Abstract
This study compared airway responsiveness in vitro, as measured in isolated bronchi, with responsiveness in vivo in patients with COPD and smokers with normal lung function. In 9 patients with COPD (mean (range) FEV1 55 (30–78) %predicted) and 8 smokers with normal lung function (FEV1 101 (89–117) %predicted), who underwent surgery for lung cancer, responses to inhaled histamine and salbutamol were assessed before surgery. Bronchial specimens of 1–4 mm internal diameter were studied in the organ bath and histamine concentration-response curves assessed. All patients with COPD and none of the control individuals were hyperresponsive to inhaled histamine. Five patients with COPD and no control patient showed a bronchodilator response to salbutamol. Opposite to these findings, bronchial rings in the organ bath demonstrated a rightward shift of histamine concentration-response curves in COPD compared to controls, (p < 0.005). Accordingly, pED50 but not Emax differed statistically (p = 0.0016) between groups, mean ± SEM values of pED50 in COPD (controls) being 4.67 ± 0.08 (5.29 ± 0.15) and of Emax 672 ± 86 (772 ± 120) mg. Patients with COPD showing hyperresponsiveness to inhaled histamine demonstrated lower responsiveness of their isolated bronchi compared to smokers with normal lung function. This suggests that in vivo hyperresponsiveness is based on other mechanisms than alterations in smooth muscle physiology.
BACKGROUND
Airway hyperresponsiveness to inhaled histamine or methacholine is not uncommon in patients with chronic obstructive pulmonary disease (COPD) [Citation[1]]. Many patients with moderate to severe disease also show obstructive airway responses to inhalation of other stimuli, such as adenosine [Citation[2]] or hypertonic saline aerosol [Citation[3]]. The mechanisms mediating these responses could comprise alterations of smooth muscle physiology as described in asthma [Citation[4]], the nervous system [Citation[5]], inflammation [Citation[6]], airway edema [Citation[7]], reduction of airway lumen [Citation[6]], alterations of airway structure [Citation[8]], or destruction of the surrounding parenchyma [Citation[9]]. These factors are difficult to disentangle both in vivo and in vitro, and most conclusions are indirect [Citation[9]].
The available data on the relationship between airway responsiveness in vivo and in vitro in patients with or without COPD do not provide a clear picture. Most investigators found no associations or disease-related differences, using histamine, methacholine, carbachol or electric field stimulation in bronchial preparations [Citation[10], Citation[11], Citation[12], Citation[13], Citation[14], Citation[15]]. In contrast, bronchiolar strips from patients with COPD developed higher force after administration of histamine but not methacholine [Citation[16]], and bronchial rings from smokers with mild airway obstruction showed stronger histamine responses than non-or ex-smokers with normal lung function [Citation[17]]. The loss of overall performance observed in patients with more advanced COPD does however not easily suggest enhanced responses of the airway smooth muscle per se in these patients, and there seems to be no correlation between airflow obstruction and the amount of smooth muscle [Citation[18]]. As some of the stimuli causing obstruction might act through mechanisms which do not require an altered response of the muscle itself, it is an open question whether hyperresponsiveness in more advanced COPD is due to increased smooth muscle reactivity. We hypothesized that by measuring reactivity of isolated bronchial rings in vitro the role of airway smooth muscle shortening for the airway responsiveness in vivo can be determined.
We therefore assessed histamine responses of isolated bronchial specimens of patients with moderate to severe COPD, compared to smokers with normal lung function. Airway responsiveness to inhaled histamine and the bronchodilator effect of inhaled salbutamol were determined before surgery, and the results were compared with the in vitro data.
METHODS
Patients
Seventeen patients undergoing lung surgery because of a peripheral lesion were included (). The control group comprised 8 patients without airway obstruction (range of FEV1 89–117% predicted [Citation[19]]). Nine patients had the diagnosis of COPD (FEV1 30–78% predicted). All patients were smokers. Patients with COPD were recruited on the basis of their clinical diagnosis and lung function impairment and categorised as stage II (moderate, n = 4) or III (severe, n = 5) according to GOLD criteria [Citation[20]]. Among the control patients, three reported symptoms of chronic bronchitis and were thus categorised as stage 0. None of the patients had lung diseases other than COPD and/or the tumor which led to surgery, or was selected for airway responsiveness or bronchodilator responses. Furthermore, none of the patients showed a positive skin prick test to a set of common allergens (Allergopharma, Reinbek, Germany). The study was approved by the local Ethics Committee and patients gave their written informed consent.
Table 1 Patients' characteristics and results of in vitro measurements
All patients with COPD were under treatment with short-acting inhaled β2-adrenoceptor agonists and/or anticholinergics, and 5 additionally with inhaled steroids, whereas the control patients did not have medication. Regarding baseline characteristics there were no other significant differences between groups, except for lung function ().
Assessment of functional characteristics
Lung function measurements including the determination of forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and single breath diffusion capacity for carbon monoxide (DLCO) were performed following established guidelines [Citation[19]] using standard equipment (Masterlab, Jaeger, Höchberg, Germany). Histamine responsiveness was assessed following a standard protocol [Citation[21]], whereby provocative concentrations PC20FEV1 of less than or equal 8 mg/ml indicated hyperresponsiveness. Bronchodilator responses were quantified as absolute and percent increase of FEV1 measured 15 min after inhalation of 200 μ g salbutamol.
Tissue preparation
Macroscopically normal bronchial specimens were taken immediately after resection as far as possible from the tumor. We prepared peripheral airways of 1–4 mm internal diameter free from alveolar tissue which were cut into rings of 2–4 mm length. After storage for 20 hours at 4°C in glass vessels containing modified Krebs buffer (NaCl 118.4 mM, KCl 4.7 mM, MgSO4 0.6 mM, CaCl2 1.3 mM, KH2PO4 1.2 mM, NaHCO3 25.0 mM, glucose 11.1 mM), specimens were transferred into 10 mL organ baths containing oxygenated (95% O2, 5% CO2) modified Krebs buffer (pH 7.4; 37°C). Prior to measurements they were equilibrated for 2 hours at a resting tension of 400 mg on average.
Measurements in isolated bronchi
Responses were assessed as changes in isometric tension in 3–8 (mean 5.9) specimens per patient. At the beginning of each experiment a single dose of isoprenaline (1 μM) was administered to achieve a standardised history for all specimens. After several washings and reequilibration, histamine concentration-response curves were determined. The curves covered a range from 10 nM to 0.3 mM, whereby concentrations were increased at half log10 intervals. Finally, the wet weight of each bronchus was determined after removal of surface fluid by filter paper.
Data analysis
Mean values of concentration-response curves from each patient's specimens were taken for analysis. The potency of histamine was calculated by fitting a sigmoid curve and expressed as pED50, i.e., the negative log10 of the cumulative dose of histamine yielding the half-maximal effect. The maximum contraction Emax was obtained by the same fitting procedure.
Concentration-response curves were compared between groups using repeated measures ANOVA, with groups as between-factor and histamine concentration as within-factor. Emax and pED50 as well as lung function variables were compared between groups by the unpaired t-test. To determine potential effects of IgE level or bronchial mass, these were included as covariates where appropriate (ANCOVA). In addition to mean values and standard errors (SEM), 95%-confidence intervals were computed. Correlation analyses were performed by Spearman's rank correlation. Statistical significance was assumed for p < 0.05.
RESULTS
Airway responses to histamine and salbutamol in vivo
None of the control patients showed airway hyperresponsiveness to inhaled histamine, whereas all patients with COPD demonstrated values of PC20FEV1 of less than 8 mg/mL (). Furthermore 5/9 patients with COPD showed a positive response to salbutamol defined as an increase in FEV1 of at least 15% and 200 mL. In contrast, maximum responses were 80 mL and 2.7% in the control group.
Responses of bronchial rings to histamine
The mass of rings did not significantly differ between groups (mean ± SEM (95%-CI): controls 53.7 ± 12.2 (23.8–83.6) mg, COPD 47.0 ± 12.2 (18.9–75.1) mg). Histamine caused concentration-dependent contraction in all specimens, and responses were significantly different between the two groups as judged from fixed effect (p = 0.0015) and interaction (p < 0.0001) terms of ANOVA using the first six concentrations (). When adding further concentrations, significance levels decreased owing to the fact that some of the airways had reached their maximum response.
Figure 1 Mean (±SEM) concentration-response curves (mg tension vs molar concentration of histamine) obtained in control smokers (open circles) and patients with COPD (closed circles).
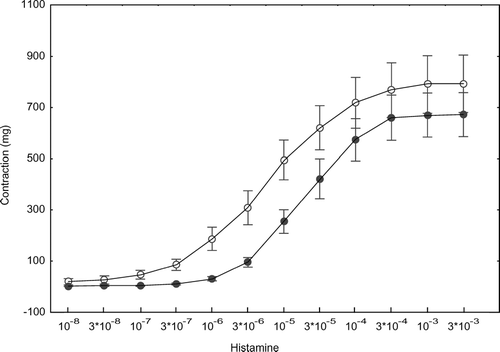
The difference in curves was reflected in a difference in pED50 (p = 0.0016). When log IgE or bronchial mass were introduced as a covariates, the difference in pED50 remained significant (ANCOVA, p = 0.0047 and p < 0.001, respectively). There was no relationship between pED50 and IgE, but a correlation between pED50 and mass in this type of analysis (p = 0.043). More specifically, in the group with COPD but not in the control group or total population, rank correlation analysis showed pED50 to be related to the mass of specimens (rs = − 0.80, p = 0.010; ). The magnitude of maximal contractions as quantified by Emax did not differ significantly between groups, independently of covariates introduced. Furthermore, the findings regarding ED50 and maximum response were not altered with regard to the presence of statistical differences between groups, when contraction was expressed per mass of bronchus ().
Figure 2 Relationship between histamine responsiveness of isolated bronchi in vitro in terms of the potency pED50 (x-axis) and responsiveness to histamine in vivo in terms of the provocative concentration PC20FEV1 (panel A, log scale), % change of FEV1 after inhalation of salbutamol (panel B), baseline FEV1 %predicted (panel C), and the mass of bronchial specimens (panel D). Control patients are represented by open circles, patients with COPD by closed circles.
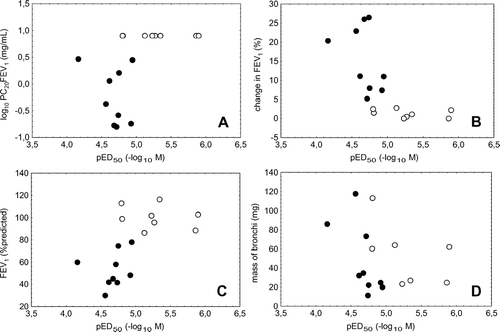
Relationship between in vitro and in vivo characteristics
As a result of the differences between groups, pED50 was correlated with PC20FEV1 (rs = 0.69, p = 0.002; ), the percent response of FEV1 to salbutamol (rs = −0.81, p < 0.001; ), as well as baseline FEV1 %predicted (rs = 0.75, p < 0.001; ) and FEV1%FVC (rs = 0.63, p = 0.007). If restricted to one of the groups, there were no significant correlations between these variables.
DISCUSSION
The present data demonstrate that histamine responsiveness of isolated bronchial rings is decreased in patients with, on average, severe COPD compared to smokers without airway obstruction. On the contrary, patients with COPD showed hyperresponsiveness to inhaled histamine and more pronounced bronchodilator responses than the control smokers. The opposite alterations of in vivo and in vitro airway responsiveness provoke the conclusion that the mechanisms mediating airway hyperresponsiveness in patients with severe COPD do not involve increased responsiveness of the airway smooth muscle itself.
A potential bias might have been the medication of patients with COPD. Isolated airways of patients with and without corticosteroids did not show apparent differences in pED50, the respective values being 4.70 and 4.53. It is furthermore known that the effect of inhaled steroids on bronchial responsiveness in vivo is small [Citation[22]]. As patients were treated with short-acting bronchodilators only, residual effects are likely to have disappeared within the 1-day interval until the start of measurements. We additionally administered isoprenaline to standardise contraction history. There is also no evidence that the disease state has influenced the result [Citation[23]], as we used histamine as agonist and both groups had lung tumors of comparable staging (tumor stadium mostly I, maximum IIa). Our data showed only a weak relationship between bronchial mass and maximum response and a negative correlation between mass and pED50 in the group with COPD, indicating higher potency with lower mass. But even when selecting only low or high mass bronchi, there remained a clear difference between groups (). We therefore believe that the result obtained was not due to noncomparability of bronchial preparations.
The majority of studies on the relationship between in vivo and in vitro airway responsiveness did not find a correlation in patients with or without airway obstruction or COPD or differences between groups [Citation[10], Citation[11], Citation[12], Citation[13], Citation[14], Citation[15]]. Most of these studies included only single patients with severe COPD, and on average patients showed only mild to moderate lung function impairment, in contrast to the present study performed in stage II-III patients [Citation[20]]. One study, however, included a well-defined group of patients with severe COPD and compared it with a group without airway obstruction [Citation[16]]. In fact, with regard to lung function groups were quite comparable to those studied by us. Different from other studies including the present one, bronchiolar strips from distal airways were taken instead of bronchial preparations from relatively proximal airways. Interestingly, in terms of maximal isometric force per tissue weight, patients with COPD showed increased responses to histamine but not methacholine compared to control patients. These results appear to be in contrast with the results obtained in bronchial rings in the present study, as we found no difference in maximum responses. Obviously, however, comparisons of large and small airways in vitro are subject to differences in methodology and probably as difficult to evaluate as the contribution of central vs peripheral airways to airway responsiveness in vivo in COPD.
A link between smoking, IgE, and hyperresponsiveness has been reported and increased serum IgE levels have been associated with increased reactivity in vitro [Citation[24]]. Smokers often show elevated serum IgE levels [Citation[25]], but the increase in airway responsiveness compared to ex-or neversmokers, both in vivo [Citation[26]] and in vitro [Citation[17]], is only partially dependent on IgE. In the present study IgE levels were > 200 U/mL in 2 subjects in the control group and in 3 patients with COPD and we found no relationship between pED50 and IgE. Smoking per se seems to be capable of inducing airway hyperresponsiveness [Citation[27], Citation[28]]. On the other hand it has been argued that the higher prevalence of hyperresponsiveness in smokers might be a consequence of the reduction in baseline airway caliber [Citation[29]]. However results form previous studies [Citation[17]] and our current findings suggest that that the difference in pED50 between groups that we found was due to a reduction of the potency of histamine in COPD and not based on an increase of responsiveness in the control group of smokers. The most likely explanation seems to be that smoking, in particular if accompanied by elevated IgE level, leads to enhanced maximum histamine responses of isolated bronchi, without a shift in potency—provided there is only mild or no COPD. With more severe disease, however, the potency of histamine and thus the responsiveness of the bronchi should decrease, without a marked change in maximum contraction.
Non-specific airway hyperresponsiveness has been reported in a great proportion of smokers with airflow limitation or patients with COPD [Citation[1]] but the underlying mechanisms so far remain unclear. Several potential reasons for increased airway reactivity in patients with COPD have been implicated, including inflammatory changes in the airways, loss of lung parenchyma, airway thickening and increased amount smooth muscle and increased smooth muscle reactivity. The inflammatory environment, particularly through the action of neutrophils, of the smooth muscle [Citation[6]] could favour an enhanced responsiveness, suggested also by finding of induced hyperresponsiveness during neutrophilic responses [Citation[30]]. Based on the marked decrease of airway reactivity in vitro in contrast to the increase reactivity in vivo in patients with COPD in the present study, the question is raised whether hyperresponsiveness as measured in inhalation challenges involves other factors than bronchial smooth muscle contractility. Obviously, the surrounding structure as well as that of the airway itself could influence contraction due to altered mechanical properties, structural constraints [Citation[29]], the amount of smooth muscle [Citation[8]] or due to emphysema. Indeed in the present study patients with COPD showed a decrease in CO-diffusion capacity which has been shown to correlate with the degree of emphysema [Citation[31]]. However, previous studies suggested that in patients with COPD emphysema is not primalry responsible for expiratory airflow limitation [Citation[31]] and in vivo airway reactivity is mainly due to altered airway geometry and not due to parenchymal destruction [Citation[32]]. In COPD airways are also thickened [Citation[6], Citation[33]] and might be even stiffer than normal but the surrounding tissue is rarefied. It could also be argued that due to the airway wall thickening perfectly normal muscle shortening could increase the resistance of the airway substantially. Whether the autonomic nervous system plays a significant role, is also difficult to decide [Citation[5]]. Increased responsiveness to histamine could also be due to effects of histamine on other tissues than bronchial smooth muscle. Evidence from viral infections indicates that plasma extravasation plays a role in airway narrowing [Citation[7]], and most patients with moderate to severe, in contrast to mild, COPD show significant airway responses to hypertonic saline aerosol which can cause airway edema [Citation[3], Citation[34], Citation[35]]. The resultant narrowing through fluid transport might compensate for a decreased response of the airway smooth muscle itself. Although these different considerations are no more than speculations, they could offer an explanation for the presence of airway hyperresponsiveness in moderate to severe COPD even with decreased airway reactivity in vitro.
In conclusion, patients with severe COPD who showed airway hyperresponsiveness to inhaled histamine demonstrated lower responsiveness of their isolated airways compared to smokers with normal lung function. This result suggests that in vivo hyperresponsiveness in these patients is not due to increased smooth muscle reactivity but involves other mechanisms than alterations in smooth muscle physiology.
LIST OF ABBREVIATIONS
| COPD: | = | chronic obstructive pulmonary disease |
| DLCO: | = | single breath diffusion capacity for carbon monoxide |
| Emax: | = | maximum contraction |
| FEV1: | = | forced expiratory volume in 1 second |
| FVC: | = | forced vital capacity |
| IgE: | = | immunoglobulin E |
| PC20FEV1: | = | provocative concentrations with a 20% decrease in FEV1 |
| pED50: | = | negative log10 of the cumulative dose of histamine yielding the half-maximal effect |
| SEM: | = | standard error of mean |
Supported by Landesversicherungsanstalt (LVA)-Freie und Hansestadt Hamburg.
REFERENCES
- Postma D S, Kerstjens H A. Characteristics of airway hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158: S187–192, [PUBMED], [INFOTRIEVE], [CSA]
- Polosa R, Rorke S, Holgate S T. Evolving concepts on the value of adenosine hyperresponsiveness in asthma and chronic obstructive pulmonary disease. Thorax 2002; 57: 649–654, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Taube C, Holz O, Mücke M, Jörres R A, Magnussen H. Airway response to inhaled hypertonic saline in patients with moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 1810–1815, [PUBMED], [INFOTRIEVE], [CSA]
- Mitchell R W, Rühlmann E, Magnussen H, Leff A R, Rabe K F. Passive sensitisation of human bronchi augments smooth muscle shortening velocity and capacity. Am J Physiol 1994; 267: 218–222, [CSA]
- Belvisi M G. Overview of the innervation of the lung. Curr Opin Pharmacol 2002; 2: 211–215, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hogg J C, Chu F, Utokaparch S, Woods R, Elliott W M, Buzatu L, Cherniack R M, Rogers R M, Sciurba F C, Coxson H O, Pare P D. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 2645–2653, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Hegele R G, Hayashi S, Hogg J C, Paré P D. Mechanisms of airway narrowing and hyperresponsiveness in viral respiratory tract infections. Am J Respir Crit Care Med 1995; 151: 1659–1665, [PUBMED], [INFOTRIEVE], [CSA]
- Tiddens H A, Hofhuis W, Bogaard J M, Hop W C, de Bruin H, Willems L N, de Jongste J C. Compliance, hysteresis, and collapsibility of human small airways. Am J Respir Crit Care Med 1999; 160: 1110–1118, [PUBMED], [INFOTRIEVE], [CSA]
- Verhoeven G T, Verbraak A F, Boere-van der Straat S, Hoogsteden H C, Bogaard J M. Influence of lung parenchymal destruction on the different indexes of the methacholine dose-response curve in COPD patients. Chest 2000; 117: 984–990, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Vincenc K S, Black J L, Yan K, Armour C L, Donnelly P D, Woolcock A J. Comparison of in vivo and in vitro responses to histamine in human airways. Am Rev Respir Dis 1983; 128: 875–879, [PUBMED], [INFOTRIEVE], [CSA]
- Armour C L, Black J L, Berend N, Woolcock A J. The relationship between bronchial hyperresponsiveness to methacholine and airway smooth muscle structure and reactivity. Respir Physiol 1984; 58: 223–233, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Armour C L, Lazar N M, Schellenberg R R, Taylor S M, Chan N, Hogg J C, Paré P D. A comparison of in vivo and in vitro human airway reactivity to histamine. Am Rev Respir Dis 1984; 129: 907–910, [PUBMED], [INFOTRIEVE], [CSA]
- Roberts J A, Raeburn D, Rodger I W, Thomson N C. Comparison of in vivo airway responsiveness and in vitro smooth muscle sensitivity to methacholine in man. Thorax 1984; 39: 837–843, [PUBMED], [INFOTRIEVE], [CSA]
- Taylor S M, Paré P D, Armour C L, Hogg J C, Schellenberg R R. Airway reactivity in chronic obstructive pulmonary disease. Failure of in vivo methacholine responsiveness to correlate with cholinergic, adrenergic, or nonadrenergic responses in vitro. Am Rev Respir Dis 1985; 132: 30–35, [PUBMED], [INFOTRIEVE], [CSA]
- Cerrina J, Le Roy Ladurie M, Labat C, Raffestin B, Bayol A, Brink C. Comparison of human bronchial muscle responses to histamine in vivo with histamine and isoproterenol agonists in vitro. Am Rev Respir Dis 1986; 134: 57–61, [PUBMED], [INFOTRIEVE], [CSA]
- de Jongste J C, Mons H, Block R, Bonta I L, Frederiksz A P, Kerrebijn K F. Increased in vitro histamine responses in human small airways smooth muscle from patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1987; 135: 549–553, [PUBMED], [INFOTRIEVE], [CSA]
- Schmidt D T, Jörres R A, Rühlmann E, Rabe K F. Isolated airways from current smokers are hyper-responsive to histamine. Clin Exp Allergy 2001; 31: 1041–1047, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Tiddens H A, Paré P D, Hogg J C, Hop W C, Lambert R, de Jongste J C. Cartilaginous airway dimensions and airflow obstruction in human lungs. Am J Respir Crit Care Med 1995; 152: 260–266, [PUBMED], [INFOTRIEVE], [CSA]
- Quanjer P H, Tammeling G J, Cotes J E, Pedersen O F, Peslin R, Yernault J C. Lung volumes and forced ventilatory flows. Eur Respir J 1993; 16: S5–S40, [CSA]
- Pauwels R A, Buist A S, Calverley P M, Jenkins C R, Hurd S S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001; 163: 1256–1276, For the update of definitions (2004) see: http://www.goldcopd.com/, [PUBMED], [INFOTRIEVE], [CSA]
- Jörres R A, Nowak D, Kirsten D, Grönke L, Magnussen H. A short protocol for methacholine provocation testing adapted to the Rosenthal–Chai dosimeter technique. Chest 1997; 111: 866–869, [CSA]
- Verhoeven G T, Hegmans J P, Mulder P G, Bogaard J M, Hoogsteden H C, Prins J B. Effects of fluticasone propionate in COPD patients with bronchial hyperresponsiveness. Thorax 2002; 57: 694–700, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Armour C L, McKay K O, Johnson P R, Glanville A R, Black J L. Does the disease state influence the responsiveness of human airways studied in vitro?. J Appl Physiol 1996; 80: 2211–2216, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Schmidt D, Watson N, Ruehlmann E, Magnussen H, Rabe K F. Serum immunoglobulin E levels predict human airway reactivity in vitro. Clin Exp Allergy 2000; 30: 233–241, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Oryszczyn M P, Annesi-Maesano I, Charpin D, Paty E, Maccario J, Kauffmann F. Relationships of active and passive smoking to total IgE in adults of the Epidemiological Study of the Genetics and Environment of Asthma, Bronchial Hyperresponsiveness, and Atopy (EGEA). Am J Respir Crit Care Med 2000; 161: 1241–1246, [PUBMED], [INFOTRIEVE], [CSA]
- Sunyer J, Antó J M, Kogevinas M, Soriano J B, Tobias A, Munoz A. Smoking and bronchial responsiveness in nonatopic and atopic young adults. Spanish Group of the European Study of Asthma. Thorax 1997; 52: 235–238, [PUBMED], [INFOTRIEVE], [CSA]
- Barrett E G, Wilder J A, March T H, Espindola T, Bice D E. Cigarette smoke-induced airway hyperresponsiveness is not dependent on elevated immunoglobulin and eosinophilic inflammation in a mouse model of allergic airway disease. Am J Respir Crit Care Med 2002; 165: 1410–1418, [PUBMED], [INFOTRIEVE], [CROSSREF], [CSA]
- Rijcken B, Schouten J P, Mensinga T T, Weiss S T, De Vries K, Van der Lende R. Factors associated with bronchial responsiveness to histamine in a population sample of adults. Am Rev Respir Dis 1993; 147: 1447–1453, [PUBMED], [INFOTRIEVE], [CSA]
- Seow C Y, Wang L, Paré P D. Airway narrowing and internal structural constraints. J Appl Physiol 2000; 88: 527–433, [PUBMED], [INFOTRIEVE], [CSA]
- De Lorme M P, Yang H, Elbon-Copp C, Gao X, Barraclough-Mitchell H, Bassett D J. Hyperresponsive airways correlate with lung tissue inflammatory cell changes in ozone-exposed rats. J Toxicol Environ Health A 2002; 65: 1453–1470, [CROSSREF], [CSA]
- Gelb A F, Schein M, Kuei J, Tashkin D P, Muller N L, Hogg J C, Epstein J D, Zamel N. Limited contribution of emphysema in advanced chronic obstructive pulmonary disease. Am Rev Respir Dis 1993; 147: 1157–1161, [PUBMED], [INFOTRIEVE], [CSA]
- Verna V K, Cockcroft D W, Dosman J A. Airway responsiveness to inhaled histamine in chronic obstructive airway disease. Chronic bronchitis vs emphysema. Chest 1988; 94: 457–461, [CSA]
- Riess A, Wiggs B, Verburgt L, Wright J L, Hogg J C, Paré P D. Morphologic determinants of airway responsiveness in chronic smokers. Am J Respir Crit Care Med 1996; 154: 1444–1449, [PUBMED], [INFOTRIEVE], [CSA]
- Hogman M, Almirall J, Mork A C, Roomans G M, Hagelqvist E, Lagerstrand L, Hedenstierna G. Nebulisation of hypertonic saline causes oedema of the airway wall. J Submicrosc Cytol Pathol 1997; 29: 59–64, [PUBMED], [INFOTRIEVE], [CSA]
- Zühlke I E, Kanniess F, Richter K, Nielsen-Gode D, Bohme S, Jorres R A, Magnussen H. Montelukast attenuates the airway response to hypertonic saline in moderate-to-severe COPD. Eur Respir J 2003; 22: 926–930, [CSA]