Abstract
Oxidative stress may play a role in chronic obstructive pulmonary disease (COPD) exacerbations. There is heterogeneity in the literature with regard to the impact of antioxidant therapy on COPD exacerbation frequency. Clinical trials of N-acetylcysteine in COPD were identified in unrestricted searches of MEDLINE, CINAHL, International Pharmaceutical Abstracts and the Cochrane Register. Randomized, controlled trials which reported exacerbations over a treatment period ≥3 months were selected. Two observers independently extracted data regarding exacerbation number over the treatment period in subjects allocated to either N-acetylcysteine or placebo. Data were analyzed using inverse-variance weighted random effects meta-analysis methodology. Meta-analysis of data from 8 trials (randomized n = 2,214) indicated that N-acetylcysteine significantly reduced the odds of experiencing one or more exacerbations over the treatment period (odds ratio = 0.49, 95% confidence interval [0.32–0.74], p = 0.001). Treatment effect was not reduced in studies which enrolled > 50% active smokers (odds ratio = 0.36 [0.24–0.55], p < 0.001), although a greater effect was observed with exclusion of subjects using concurrent inhaled corticosteroids (odds ratio = 0.42 [0.32–0.54], p < 0.0001), suggesting that inhaled steroids attenuate the effect of N-acetylcysteine. The use of N-acetylcysteine significantly reduces the odds of exacerbation in patients with COPD, an effect possibly attenuated by inhaled steroids but not smoking. This analysis suggests treatment with N-acetylcysteine may be beneficial in a subset of patients with COPD.
Key words: :
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a syndrome of chronic airway inflammation, initiated in most cases by chronic tobacco smoke exposure (Citation[1], Citation[2]). Chronic airway inflammation damages the airways and lung parenchyma over many years, leading to a doubling in the rate of decline in the forced expiratory volume in one second (FEV1) from 30 to approximately 60 mL/year (Citation[3]). This accelerated functional deterioration is accompanied by the development of cough, sputum production, dyspnea and abnormal gas exchange and leads to an increased risk of exacerbations. Exacerbation frequency increases as the disease progresses, further accelerating lung function decline (Citation[4]).
Although a variety of drugs are employed in COPD with the goal of improving airflow, symptoms and quality of life, only smoking cessation has been shown to significantly modify the long-term progression of airflow limitation in this disorder (Citation[5]). This beneficial effect of smoking cessation is likely mediated in part by a reduction in oxidative stress and thereby airway inflammation. Other attempts to modify airway inflammation in COPD have met with limited success, and while inhaled corticosteroids have modest physiologic and clinical benefits in some patients (Citation[6]), there are no highly effective modulators of inflammation currently available for use in COPD.
Oxidative stress (an excess of oxidants relative to antioxidants) is postulated to play an important role in the pathogenesis and progression of COPD. Tobacco smoke contains numerous oxidants such as hydrogen peroxide, superoxide, hydroxyl radical and nitric oxide (Citation[7]), and studies have demonstrated that cigarette smoking induces oxidative stress in the airways and blood, while COPD itself is marked by oxidative stress independent of smoking status (Citation[8], Citation[9], Citation[10]). Tobacco smoke may ultimately lead to COPD by creating an oxidizing environment in the airways and parenchyma, resulting in activation of transcription factors, enhanced production of cytokines and inflammatory cell recruitment (Citation[11]). Oxidative stress is also increased by COPD exacerbations, but it remains uncertain whether long-term modulation of oxidant:antioxidant balance decreases airway inflammation or exacerbation frequency.
N-acetylcysteine (NAC), an inexpensive and widely-available non-prescription drug, increases intracellular glutathione stores and may augment antioxidant defenses. The assessment of its effect has been complicated by heterogeneity in published studies with regard to inclusion criteria, duration of intervention, primary outcome and overall efficacy. For example, while prior clinical trials and systematic reviews have shown a beneficial effect of NAC with regard to COPD exacerbations, the largest and most recently-published of these clinical trials concluded that NAC was ineffective at preventing deterioration of lung function or exacerbations (Citation[12]). We hypothesized that oral NAC would reduce exacerbations in patients with COPD and that this effect could possibly be modified by concomitant smoking or use of inhaled corticosteroids. We tested this hypothesis by means of an inverse-variance weighted random effects meta-analysis of published long-term randomized, controlled trials.
MATERIALS AND METHODS
This meta-analysis was conducted according to QUOROM guidelines (Citation[13]).
Data sources and study selection
A literature search was conducted by crossing Medical Subjects Headings chronic obstructive pulmonary disease, chronic bronchitis, pulmonary emphysema with acetylcysteine in MEDLINE (1966–November 2005 (week 3)), CINAHL (1982–November 2005 (week 3)), International Pharmaceutical Abstracts (1970–November 2005 (week 3)) and the Cochrane trials register (third quarter, 2005). The search was restricted to clinical trials but not specific journal subsets or languages. Bibliographies of retrieved articles were reviewed to identify additional candidate studies, and discussions with experts were undertaken to uncover additional relevant data.
Included studies met the following criteria—Design: randomized, controlled clinical trial of orally-administered N-acetylcysteine plus standard therapy versus placebo plus standard therapy in subjects with COPD; Duration of follow-up: ≥3 months; Reported outcome: numbers of exacerbations in one of two treatment arms: N-acetylcysteine + standard care or placebo + standard care; and Publication venue: not published solely in abstract form.
Using the criteria above, search results were reviewed and selected for inclusion as follows: citations were evaluated using a sequential screening approach which determined relevance first by title, then by abstract and finally by manuscript review. Screening was performed independently by two authors (ERS, RPB) and trials were selected and agreed upon by consensus of all three authors.
Outcome variable and data extraction
The primary outcome variable for this analysis was the odds of experiencing ≥1 exacerbation over the duration of the study. Where possible, primary count data were extracted in 2 × 2 form, with patients grouped by treatment allocation (N-acetylcysteine versus placebo) and by the dichotomous outcome of 0 versus ≥ 1 exacerbations. Inability to obtain primary 6-month count data from one large trial (Citation[12]) in which frequency rather number of exacerbations was reported required that count data be extrapolated by halving the annualized exacerbation frequency and multiplying the number of subjects allocated to either placebo or N-acetylcysteine by this 6-month exacerbation frequency. Number of subjects, age, baseline lung function, smoking prevalence, treatment regimens, study duration and study-specific definition of exacerbations were also extracted to facilitate assessment of between-study comparability of inclusion and demographic criteria.
Statistical analysis
Stata version 9 (Stata Corporation, College Station, Texas) was used for all analyses. Inverse-variance weighted meta-analysis (Citation[14]) (Stata command meta logor selogor, eform) was used to generate random effects summary estimates. Random effects methodology was chosen to account for both within-study and between-study variation. Heterogeneity of data was evaluated using the Q statistic (Citation[15]). Summary odds ratios were represented as a point estimate and 95% confidence intervals on a forest plot (Citation[16]), and publication bias was evaluated by means of a funnel plot (Citation[17], Citation[18]). Based on the hypothesis that smoking or inhaled steroids might modify N-acetylcysteine effect, an a priori determination was made to stratify analyses, where possible, on clinically important variables including baseline lung function, current smoking prevalence and inhaled corticosteroid use. Number needed to treat was calculated as [1/absolute risk reduction] (Citation[19]).
RESULTS
Study selection
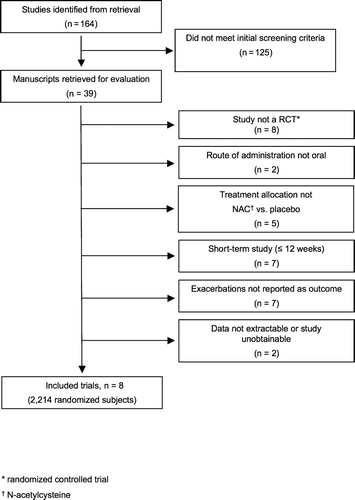
The search strategy () identified 164 unique and potentially relevant citations. Of these, 125 citations did not meet initial title screening criteria, leaving 39 citations for which manuscripts were obtained and their abstracts reviewed for inclusion. Of these abstracts, 31 were excluded for the following reasons: 8 were not randomized, controlled clinical trials, 2 did not administer N-acetylcysteine via the oral route, 5 did not compare N-acetylcysteine plus standard care versus placebo plus standard care, 7 were short-term studies (range 4 hours–3 months), 7 did not report exacerbations as an outcome, data could not be extrapolated or obtained from 1 study, and 1 manuscript was unobtainable. The 8 remaining studie (Citation[12], Citation[20], Citation[21], Citation[22], Citation[23], Citation[24], Citation[25], Citation[26]) were included in this meta-analysis.
Subjects and individual trial characteristics
The included studies randomized 2,214 subjects, and data from 1,819 subjects (82% of randomized subjects) were available for inclusion in the meta-analysis. reports age, baseline forced expiratory volume in one second (FEV1) and smoking prevalence for the placebo and N-acetylcysteine arms of each study. The majority of subjects were between 51 and 70 years of age, with a range of reported FEV1 percent predicted between 29% and 81% and a range of current smoking prevalence between 23 and 95%. reports the number of randomized subjects by treatment allocation, N-acetylcysteine dose used and overall study duration. Defining characteristics of exacerbations () varied minimally across studies, and with the exception of one study (Citation[22]) the diagnosis required the presence of a new or worsened cough and increased or purulent mucus, characteristics which current guidelines propose as clinical manifestations of COPD exacerbation (Citation[2]). Exacerbation data for subjects who completed each study were extracted and are reported in .
Table 1 Age, physiologic and smoking status of study subjects randomized to placebo or N-acetylcysteine
Table 2 Number of randomized subjects and treatment allocation by study
Table 3 Definition of exacerbation by study
Table 4 Outcomes and odds of exacerbation with N-acetylcysteine therapy, by study
Overall effect of N-acetylcysteine on exacerbations
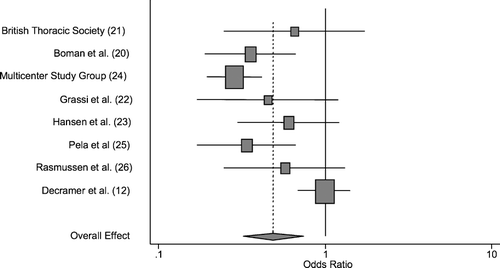
Inverse-variance weighted random effects meta-analysis methodology of data from all randomized subjects indicated that N-acetylcysteine significantly reduced the odds of COPD exacerbation by approximately 50%, with an odds ratio for experiencing ≥1 exacerbation over 6 months of 0.49, with a 95% confidence interval of 0.32–0.74 and p = 0.001. Weighting by inverse-variance methodology gave greatest weight to the BRONCUS study (Citation[12]) (a study that showed no significant effect, with an odds ratio of 0.99) and the Multicenter Study Group study (Citation[24]) (a study which showed a significant effect, with an odds ratio of 0.28 favoring NAC). Data from all individual studies and the summary effect estimate are summarized in .
Impact of baseline lung function and smoking status on N-acetylcysteine effect
Due to heterogeneity among studies with regard to reporting of absolute FEV1 or FEV1 % predicted, quantitative analysis of N-acetylcysteine effect stratified on lung function could not be performed. However, in those studies which did report baseline FEV1 % predicted, unweighted simple linear regression suggested that there was not a significant correlation (r = 0.38, p = 0.6) between lung function and the effect of N-acetylcysteine.
As it is not known if the effects of N-acetylcysteine are modified by cigarette smoking, a stratified analysis of studies by smoking prevalence was performed. In the subset of 3 studies (Citation[20], Citation[23], Citation[24]), which reported current smoking in > 50% of subjects, the odds ratio for experiencing one or more exacerbations was 0.36 (95% confidence interval 0.24–0.55, p < 0.001), suggesting that ongoing smoking does not attenuate the effect of N-acetylcysteine.
Impact of inhaled corticosteroid use on N-acetylcysteine effect
With the exception of the BRONCUS study (Citation[12]) and the study of Pela and colleagues (Citation[25]), all analyzed studies were conducted in the time period before inhaled corticosteroids were either available or widely employed in the treatment of COPD. To evaluate the impact of inhaled corticosteroids on the therapeutic effect of N-acetylcysteine, a separate meta-analysis was performed in which the data from the Pela study were excluded and only those BRONCUS subjects who were not using inhaled corticosteroids at the time of randomization (n = 158, 30% of study cohort) were included in an analysis. This analysis yielded a stronger overall estimate of effect, with an odds ratio of 0.42 and a 95% confidence interval of [0.32 – 0.54] (p < 0.0001), suggesting that concurrent inhaled corticosteroid use attenuated the effect of N-acetylcysteine.
Statistical heterogeneity and publication bias
For the analysis of all 8 studies, there was evidence of significant statistical heterogeneity (Q = 25.0 on 7 degrees of freedom, p = 0.001). However, when the 8 studies were analyzed excluding BRONCUS subjects who received inhaled corticosteroids, there was no evidence of statistical heterogeneity (Q = 7.2 on 7 degrees of freedom, p = 0.41), indicating that inclusion of inhaled corticosteroid-using subjects introduced both clinical and statistical heterogeneity to the overall estimate of effect. There was no evidence of significant statistical heterogeneity in the analysis of studies with a high proportion of smokers (Q = 3.4, p = 0.2). Given the absence of significant statistical heterogeneity in some cases, meta-analysis was repeated using fixed-effects methodology. Fixed-effects meta-analysis yielded results that were in all cases nearly identical to those obtained with random-effects meta-analysis (data not shown).
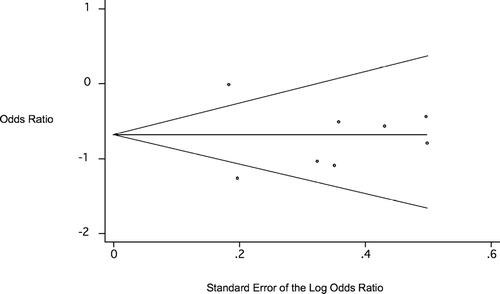
Analysis was performed to evaluate for publication bias. The distribution of studies on the funnel plot () was symmetric, and Begg's test was not statistically significant (p = 0.62 uncorrected for continuity, p = 0.71 corrected), indicating the absence of any evidence of publication bias.
Number needed to treat
Of the 1,820 subjects who completed the studies, 900 were allocated to N-acetylcysteine and 920 were allocated to placebo. In the 900 treated subjects, 509 (57%) had ≥1 exacerbation and 391 (43%) had 0 exacerbations over the study period. In the 920 untreated subjects, 657 (71%) had ≥1 exacerbation and 263 (29%) had 0 exacerbations over the study period. Thus, an absolute risk reduction of 0.14 (from 0.71 to 0.57) was observed in response to treatment, yielding a number needed to treat of 7.
DISCUSSION
This analysis suggests that oral administration of N-acetylcysteine significantly reduces the odds of one or more COPD exacerbations when used for a period of approximately 6 months. The magnitude of the odds reduction is numerically large, statistically-significant and does not appear to be attenuated by active smoking. However, concurrent use of inhaled corticosteroids may diminish the beneficial effect of N-acetylcysteine and may have explained the difference between a recent large trial of N-acetylcysteine in COPD (Citation[12]) and prior trials (Citation[20], Citation[21], Citation[22], Citation[23], Citation[24], Citation[25], Citation[26]). Viewed in aggregate, these data suggest that N-acetylcysteine reduces exacerbation frequency and may thereby modify the natural history of moderate to severe COPD.
While the mechanism by which N-acetylcysteine reduces exacerbations is unknown, its effect may be mediated by its ability to replete intracellular glutathione stores. Glutathione is found in plasma and airway epithelial cells (Citation[27]), but levels are greatest in the airway epithelial lining fluid, exceeding plasma levels by approximately 100-fold (Citation[28]). The airways are also rich in extracellular superoxide dismutase (Citation[29]) and other antioxidants, suggesting that the healthy airway is armed with a host of antioxidant defenses. Thus one mechanism that NAC may attenuate COPD exacerbations is by restoring the thiol balance in the airway epithelial lining fluid (Citation[30], Citation[31]). However, NAC may also have mucolytic properties that may be responsible for its beneficial effects (Citation[32]). Regardless of whether NAC exerts its effects by increasing antioxidant defenses or by mucolysis, NAC is cheap, effective and well tolerated.
Other systematic reviews have addressed the role of N-acetylcysteine in COPD (Citation[33], Citation[34], Citation[35]), but this analysis is distinguished from others by inclusion of data from the BRONCUS study as well as through differences in design and statistical methodology with regard to data extraction and analysis (Citation[33]), the choice to focus exclusively on N-acetylcysteine (Citation[34]) and a focus on studies of a duration more likely be consistent with chronic therapy (Citation[35]). The focus on studies of longer duration is critical when evaluating the COPD exacerbation as an outcome variable, for exacerbations are relatively infrequent events (median of 1.32 per year) even in patients with FEV1 of approximately 50% of predicted (Citation[36]). Thus, we chose the longest-term subset of studies (approximately 6 months duration) in an attempt to gain the most accurate data available on exacerbation frequency and long-term therapeutic effect.
Of particular interest with regard to currently employed COPD pharmacotherapy is the apparent impact of concurrent inhaled corticosteroid use on the effect of N-acetylcysteine, in which use of inhaled corticosteroids introduces both clinical and statistical heterogeneity to the results of our analysis. As noted above, inclusion of only those BRONCUS subjects who did not receive inhaled corticosteroids yields a result virtually identical to that obtained when all BRONCUS data are excluded. This result is not associated with significant statistical heterogeneity. When all BRONCUS subjects (on and off inhaled corticosteroids) are included in the analysis, there is an attenuation of N-acetylcysteine effect and a significant increase in statistical heterogeneity. Thus, there may differential effects of N-acetylcysteine in COPD patients based on the presence or absence of concurrent inhaled corticosteroid use, a phenomenon on which the current literature can provide little clarification due to the absence in the BRONCUS study of stratified randomization based on inhaled steroid use. Rather than being ineffective (Citation[37]), N-acetylcysteine may be effective only in certain subsets of COPD patients, e.g., those not receiving inhaled corticosteroids.
Although the effect of N-acetylcysteine on exacerbations observed in this meta-analysis is greater than that reported in a prior meta-analysis of inhaled steroids and COPD exacerbations (Citation[38]), the conclusion that NAC is superior to inhaled corticosteroids or other therapies must be made cautiously due to the overall quality of evidence and deficiencies in study design and reporting (particularly with regard to criteria for diagnosis of both COPD and exacerbations) in some of the N-acetylcysteine literature. However, particularly in areas of the developing world where access to newer or more costly therapies is limited, the use of older and less costly therapies such as albuterol, theophylline and N-acetylcysteine may be associated with positive therapeutic effect in patients with COPD.
Funding from NIH: K23 HL04385 (Sutherland), P01 HL31992 (Crapo) and K08 HL04407 (Bowler).
REFERENCES
- Barnes P J. Chronic obstructive pulmonary disease. N Engl J Med 2000; 343: 269–280, [INFOTRIEVE], [CSA]
- Celli B R, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23: 932–946, [INFOTRIEVE], [CSA]
- Anthonisen N R, Connett J E, Murray R P. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med 2002; 166: 675–679, [INFOTRIEVE], [CROSSREF], [CSA]
- Donaldson G C, Seemungal T A, Bhowmik A, Wedzicha J A. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002; 57: 847–852, [INFOTRIEVE], [CROSSREF], [CSA]
- Sutherland E R, Cherniack R M. Management of chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 2689–2697, [INFOTRIEVE], [CROSSREF], [CSA]
- Sutherland E R, Allmers H, Ayas N T, Venn A J, Martin R J. Inhaled corticosteroids reduce the progression of airflow limitation in chronic obstructive pulmonary disease: A meta-analysis. Thorax 2003; 58: 937–941, [INFOTRIEVE], [CROSSREF], [CSA]
- Pryor W A, Stone K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann N Y Acad Sci 1993; 686: 12–27, [INFOTRIEVE], [CSA]
- Pratico D, Basili S, Vieri M, Cordova C, Violi F, Fitzgerald G A. Chronic obstructive pulmonary disease is associated with an increase in urinary levels of isoprostane F2alpha-III, an index of oxidant stress. Am J Respir Crit Care Med 1998; 158: 1709–1714, [INFOTRIEVE], [CSA]
- Rahman I, Morrison D, Donaldson K, MacNee W. Systemic oxidative stress in asthma, COPD, and smokers. Am J Respir Crit Care Med 1996; 154: 1055–1060, [INFOTRIEVE], [CSA]
- Tager M, Piecyk A, Kohnlein T, Thiel U, Ansorge S, Welte T. Evidence of a defective thiol status of alveolar macrophages from COPD patients and smokers. Chronic obstructive pulmonary disease. Free Radic Biol Med 2000; 29: 1160–1165, [INFOTRIEVE], [CROSSREF], [CSA]
- Crapo J D. Oxidative stress as an initiator of cytokine release and cell damage. Eur Respir J Suppl 2003; 44: 4s–6s, [INFOTRIEVE], [CROSSREF], [CSA]
- Decramer M, Rutten-van Molken M, Dekhuijzen P N, Troosters T, van Herwaarden C, Pellegrino R, van Schayck C P, Olivieri D, Del Donno M, De Backer W, Lankhorst I, Ardia A. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet 2005; 365: 1552–1560, [INFOTRIEVE], [CROSSREF], [CSA]
- Moher D, Cook D J, Eastwood S, Olkin I, Rennie D, Stroup D F. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of Meta-analyses. Lancet 1999; 354: 1896–1900, [INFOTRIEVE], [CROSSREF], [CSA]
- Sterne J AC, Bradburn M J, Egger M. Meta-analysis in Stata. Systematic reviews in health care: meta-analysis in context, M Egger, D G Altman, G D Smith. BMJ Books, London 2001
- Cochran W G. the combination of estimates from different experiments. Biometrics 1954; 10: 101–129, [CROSSREF], [CSA]
- Light R J, Singer J D, Willett J B. The visual presentation and interpretation of meta-analysis. The handbook of research synthesis, H Cooper, L V Hedges. Russell Sage Foundation, New York 1994; 439–454
- Begg C B, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101, [INFOTRIEVE], [CROSSREF], [CSA]
- Light R J, Pillemar D B. Summing up: the science of reviewing research. Harvard University Press, Cambridge, MA 1984
- Sackett D L, Haynes R B, Guyatt G H, Tugwell P. Clinical epidemiology: a basic science for clinical medicine. Little, Brown and Company, Boston 1991
- Boman G, Backer U, Larsson S, Melander B, Wahlander L. Oral acetylcysteine reduces exacerbation rate in chronic bronchitis: report of a trial organized by the Swedish Society for Pulmonary Diseases. Eur J Respir Dis 1983; 64: 405–415, [INFOTRIEVE], [CSA]
- British Thoracic Society Research Committee. Oral N-acetylcysteine and exacerbation rates in patients with chronic bronchitis and severe airways obstruction. Thorax 1985; 40: 832–835, [CSA]
- Grassi C, Morandini G C. A controlled trial of intermittent oral acetylcysteine in the long-term treatment of chronic bronchitis. Eur J Clin Pharmacol 1976; 09: 393–396, [INFOTRIEVE], [CROSSREF], [CSA]
- Hansen N C, Skriver A, Brorsen-Riis L, Balslov S, Evald T, Maltbaek N, Gunnersen G, Garsdal P, Sander P, Pedersen J Z, Ibsen T B, Rasmussen F V. Orally administered N-acetylcysteine may improve general well-being in patients with mild chronic bronchitis. Respir Med 1994; 88: 531–535, [INFOTRIEVE], [CROSSREF], [CSA]
- Multicenter Study Group. Long-term oral acetylcysteine in chronic bronchitis. a double-blind controlled study. Eur J Respir Dis 1980; 61: 93–108, Suppl. 111[CSA]
- Pela R, Calcagni A M, Subiaco S, Isidori P, Tubaldi A, Sanguinetti C M. N-acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPD. Respiration 1999; 66: 495–500, [INFOTRIEVE], [CROSSREF], [CSA]
- Rasmussen J B, Glennow C. Reduction in days of illness after long-term treatment with N-acetylcysteine controlled-release tablets in patients with chronic bronchitis. Eur Respir J 1988; 1: 351–355, [INFOTRIEVE], [CSA]
- Halliwell B, Gutteridge J MC. Free radicals in biology and medicine. 3rd ed. Oxford University Press, OxfordEngland 1999
- van d er, Vliet A, O'Neill C A, Cross C E, Koostra J M, Volz W G, Halliwell B, Louie S. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am J Physiol 1999; 276: L289–296, [CSA]
- Kinnula V L, Crapo J D. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 2003; 167: 1600–1619, [INFOTRIEVE], [CROSSREF], [CSA]
- Linden M, Hakansson L, Ohlsson K, Sjodin K, Tegner H, Tunek A, Venge P. Glutathione in bronchoalveolar lavage fluid from smokers is related to humoral markers of inflammatory cell activity. Inflammation 1989; 13: 651–658, [INFOTRIEVE], [CROSSREF], [CSA]
- Muller T, Gebel S. The cellular stress response induced by aqueous extracts of cigarette smoke is critically dependent on the intracellular glutathione concentration. Carcinogenesis 1998; 19: 797–801, [INFOTRIEVE], [CROSSREF], [CSA]
- Verstraeten J M. Mucolytic treatment in chronic obstructive pulmonary disease. Double-blind comparative clinical trial with N-acetylcysteine, bromhexine and placebo. Acta Tuberc Pneumol Belg 1979; 70: 71–80, [INFOTRIEVE], [CSA]
- Grandjean E M, Berthet P, Ruffmann R, Leuenberger P. Efficacy of oral long-term N-acetylcysteine in chronic bronchopulmonary disease: a meta-analysis of published double-blind, placebo-controlled clinical trials. Clin Ther 2000; 22: 209–221, [INFOTRIEVE], [CROSSREF], [CSA]
- Poole P J, Black P N. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2003, CD001287[CSA]
- Stey C, Steurer J, Bachmann S, Medici T C, Tramer M R. The effect of oral N-acetylcysteine in chronic bronchitis: a quantitative systematic review. Eur Respir J 2000; 16: 253–262, [INFOTRIEVE], [CROSSREF], [CSA]
- Burge P S, Calverley P M, Jones P W, Spencer S, Anderson J A, Maslen T K. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000; 320: 1297–1303, [INFOTRIEVE], [CROSSREF], [CSA]
- Donohue J F. Still looking for answers in COPD. Lancet 2005; 365: 1518–1520, [INFOTRIEVE], [CROSSREF], [CSA]
- Alsaeedi A, Sin D D, McAlister F A. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomized placebo-controlled trials. Am J Med 2002; 113: 59–65, [INFOTRIEVE], [CROSSREF], [CSA]