Abstract
The purpose of this endeavor is to compare the morbidity, mortality and costs of LVRS versus transplantation in severe emphysema. This was a retrospective review of severe emphysema patients who received LVRS (n = 70) from 1994–1999, or transplant (n = 87) from 1994–2004. Change in functional status was calculated by the change in modified BODE (mBODE) score. Financial data included physician, hospital and medication costs. Preoperatively, there was no significant difference between the transplant and LVRS groups (mean ± SD) in age (57.7 ± 5.7 vs. 59.1 ± 8.3 years), BMI, Borg dyspnea score, 6-minute walk distance or mBODE (10.4 ± 2.6 vs. 9.6 ± 2.7, p = 0.4). Preoperatively, FEV1% (23.6 ± 8.5 vs. 31.9 ± 17.7, p = 0.008) was significantly lower in the transplant group. One year post-operatively, transplantation patients had a significantly greater improvement in mBODE (−5.7 vs. −2.0, p = 0.0004), FEV1% (43.4 vs. 2.2%, p = 0.0004) and Borg score (−3.0 vs. −1.4, p = 0.04). Transplantation patients had lower long-term survival compared to LVRS patients (p = 0.01). The only variable that affected survival was type of surgery favoring LVRS (hazard ratio 1.7, 95% confidence limits 1.05–2.77). During a mean follow-up of 2.4 ± 2.5 years after transplant and 5.0 ± 3.1 years after LVRS, transplantation mean total costs were greater ($381,732 vs. $140,637, p < 0.0001). Transplantation patients spent more time in the hospital (74.3 ± 81.3 vs. 39.5 ± 66.7 days, p = 0.009) and had more outpatient visits (29.9 ± 28.8 vs. 12.3 ± 12.6 visits, p < 0.0001). In patients who survive over 1 year, transplantation provides a higher level of functional status and a greater improvement in airflow obstruction, dyspnea, exercise tolerance, and mBODE score, but costs more and carries greater mortality.
INTRODUCTION
Lung volume reduction surgery and lung transplantation are heroic and expensive measures aimed at improving the quality of life in maximally treated severe emphysema. Prior studies have shown an improvement in quality of life after lung volume reduction surgery (LVRS) (Citation[1], Citation[2]) or lung transplantation in severe emphysema (Citation[3]). However, there is no survival benefit in emphysema following lung transplantation when compared with medical therapy (Citation[4]), whereas prolonged life expectancy is found only in a select subset of patients receiving LVRS (Citation[5]).
It is often difficult for patients to decide to opt for surgical therapy for emphysema. Whether surgery will be helpful, when to operate, and which type of surgery to perform are difficult decisions for the patient as well as the physician. Compounding the problem are the differences in physiologic criteria for performing LVRS or transplantation. Lung transplantation is generally considered for COPD when the FEV1 drops below 25% predicted with an age limitation of 65 for single lung transplant (Citation[6]). LVRS is considered when the FEV1 is less than 45% predicted with the exception of patients that have a FEV1 less than 20% predicted with either a DLCO less than 20% predicted or diffuse disease by chest CT imaging (Citation[7]). Patients with low baseline exercise tolerance and upper lobe disease have been reported to have a survival advantage after LVRS. Moreover, there is a select group of patients with FEV1 > 20 and < 30% predicted who are candidates for both types of surgery.
Both surgeries are also expensive endeavors with different aspects that contribute to their respective costs. Transplantation not only includes the cost of surgery, but also includes immunosuppressive agents. However, there are no data that directly compare the costs of transplantation with LVRS in comparable groups of emphysema patients. We sought to retrospectively compare the physiologic outcome, morbidity, mortality and costs of LVRS versus lung transplantation in a large cohort of patients followed for several years at a single center.
METHODS
Design
The study is a retrospective chart and database review of all patients undergoing LVRS at Temple University Hospital between 1994 and 1999, and lung transplantation between 1994 and 2004. The study included 144 patients with advanced COPD who received 157 procedures, either LVRS (n = 70) or lung transplantation (n = 87).
Patient selection
All procedures were performed at Temple University Hospital in Philadelphia, PA. Lung transplantation included single lung, double-lung and heart-lung transplantation. The LVRS patients were censored for inclusion into this study after 1999 secondary to involvement of our center with the National Emphysema Treatment Trial.
Patient selection—Transplantation
Referral criteria for lung transplantation were age ≤65 years, FEV1 < 25% predicted, patient on maximum medical therapy including oxygen and pulmonary rehabilitation, and the presence of hypercapnia or cor pulmonale with death considered likely within 2–3 years. Exclusion criteria included intubation and invasive mechanical ventilation, tobacco use within the prior 6 months, or evidence of HIV, Hepatitis C, or active bacterial infection.
Patient selection—LVRS
Referral criteria for LVRS included FEV1 < 45% predicted (> 15% if > 70 years old), Total lung capacity > 100% predicted, residual volume > 150% predicted, high resolution computed tomography scan showing evidence of bilateral emphysema, PaCO2 < 60 mmHg, PaO2 > 45 mmHg, Post-rehabilitation 6-minute walk distance > 140 m, and the ability to complete 3 minutes of unloaded pedaling on an exercise tolerance test.
Outcome measures
The primary outcome was survival after surgery and change in functional status at 1 year as measured by a change in FEV1%, 6-minute walk distance, Borg dyspnea scale and modified composite BODE. Secondary outcomes were length of stay for the hospitalization that included the initial hospitalization at the time of the surgery, total hospital days following the original hospitalization for the surgery, total number of hospitalizations, number of outpatient visits after surgery, the number of outpatient short procedures, and total costs including hospital, medication, and physician costs. After analyzing the whole group of patients, a subset of patients who could conceivably be referred for either LVRS or transplant with a baseline FEV1 between 20 and 30% had all of the above measurements additionally compared.
Physiologic measurements
Pulmonary function testing
Pulmonary function testing was performed with a system 6200 Autobox DL Plethysmograph (Sensor-Medics, Yorba Linda, CA), using American Thoracic Society guidelines (Citation[8]). FVC, FEV1, and FEV1/FVC were measured. Thoracic gas volumes were measured in a body plethysmograph. All post-bronchodilator pulmonary function data are reported as percent of normal predicted values.
Exercise capacity
Six-minute walks were performed in accordance with American Thoracic Society guidelines (Citation[9]).
BODE index
The BODE index was used to compare physiologic function post-procedure between groups (Citation[10]). In our transplant and LVRS groups, the MMRC scale was not a part of the database; a Borg dyspnea questionnaire was substituted to create a modified BODE (mBODE) index. The Borg scale grades dyspnea 0–10, based upon increasing severity (Citation[11]).
Economic data
Financial analysis
Costs were split into physician, hospital, and medication costs. All physician and hospital charges were captured in our hospital financial database, from 1 month prior to either lung transplant or LVRS until the time of death or completion of study data collection in October, 2004. CPT codes for physician charges were converted into physician costs utilizing the standardized reimbursement utilizing the Medicare web site for the Philadelphia metropolitan area in 2004 〈www.cms.hhs.gov〉. Hospital costs were calculated using a ratio of costs-to-charges employed by the Financial Department of Temple University Hospital. Ratios of hospital costs to charges were developed to encompass both direct costs (nursing salaries, supplies, etc.) and indirect costs (administration, housekeeping, etc.) using the most recent Medicare cost report. All hospital costs were adjusted to 2004 dollars using the consumer price index for medical care. Medication costs were estimated using the 2004 drug cost scale at Temple University Hospital's outpatient pharmacy for medications used in LVRS and transplantation. Daily cost for medications was multiplied by the number of days alive and out of the hospital after surgery until October, 2004.
Statistical analysis
Analysis of variance for repeated measures was employed for comparison among groups. Kaplan–Meier analysis with the log-rank test was used for survival time. Comparisons of changes from baseline to 1-year post-surgery between the LVRS and transplant groups are displayed in a four-quadrant box-plot form. LVRS patients are displayed on the left half of the plot and transplant patients are displayed on the right half. The length of the bar is proportional to the number of patients in a specific range. The x-axis represents no change in function. Above the x-axis is an improvement while below the x-axis is worsening function, missing patients including those who were too sick for follow-up testing, deceased patients, and two patients who received transplantation within a year of LVRS. The four-quadrant box plots were compared using McNemar's test of symmetry.
RESULTS
Baseline characteristics
Baseline characteristics are shown in . Patients in both groups were statistically similar in terms of age, mBODE, 6-minute walk distance, Borg dyspnea scale, BMI, cardiac index, pulmonary artery pressure and cardiac ejection fraction. The only difference was in the degree of airflow obstruction that was worse in the patients who received transplantation.
Table 1 Baseline characteristics
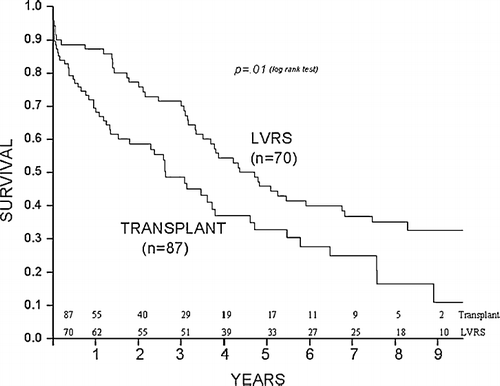
Survival
In comparison to lung transplantation, survival after LVRS was statistically better (p = 0.01) as shown in . Among age, sex, type of surgery, BMI, mBODE, FEV1, 6-minute walk distance, and Borg dyspnea score, the type of surgery was the only predictor of survival and favored LVRS (hazard ratio 1.7, 95% confidence limits 1.05–2.77).
Primary outcomes
Compared to baseline, LVRS and lung transplantation patients who survived at least 1 year post-surgery had an improvement in airflow obstruction, dyspnea, and mBODE. Transplantation patients also had an improvement in 6-minute walk distance while LVRS patients had a trend towards improvement in 6-minute walk distance. Transplantation patients who survived 1 year also had significantly better FEV1%, dyspnea and mBODE scores compared to LVRS patients at 1 year ().
Table 2 1-year postoperative data of surviving patients
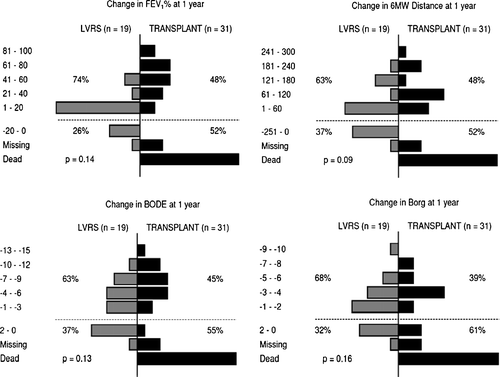
shows the change in functional status 1 year following surgery. The plots had similar distributions. Both groups had similar improvements in FEV1%, 6-minute walk distance, dyspnea and mBODE, but patients undergoing transplantation had more significant increases in these measures. Forty percent (32/80) of transplantation patients had a > 20% increase in FEV1% versus 8.6% (6/70) of LVRS patients. 28.8% (23) of transplantation patients had a > 60 meter improvement in 6-minute walk distance versus 17.1% (Citation[12]) of LVRS patients. 27.5% (22) of transplantation patients had a greater than 2 scale improvement in Borg versus 21.4% (Citation[15]) of LVRS patients. 32.5% (26) of transplantation patients had a better than 3 scale improvement in mBODE versus 21.4% (Citation[15]) of LVRS patients.
Figure 2 Compares the changes in FEV1, 6-minute walk test, mBODE and Borg from before surgery to 1-year post-surgery between LVRS patients (shown on the left) and transplantation patients (shown on the right). More transplantation patients are missing or dead at 1-year (below dotted line), while more LVRS patients did not improve following surgery (also below dotted line). Of patients who survived 1 year post-surgery, the transplantation patients had greater improvements in functional status (above dotted line).
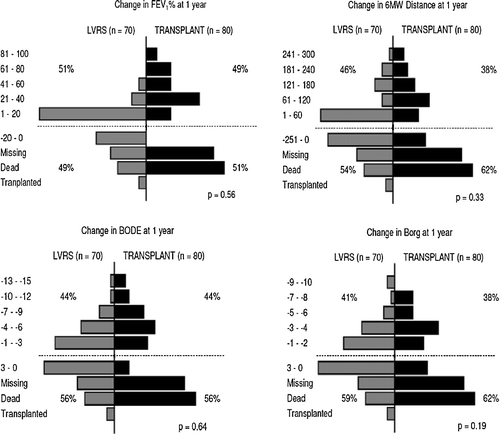
While the transplantation patients had greater improvements in functional status at 1-year post-surgery, there were more transplantation patients who were missing data or deceased at the time of follow-up. There were 10 patients (14.3%) missing data, 8 (11.4%) deceased and 2 (2.9%) who were transplanted 1-year post-LVRS, while there were 19 (23.8%) patients missing data and 22 (27.5%) deceased 1-year post-transplantation.
Secondary outcomes
The Temple University Hospital Financial Department had data on 79 transplant and 57 LVRS patients enrolled in our study. Secondary outcomes included cost, length of hospital stay after surgery, total number of days in the hospital through October, 2004, and outpatient care are shown in . Over a mean follow-up of 2.4 ± 2.5 years after transplant and 5.0 ± 3.1 years after LVRS, transplantation patients spent a median of 23.5 days after surgery compared to 14 days after LVRS. Transplantation patients spent 74.3 ± 81.3 total days in the hospital over an average of 3.9 ± 3.4 hospitalizations while LVRS patients spent 39.5 ± 66.7 days (p = 0.009) in the hospital over an average of 2.3 ± 2.7 hospitalizations (p = 0.003) after surgery. Transplantation patients had 29.9 ± 28.8 outpatient follow-up visits compared to 12.3 ± 12.6 (p < 0.0001) for LVRS. Transplantation patients had an average of 3.3 ± 3.0 same day procedures (generally fiberoptic bronchoscopy with transbronchial biopsy) compared to 0.1 ± 0.4 for LVRS patients (p < 0.0001). The mean hospital costs from the time of surgery to the time of death, loss to follow-up or end of the study in October, 2004 were $246,666 for transplantation and $97,010 for LVRS (p < 0.0001).
Table 3 Secondary outcomes
The mean physician costs during that time were $42,262 for transplant and $14,551 for LVRS (p < 0.0001). The mean cost for medications was $92,804 after transplant and $29,076 after LVRS (p < 0.0001). The average total cost following surgery including physician reimbursement, hospital cost and medication cost was $381,732 following transplant and $140,637 following LVRS (p < 0.0001).
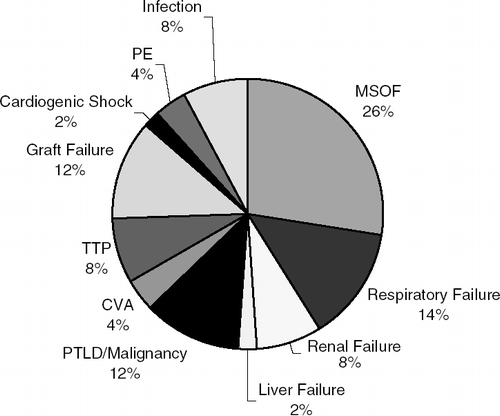
Fifty-one of the lung transplant patients were deceased at the end of data collection in October, 2004. The causes of death are shown in . We see that 34% of patients died from either multi-system organ failure or infection, 14% from chronic respiratory failure, 12% from graft failure, 12% from post-transplant lymphoproliferative disorder or malignancy, 8% from complications due to TTP, and 4% each from pulmonary embolism and stroke. Several of these causes of death occur as complications of transplant, which may explain why transplant has a higher mortality than LVRS.
Analysis of patients with FEV1 between 20–30%
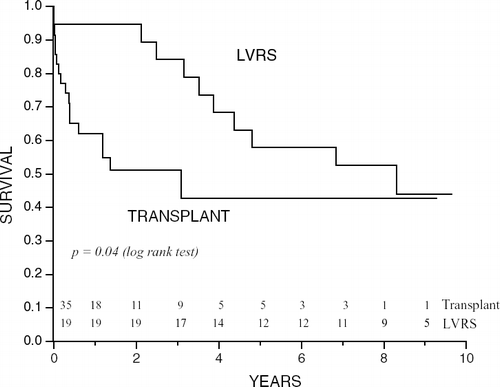
There were 19 LVRS and 35 transplant patients who had baseline FEV1 between 20 and 30% predicted. These patients were statistically similar in terms of age, mBODE, 6-minute walk distance, Borg dyspnea scale, BMI, cardiac index, pulmonary artery pressure and cardiac ejection fraction. Kaplan–Meier survival comparing this subgroup of patients after surgery was also superior for LVRS compared to transplantation (p = 0.04) ().
Figure 4 Kaplan–Meier analysis showing that survival is greater following LVRS than following transplantation in patients with baseline FEV1 20–30% at all times with a p = 0.04 by the log-rank test.
shows box plots for the group of patients with FEV1 between 20 and 30%. Although all plots had symmetrical distributions, a greater proportion of patients had an improvement in airflow obstruction, exercise capacity, dyspnea and mBODE score after LVRS compared to transplantation. This difference was largely due to the fact that 13 patients died < 1 year after transplantation. Of the transplantation patients who survived, most had a significantly larger magnitude of improvement in functional status than the LVRS patients.
Figure 5 Compares changes in FEV1, 6-minute walk distance, mBODE and Borg from before surgery to 1-year post-surgery between LVRS (shown on the left) and transplantation patients (shown on the right) with a baseline FEV1 20–30%. More transplantation patients are missing or dead at 1-year (below dotted line). While no LVRS patients died within 1 year following surgery, more did not improve following surgery (also below dotted line). Of patients who survived 1 year post-surgery, the transplantation patients had greater improvements in functional status (above dotted line).
DISCUSSION
This study shows that survival, even when adjusted for FEV1, is better after LVRS compared with lung transplantation in patients with severe emphysema. Lung transplantation costs more, requires more time in the hospital and doctor's office and has more expensive and complicated medications after surgery. LVRS patients had an improvement in physiological function compared with their baseline function; however, these improvements were not as great as in the transplantation patients who survived 1 year following surgery.
This study has the strength of utilizing the same surgeons, pulmonologists and nursing care for the 144 patients who received 157 procedures. This resulted in a standard approach to the evaluation and surgical and medical treatment of patients receiving either transplantation or LVRS. Limitations of the study are the consequence of the retrospective nature of the study. We interrupted LVRS patient enrollment secondary to the beginning of the National Emphysema Treatment Trial. This may have altered patient outcome secondary to treatment changes over time, but this would have been biased against LVRS.
Our center reports a lower survival than reported by the International Society for Heart and Lung Transplant. However, our patients were selected over a relatively long time period from 1994–2004. We expect improvement in outcome secondary to improvements in patient selection, pre and post-operative care, improved transplant medications, supportive care and surgical technique with experience over time. At our center, the 1-year survival of transplant patients for surgeries performed after 2000 was 75.9% versus 67.3% for transplants from 1994–1999.
The ISHLT shows an improvement in survival with era of procedure and a statistically significant increase in mean survival by almost 8 months over the past decade (Citation[12]). The relatively small number of transplant patients in our center required us to report data over a longer period of time and this could account for our difference in outcome compared to the large ISHLT data. We expect that with more time and numbers of patients, we will be able to show a statistically significant improvement in survival over time. The small number of patients in the subgroup analysis may have also affected our survival after transplant in the group of patients with an FEV1 between 20–30%. Although this is a distinct limitation of our data, in lieu of a prospective randomized controlled trial of LVRS vs. transplant, this is the best that can be afforded by retrospective analysis of single center data.
What is not known is if there would be a greater improvement in airflow obstruction, exercise capacity and dyspnea in more recent LVRS patients. There were patients who received transplantation who had an FEV1 < 20%. These patients would not be considered candidates for LVRS. To rectify this, we additionally looked at any patients with the same degree of airflow obstruction that could be considered candidates for LVRS or transplantation.
The subgroup of patients with an FEV1 between 20 and 30% could conceivably qualify for either surgery. Fifty-four out of our 144 patients had a FEV1 between 20 and 30% predicted. This subset of patients showed a dramatic difference in outcomes for the transplant patients at 1-year post surgery. Also, 13 patients were deceased and 3 were missing at the 1-year follow-up time. All of the other 19 patients had significant improvements in FEV1 and 6-minute walk distance while 16 had an improvement in dyspnea and 18 had an improvement in mBODE. None of the LVRS patients died in the first year post-surgery and only one was missing at 1-year follow-up. 14 patients had an improvement in FEV1, 12 in 6-minute walk distance, 13 in dyspnea and 12 in mBODE, though not to the same extent as the transplantation patients who did well.
Transplantation patients who survived for a year after surgery had greater improvements in functional status than the matched LVRS patients, but the transplantation patients also had a much higher risk of not surviving for 1 year after surgery. This shows a dramatic difference in mortality and physiologic improvements after LVRS or transplant in severe emphysema. Interpretation of this subgroup data should be taken with caution in lieu of the smaller numbers and missing data. However, this may be the most important group of patients to consider for comparison since they are the ones who may have a difficult choice to make between two very different surgical treatments. A larger study comparing LVRS, transplant and medical therapy in the subgroup of patients with FEV1 between 20–30% may help in the decision of treatment options.
The BODE score is becoming a more reported measure of outcome to an intervention. Imfeld et al. recently reported that postoperative BODE is a powerful predictor of survival in COPD patients after LVRS (Citation[13]). In our retrospective analysis we did not have data for the MMRC dyspnea scale used in the original description of the BODE index. We modified the BODE using the Borg dyspnea scale which is a 0–10 scale allowing for higher mBODE scores than described by Celli et al. Martinez et al. recently published a study of predictors of mortality in patients with emphysema and severe airflow obstruction (Citation[14]). They modified the BODE using the University of California at San Diego Shortness of Breath Questionnaire (UCSD SOBQ).
While it would be preferable to use the BODE index as reported by Celli et al. for comparison's sake, there seems to be a clear correlation between body mass index, airflow obstruction, degree of dyspnea and exercise capacity and mortality in emphysema and after LVRS. Further studies are needed to determine if the BODE is a predictor of mortality after lung transplant.
Transplant or LVRS improves patient quality of life and in some patients it is the selection factor for choosing surgery. We did not have access to this data pre-and post-intervention that would have been important. It is a clear limitation of our retrospective study that we did not have quality of life information or MMRC dyspnea data and a prospective study comparing LVRS and transplant in emphysema would be beneficial.
Transplant patients had a significantly higher increase in FEV1, Borg and mBODE at 1 year post-transplant, but no difference in 6-minute walk distance. This may be related to other factors affecting the 6-minute walk test such as delayed recovery of peripheral muscle strength and effects of transplant medications on peripheral muscles.
Transplant should cost more at the time of surgery and transplant medications are costly. The cost-effectiveness ratio for lung volume reduction surgery as compared with medical therapy was $190,000 per quality-adjusted life-year gained at 3 years and $53,000 per quality-adjusted life-year gained at 10 years (Citation[15]). Not surprisingly, we found that transplantation costs more than LVRS. Transplantation patients take more medications, spend more time in the hospital, doctor's office and short-procedure unit thus generating more cost even though they do not survive as long as LVRS patients. A large portion of the increased cost of transplant is related to hospital length of stay. There were 4 patients that had prolonged hospital stays after transplant of greater than 140 days with one patient staying in the hospital for 312 days. This may skew the data for length of stay and cost and explains the broad standard deviations seen in . This again illustrates the point that in our relatively small study a few patients can significantly skew the mean.
Patients who do qualify for both procedures need to understand the risks and benefits of each. Patients who choose lung transplant have a greater chance of more substantial increase in FEV1, exercise tolerance and dyspnea at the risk of greater morbidity and mortality. Mortality is higher secondary to the complications of transplant and immunosuppression. COPD patients die from respiratory failure, infection and its complications such as sepsis and multisystem organ failure. Transplant patients who survive should do well with new lungs, but they die from infection, obliterative bronchiolitis or medication related problems. Also, 40% of our transplantation patients died from graft failure, PTLD and malignancy, TTP, and thromboembolic complications.
Similar patients have different treatment goals. Some patients who are so dyspneic that they are unable to enjoy life may be willing to accept the higher risks that come with transplant in the hopes of becoming more functional. Others may not be ready to accept the higher risk of death but are willing to take a chance at a smaller improvement in functional capacity that comes from LVRS. Still others may want to try LVRS first, and then pursue transplant should they have no improvement after LVRS, or deteriorate to the point that they are willing to attempt transplant. Some patients may not be willing even to risk LVRS, but may consider newer treatments such as endobronchial valve placement (Citation[16]). Prior to choosing a treatment plan, the physician and patient need to discuss each option and the patient's goals.
CONCLUSION
COPD patients have improvement in FEV1, dyspnea, 6-minute walk test distance, and mBODE following LVRS or transplantation. The magnitude of changes is greater in patients who survive at least 1 year after transplantation. However, mortality is substantially higher in the group following transplantation due to infection, chronic rejection and other treatment-related factors. Costs also are substantially higher for transplantation. These data may be useful for patients and doctors considering the risks and benefits of LVRS or transplantation in emphysema.
The authors claim no financial relationships.
REFERENCES
- Leyenson V, Furukawa S, Kuzma A M, Cordova F, Travaline J, Criner G J. Correlation of changes in quality of life after lung volume reduction surgery with changes in lung function, exercise, and gas exchange. Chest 2000; 118: 728–735
- Criner G J, Cordova F C, Furukawa S, Kuzma A M, Travaline J M, Leyenson V, O'Brien G M. Prospective randomized trial comparing bilateral lung volume reduction surgery to pulmonary rehabilitation in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160: 2018–2027
- Gross C R, Savik K, Bolman R M, Hertz M I. Long-term health status and quality of life outcomes of lung transplant recipients. Chest 1995; 108: 1587–1593
- Hosenpud J D, Bennett L E, Keck B M, Edwards E B, Novick R J. Effect of diagnosis on survival benefit of lung transplantation for end-stage lung disease. Lancet 1998; 351: 24–27
- National Emphysema Treatment Trial Research Group. A randomized trial comparing lung volume reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348: 2059–2073
- Maurer J R, Frost A E, Estenne M, Higgenbottam T, Glanville A R. International guidelines for the selection of lung transplant candidates. J Heart Lung Transplant 1998; 17: 703–709
- National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung volume reduction surgery. N Engl J Med 2001; 345: 1075–1083
- American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 1995; 152: 1107–1136
- American Thoracic Society. Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117
- Celli B R, Cote C G, Marin J M, Casanova C, Montes de Oca M, Mendez R A, Pinto Plata V, Cabral H J. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350: 1005–1012
- Borg G. Psychophysical basis of perceived exertion. Med Sci Sports Exer 1982; 14: 377–381
- http://〈www.ishlt.org〉(accessed July 2006)
- Imfeld S, Bloch K E, Weder W, Russi E W. The BODE index after lung volume reduction surgery correlates with survival. Chest 2006; 129: 873–878
- Martinez F J, Foster G, Curtis J L, Criner G, Weinmann G, Fishman A, DeCamp M M, Benditt J, Sciurba F, Make B, Mohsenifar Z, Diaz P, Hoffmann E, Wise R; for the NETT Research Group. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006; 163: 1326–1334
- National Emphysema Treatment Trial Research Group. Cost effectiveness of lung volume reduction surgery for patients with severe emphysema. N Engl J Med 2003; 348: 2092–2102
- Maxfield R A. New and emerging minimally invasive techniques for lung volume reduction. Chest 2004; 2: 777–783