able to adapt appropriately to the exercise stimulus. Perhaps if patients begin exercise training earlier in the disease process, it may be possible to modify disease-related changes in peripheral muscle.
Therefore, the purposes of this investigation were: 1) to determine the effect of disease duration on peripheral skeletal muscle composition and 2) to determine the influence of muscle activity on disease-related changes in muscle.
MATERIALS AND METHODS
All procedures were approved by the Animal Care and Use Committee at West Virginia University.
Animals
Male Syrian golden hamsters aged 7–9 weeks were obtained (Harlan Labs, Indianapolis, IN) and housed individually in standard plastic bottom cages with free access to water and rodent chow. A standard 12-hour dark/light cycle was used throughout the study. Animals were handled, weighed and observed daily for a 2-week baseline period.
A series of three experiments were performed to examine the effects of disease duration on peripheral muscle. Animals were sacrificed at 1-month, 3-months and 5-months following induction of emphysema and compared to control animals at the same time period. These time points were chosen to correspond to the earliest significant increase in lung volume seen previously [Citation[12]] (1 month), the most commonly studied time point, (5 months) [Citation[16], Citation[18], Citation[19], Citation[20], Citation[21]] and a point midway between (3 months). We felt that examination of peripheral muscle at these points allowed us to determine changes related to disease progression. Animals were randomly assigned to the emphysema (elastase-instilled) or saline control groups, allotting a greater number of animals to the emphysema condition. This was done based on mortality reported by other investigators using elastase instillation [Citation[20], Citation[21], Citation[22]]. Based on power analysis from our 1-month groups, two animals were required in each group to allow 80% power to detect a 10% change in fiber type composition. We therefore allotted two animals to the saline control group and four animals to the emphysema group for the 3-month and 5-month durations. We had no premature mortality in our animals, thus we had four animals in each emphysema group and two animals in each saline-instilled group.
Induction of emphysema
Animals were given a single intraperitoneal injection of ketamine/xylazine to produce deep sedation (0.2 ml of 50 mg/ml Ketamine with 0.02 ml of 50 mg/ml Xylazine). Animals were then placed on a 45-degree slant board, suspended supine on a metal wire by their incisors and supported by a strap across the abdomen. A 24-gauge feeding needle was placed into the trachea under direct observation and instillation of either 0.9% sterile saline (0.3 ml/100 g body weight) (n = 6) or porcine elastase (40 IU Sigma E-6883 in 0.3 ml sterile normal saline/100g body weight) (n = 12) was performed, coordinating with spontaneous inspirations. This dosage has been shown to produce significant increases in lung volume beginning 1 month after instillation [Citation[12]]. Each animal was removed from the slant board and rotated in a head-up position for 3–5 minutes to distribute the liquid around the lungs, then returned to its cage in a head-up position to recover.
Muscle overload procedure
Exercise was modeled in this study by selective tenotomy of the gastrocnemius and soleus muscles. This model allows the remaining intact plantaris muscle in the hindlimb to be subjected to an overload stimulus with each step taken by the animal. The application of the overload stimulus in the animal model and has been shown to produce reliable changes in muscle fiber composition [Citation[23]]. Tenotomy surgery was performed one week following instillation. Anesthesia was induced and maintained using 2–3% isoflurane with oxygen. The right hindlimb of each animal was shaved and sterile technique used to open the skin from just proximal to the knee to the calcaneus. The gastrocneumius and soleus muscle bellies and tendons were visualized and separated from the deeper plantaris tendon. The joint gastrocnemius and soleus tendon was cut distally from the calcaneus, while ensuring that the plantaris tendon remained intact. The distal one-third of the gastrocnemius muscle and the distal one-half of the soleus muscle were removed, to ensure that tendon reattachment could not occur. Skin was closed using metal sutures and the animal returned to its cage to recover. The left hindlimb of each animal served as the intact control limb. Animals were monitored until they were freely ambulating in their cages and grooming themselves normally, then were returned to the housing area. Animals were observed twice per day for behaviors indicating discomfort, and given analgesic as needed for 1–2 days (0.5 ml/kg of 0.3 mg/ml buprenorphine). At 5–7 days following surgery, sutures were removed.
Muscle preparation
Animals were sacrificed via carbon dioxide inhalation. Following euthanasia, the plantaris muscles from each hindlimb were removed, cleaned of obvious fat and tendon, weighed and frozen in isopentane cooled to the temperature of liquid nitrogen. Tissue samples were stored at −80°C until analysis.
Lung preservation and measurement
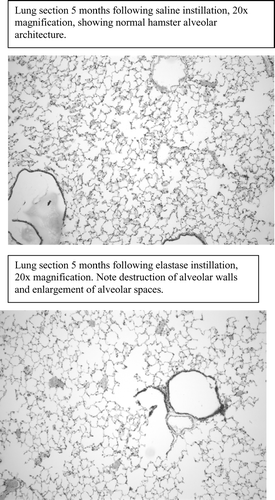
Following removal of hindlimb muscles, the trachea of each animal was cannulated with small-diameter polyethylene tubing attached to a reservoir of 4% formalin clamped 50 cm above the level of the trachea. The diaphragm was punctured to allow deflation of the lungs and formalin was allowed to flow by gravity to the trachea and observed to completely fill the lungs. Fixation was maintained for two hours at room temperature, and then the heart-lung block was removed and kept in formalin for 3–5 days at 4°C. Lungs were imbedded in paraffin, sectioned saggitally and placed on slides. Three sections taken at equally spaced intervals from the peripheral, mid-lung and central portions of the right and left lung of each animal were imaged at 20× magnification. Digital images were printed and an overlay grid 1 cm × 1 cm was placed onto the photos. Ten random vertical 1 cm lines were selected for each image and the number of times an alveolar wall was seen to intercept the line were counted (alveolar wall intercepts). These counts were summed for each lung section. This stereologic method to quantify lung damage was modified from earlier work done by other investigators using the elastase-instilled hamster model [Citation[22], Citation[24]].
Fiber composition determination
Cryosections from the mid-belly of each muscle (10 μ m) were stained for myofibrillar ATPase at a preincubation of pH = 4.58 for 3.5 minutes, followed by incubation in ATP solution at pH = 9.40 for 45 minutes at 37°C [Citation[23], Citation[24]]. Each complete muscle section was imaged (a total of 4–6 non-overlapping fields) at 20× magnification (Nikon Eclipse E800) and analyzed using software (Scion Image, Scion Corporation, Fredrick, MD, USA) to determine number of type I, IIA and IIB fibers present in the muscle section.
Capillary analysis
Cryosections (10 μ m) were stained for capillaries using a modified alkaline phosphatase reaction [Citation[25]]. Digital images of non-overlapping fields across the entire cross-section were then captured at 20× objective magnification. The number of capillaries and number of fibers present in all fields of each muscle section were counted. The capillary-to-fiber ratio (CFR) was then determined for each muscle section by dividing the sum of capillaries from each section by the sum of fibers in each section.
Statistical analysis
Analysis of variance procedures were performed to determine differences in fiber type composition and CFR due to emphysema alone as well as the effect of overload on disease-induced changes in each experiment. The effect of emphysema was determined by comparing the intact control (IC) limb plantaris muscle of animals with emphysema to the IC limb of the saline-instilled animals. The influence of overload on disease-related muscle changes was determined by comparing the overloaded (OV) plantaris muscle in the emphysema group to the OV muscle in the saline-instilled group. Analysis of variance was also used to compare alveolar wall intercepts between animals with emphysema and saline-instilled animals in each experiment. Significance was set at p < 0.05 for all analyses.
RESULTS
Alveolar wall intercepts
Lung damage due to elastase instillation was seen by a significant decrease in alveolar wall intercepts compared to saline-instilled animals, regardless of disease duration. A representative image of lung sections from animals with emphysema is shown in comparison to saline-instilled lungs in the . Alveolar wall intercepts of animals exposed to emphysema for 1 month were significantly less than saline-instilled animals in the same group (17.5 ± 3.6 vs. 21.4 ± 5.9 intercepts). Animals sacrificed after 3 months of emphysema had fewer intercepts than saline-instilled animals (16.0 ± 3.3 vs. 19.1 ± 3.4 intercepts). The same finding occurred in animals exposed to emphysema or saline for 5-months (15.2 ± 4.0 vs. 21.2 ± 3.2 intercepts).
Fiber type composition
There were no significant differences in fiber type composition due to emphysema in the 1-month emphysema experiment. Disease-related changes in fiber composition were seen in the IC limb in two of our experiments. As seen in , there was a significant increase in the percentage of type IIA fibers and concurrent decrease in type IIB fibers in animals with emphysema for 3-months. In animals exposed to emphysema for 5 months, there was a significantly lower percentage of type I fibers than the saline group.
Table 1 Fiber-type percentages in intact control muscle for different disease durations
Fiber type composition was not different between the emphysema and saline groups for any fiber type in the OV limb in any of the experiments. These results can be seen in .
Table 2 Fiber-type percentages in overloaded muscle
Capillary-to-fiber ratio
There were no significant changes in any of the experiments in CFR of the IC limb of animals with emphysema compared to saline animals. However, as seen in , the overloaded limb of animals with 5 months duration of emphysema had a significantly lower CFR than saline-instilled animals.
Table 3 Capillary-to-fiber ratio
DISCUSSION
The most important findings in this study were: [Citation[1]] emphysema resulted in peripheral muscle fiber composition changes beginning as early as 3 months after induction of emphysema; [Citation[2]] OV prevented peripheral muscle effects of emphysema regardless of disease duration; and [Citation[3]] OV decreased the CFR in peripheral muscle of animals with long duration disease.
Fiber type composition changes due to emphysema
This study is the first to examine peripheral muscle at time points less than 5 months after elastase-induced emphysema. Based on our analysis of lung sections, we found evidence of alveolar destruction 1 month following disease induction, however, we did not see changes in muscle fiber composition until 3 months. It is likely that events occurred in the lungs in response to direct contact with elastase that preceded any systemic factors that would change muscle composition. It has been theorized that systemic inflammation, high levels of inflammatory cytokines or tissue hypoxia may be responsible for peripheral muscle changes in humans with COPD [Citation[1], Citation[8], Citation[10]]. These same processes may be at work in the animal model, but may take longer to affect the muscle than the lung tissues. As the duration of emphysema is often unknown in studies of peripheral muscle in humans, it is also unclear if, and by how much, the lung changes precede muscular compositional changes.
The finding of muscle fiber composition changes 3 months following induction of emphysema suggests that the factors influencing peripheral muscle in COPD may be at work early in the disease process. This theory is supported by the work of Clark et al. [Citation[26]], who found that subjects with COPD had impairments in muscle strength even though they had only mild lung disease (mean FEV1 77% predicted). The shift of fiber type from IIB to IIA seen following 3 months exposure to emphysema suggests that peripheral muscle may be adapting in ways that could preserve oxidative capacity. This finding was surprising, as previous evidence in humans with severe COPD and in animals with longer duration emphysema has been a shift away from oxidative fiber composition. Perhaps there is a stimulus of early emphysema that induces shifts in muscle fiber types to help preserve endurance capacity. In contrast, the animals exposed to emphysema for 5 months showed a decrease in the percentage of type I fibers, which might correspond to impaired muscle oxidative capacity. This finding is consistent with other studies in animals and humans with severe COPD, and may indicate a limited capacity of the peripheral muscle to maintain type I fibers in the face of prolonged emphysema. More study is needed regarding the mechanisms at work related to duration of emphysema on peripheral muscle fiber composition as well as determining any effects on oxidative capacity. The animal model utilized in the current study could provide an ideal model for these studies.
Effect of muscle overload on disease-induced fiber type changes
Our model of muscle overload, selective tenotomy of synergistic muscles, was chosen to simulate increased muscle activity, such as exercise in humans. We found evidence that such activity prevented fiber type composition changes related to emphysema. If the fiber shifts seen following exposure to emphysema for 3 months (increased percentage of type IIA fibers) in the IC limb correspond with increases in muscle oxidative capacity, then preventing these shifts may not be desirable. However, chronic OV was clearly able to prevent the loss of type I fibers seen due to longer duration of emphysema. Since a decrease in type I fibers might correlate with a decline in oxidative capacity in the muscle, our data suggest that chronic overload may preserve peripheral muscle type I fiber content. Studies are needed to determine if the fiber composition changes seen do indeed correlate with changes in muscle oxidative capacity, as well as to examine in humans, whether increasing muscle activity has the same effects on muscle structure and function as in the animal model.
Effects of disease and overload on capillary-to-fiber ratio
The induction of emphysema alone had little effect on CFR in the plantaris muscle. As the plantaris muscle in the hamster is predominately type IIA and IIB fibers [Citation[25], Citation[27]], which have low CFR [Citation[25]], we may not have induced a large enough change in fiber type composition to cause a significant increase in the whole muscle CFR. In the OV limb of animals with 5 months duration of emphysema, there was a significant decline in CFR. This finding suggests that CFR in the muscle may be decreased either by chronic muscle overload or by other factors related to duration of the disease. Perhaps systemic factors, such as increases in inflammatory cytokines or hypoxia, may alter CFR adaptation to overload at this time point. Further studies should investigate the effects of emphysema on peripheral muscle angiogenesis in response to activity.
In conclusion, this study provided preliminary evidence that peripheral muscle is affected during the early stages of emphysema in ways that differ from those seen later in the disease process. Chronic muscle overload appears to be a powerful stimulus to prevent disease-related changes in fiber composition, but not CFR. More study is needed to determine the effects of disease duration and severity, as well as the influence of increased muscle activity, on muscle structure and function. Research using the animal model then needs to be translated to human studies to help determine the optimal type and timing of exercise interventions to preserve peripheral muscle in patients with COPD.
REFERENCES
- Mador M, Bozkanat E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Respir Res 2001; 2: 216–224, [PUBMED], [INFOTRIEVE], [CSA]
- Society. Skeletal muscle dysfunction in chronic obstructive pulmonary disease: a statement of the American Thoracic Society and European Respiratory Society. American Journal of Respiratory and Critical Care Medicine 1999; 159: S1–S40, [CSA]
- Troosters T, Gosselink R, Decramer M. Chronic obstructive pulmonary disease and chronic heart failure: two muscle diseases?. J Cardiopulm Rehab 2004; 24: 137–145, [CROSSREF], [CSA]
- Allaire J, Maltais F, Doyon J, Le Blanc P, Carrier G, Simard C, Jobin J. Peripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPD. Thorax 2004; 59: 673–678, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Jakobsson P, Jorfeldt L, Brundin A. Skeletal muscle metabolites and fibre types in patients with advanced chronic obstructive pulmonary disease (COPD), with and without chronic respiratory failure. Eur Respir J 1990; 3: 192–196, [PUBMED], [INFOTRIEVE], [CSA]
- Jobin J, Maltais F, Doyon J, Le Blanc P, Simard P, Simard A, Simard C. Chronic obstructive pulmonary disease: capillarity and fiber-type characteristics of skeletal muscle. J Cardiopulmonary Rehabil 1998; 18: 432–437, [CROSSREF], [CSA]
- Gerald L, Bailey W. Global initiative for Chronic Obstructive Lung Disease. J Cardiopulm Rehab 2002; 22: 234–244, [CROSSREF], [CSA]
- Casaburi R. Skeletal muscle function in COPD. Chest 2000; 117(5)267S–271S, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Maltais F, LeBlanc P, Jobin J, Casaburi R. Peripheral muscle dysfunction in chronic obstructive pulmonary disease. Clin Chest Med 2000; 21(1)665–677, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Agusti A, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 2003; 21: 347–360, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hayes J, Christensen T, Snider G. The hamster as a model of chronic bronchitis and emphysema in man. Lab Animal Science 1977; 27(5)762–770, [CSA]
- Lucey E, Stone P, Christensen T, Breuer R, Snider G. An 18-month study of the effects on hamster lungs of intratracheally administered human neutrophil elastase. Exp Lung Res 1988; 14: 671–686, [PUBMED], [INFOTRIEVE], [CSA]
- Farkas G, Roussos C. Diaphragm in emphysematous hamsters: sarcomere adaptability. J Appl Physiol 1983; 54(6)1635–1640, [PUBMED], [INFOTRIEVE], [CSA]
- Lewis M, Zhan W, Sieck G. Adaptations of the diaphragm in emphysema. J Appl Physiol 1992; 72(3)934–943, [PUBMED], [INFOTRIEVE], [CSA]
- Snider G, Sherter C, Koo K, Karlinsky J, Hayes J, Franzblau C. Respiratory mechanics in hamsters following treatment with endotracheal elastase or collagenase. J Appl Physiol 1977; 42(2)206–215, [PUBMED], [INFOTRIEVE], [CSA]
- Mattson J, Poole D. Pulmonary emphysema decreases hamster skeletal muscle oxidative enzyme capacity. J Appl Physiol 1998; 85(1)210–214, [PUBMED], [INFOTRIEVE], [CSA]
- Mattson J, Delp M, Poole D. Differential effects of emphysema on skeletal muscle fibre atrophy in hamsters. Eur Respir J 2004; 23: 703–707, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Farkas G, Roussos C. Histochemical and biochemical correlates of ventilatory muscle fatigue in emphysematous hamsters. J Clin Invest 1984; 74: 1214–1220, [PUBMED], [INFOTRIEVE], [CSA]
- Fournier M, Lewis M. Functional, cellular, and biochemical adaptations to elastase-induced emphysema in hamster medial scalene. J Appl Physiol 2000; 88: 1327–1337, [PUBMED], [INFOTRIEVE], [CSA]
- Poole D, Leiber R, Mathieu-Costello O. Myosin and actin filament lengths in diaphragms from emphysematous hamsters. J Appl Physiol 1994; 76(3)1220–1225, [PUBMED], [INFOTRIEVE], [CSA]
- Poole D, Kindig C, Behnke B. Effects of emphysema on diaphragm microvascular oxygen pressure. Am J Respir Crit Care Med 2001; 163: 1081–1086, [PUBMED], [INFOTRIEVE], [CSA]
- Hayes J, Korthy A, Snider G. The pathology of elastase-induced panacinar emphysema in hamsters. J Path 1975; 117(1)1–14, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Tamaki T, Shiraishi T. Characteristics of compensatory hypertrophied muscle in the rat: II. Comparison of histochemical and functional properties. Anatomical Record 1996; 246: 335–342, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Snider G, Korthy A. Internal surface area and number of respiratory air spaces in elastase-induced emphysema in hamsters. Am Rev Resp Dis 1978; 117: 685–693, [PUBMED], [INFOTRIEVE], [CSA]
- Swisher A, Alway S, Yeater R. Capillary-to-fiber ratio of hind limb muscles in the male Syrian golden hamster. Anat Record 2004; 227A(2)272–274, [CROSSREF], [CSA]
- Clark C, Cochrane L, Mackay E, Paton B. Skeletal muscle strength and endurance in patients with mild COPD and the effects of weight training. Eur Respir J 2000; 15: 92–97, [PUBMED], [INFOTRIEVE], [CSA]
- Mattson J, Miller T, Poole D, Delp M. Fiber composition and oxidative capacity of hamster skeletal muscle. J Histochem Cytochem 2002; 50: 1685–1692, [PUBMED], [INFOTRIEVE], [CSA]