INTRODUCTION
COPD is a major public health problem worldwide and the impact is expected to increase in the next several decades (Citation[1]). In the United States, more than 10 million people have been diagnosed with COPD and another 14 million show signs of the disease, although they have not yet been diagnosed (Citation[2]). The COPD death rate for women has more than doubled in the past 20 years (from 20.1 to 56.7/100,000), and the financial burden of COPD is significant, costing an estimated $14.7 billion annually for direct medical costs, and an estimated $15.7 billion annually for indirect costs (loss of productivity and premature mortality) (Citation[3]). Despite increasing knowledge of disease pathogenesis, prevention and intensified efforts at patient education and early detection, the prevalence of COPD and COPD mortality is on the rise, especially in women and African Americans (Citation[4]).
For primary care physicians this means that the need to provide care at all stages of COPD will increase, requiring us to develop new skills, find new tools for diagnosis and management and gather new knowledge for integrating COPD into chronic care treatments. This article reviews the epidemiology, patho-physiology, recognition, diagnosis and management of COPD from a primary care perspective. Where feasible primary care focused tools and recommendations for COPD care are presented.
PUTTING COPD IN PERSPECTIVE
Despite the fact that COPD is the fourth-leading cause of death in the United States, behind only heart disease, cancer and stroke, it is underrecognized and undertreated () (Citation[5], Citation[6], Citation[7]). Many of the people with unrecognized disease are in the earlier stages of the disease when symptoms are often blamed on aging or the side effects of smoking such as a chronic smoker's cough. Many of these patients come to our office once or twice a year but fail to report symptoms, just as we often fail to specifically attempt to illicit those symptoms (Citation[8]). Even when we have diagnosed the COPD, patients make far fewer COPD management visits than they do for other chronic diseases (). The result of this late diagnosis may be part of the reason for COPD to be the only common chronic disease in which the age adjusted mortality rate continues to climb ().
Figure 1 COPD compared to other chronic diseases treated in primary care. Information taken from: *All About Diabetes. American Diabetes Association Web site: www.diabetes.org. (90% to 95% of Americans with diabetes have type 2 diabetes.) AHA. Heart Disease and Stroke Statistics—2004 Update. Dallas, TX: AHA, 2003. Overweight and Obesity FAQs. CDC Web site: <www.cdc.gov/nccdphp/dnpa/|obesity/|faq.htm#adults>.
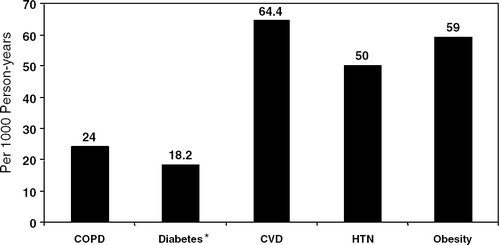
Figure 2 COPD competes for our attention and loses. *For COPD, physician office visits and hospital outpatient visits are combined. Information taken from Mannino DM. MMWR Surveill Summ 2002; 51(6):1–16. Fast Stats A to Z. National Center for Health Statistics. CDC Web site: www.cdc.gov/nchs/fastats/default.htm#A.
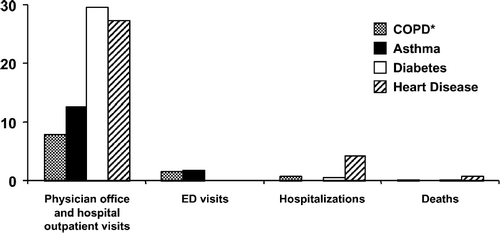
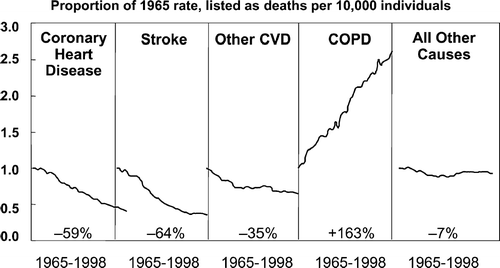
Figure 3 Percent change in age-adjusted death rates, U.S., 1965–1998. Information taken from NHLBI/NIH/DHHS.
In 2002 a group of health care professionals and patients with an interest in COPD met to identify the major barriers to the recognition and care of COPD. They summarized the issues into five major topics:
There is a widespread lack of awareness and understanding of the adverse impact of COPD on the health and quality of life of Americans.
Primary care physicians have a therapeutic nihilism that prevents aggressive treatment of COPD in part reflecting the limited therapeutic options available.
Lack of symptom awareness by both patients and clinicians, lack of spirometry equipment in primary care offices, and lack of diagnostic biomarkers results in late diagnosis of COPD.
COPD care is seldom coordinated among health care professionals and co-morbid conditions are often overlooked.
Reimbursement favors procedures over careful history, monitoring, and education (Citation[9]).
As individual primary care physicians, we can begin to address the first four of these concerns. In fact, we may be able the best specialty to provide expertise in the management of people with multiple morbidities. However, first we must master the skills to recognize, assess and diagnosis COPD. One of the first steps is to understand who may be at risk for COPD.
THE EPIDEMIOLOGY OF COPD
Cigarette smoking causes 80% to 90% of all cases of COPD and a smoker is 10 times more likely to die of COPD than a non-smoker. COPD is more common among women who smoke and those who have a strong family history of COPD (Citation[10]). Currently no tests can predict which smoker will develop COPD but all smokers are candidates for smoking cessation.
Smoking irreversibly changes the structure of the lungs and accelerates the decline in lung function that normally accompanies aging. However, if a person stops smoking even after COPD is established, his or her rate of lung function decline will return to that of a non-smoker, prolonging life (Citation[11]). Thus, detecting COPD is important so that aggressive smoking cessation intervention supported by counseling and medications can be started. Remember simply asking your patients to quit and providing smoking cessation treatments or referral makes a difference.
Other risk factors for COPD include:
middle/old age,
genetic factors (including deficiency of the anti-protease enzyme alpha-1-antitrypsin),
passive smoking, and
prolonged exposure to air pollution <www.GOLDCOPD.org>.
People with > 30-or 40-year packs of smoking and patients who are exposed to respiratory irritants (e.g., fumes and dust) for extended periods, such as during work, are at greatest risk of COPD. Those with family history of early-onset COPD or of respiratory death in the second and third decades may be at risk due to alpha-1-antitrypsin deficiency.
While cigarette smoking is the most common and widely recognized risk factor for the development of COPD, a number of other factors have been shown to be associated with developing COPD (Citation[12], Citation[13]). These factors include environmental exposures such as the occupational dust found in carpentry factories, fumes and vapors found in metal polishing factories, outdoor air pollution, indoor air pollution such as second-hand smoke and smoke from wood or biomass fuel cooking fires, a history of severe childhood respiratory infection(s) and lower socioeconomic status. Of these factors primary care physicians can have impact on the occupational related exposures by helping workers to select and wear appropriate protective gear including masks or respirators (Citation[14]) as well as work with families to reduce the secondhand smoke and make sure that cooking and heating fires are well vented. It is likely that living in poverty results in families being exposed to several of the problems as well as inadequate nutrition, increased exposure to in-utero cigarette smoke and increased exposure to noxious stimuli for the lungs from poorly maintained and ventilated rental housing and to smoke and fumes from surrounding industrial areas.
In addition to environmental factors, a group of host factors have been identified including a hereditary deficiency of a serum protein α-1 antitrypsin (Citation[15]). This protein protects lung tissue from proteolytic attack by the proteinase neutrophil elastase, which targets elastin, a major component of alveolar walls. A hereditary deficiency of α-1 antitrypsin increases the risk of destroying alveolar walls, developing emphysema and, thus, COPD (Citation[16], Citation[17]). This is a recessive trait seen mostly in individuals of Northern European origin. When combined with smoking, α-1 antitrypsin deficiency represents a classic case of host factors working in conjunction with environmental factors—in this case smoking—to contribute to the development of COPD (Citation[15]). Other genetic factors influencing the development of COPD include asthma and airway hyper-responsiveness, and lung growth related to preterm births and low birth weight (Citation[16], Citation[17]). Primary care physicians cannot currently reverse genetic factors but can continue to be vigilant in urging smoking prevention and smoking cessation in pregnant women, people with a family history of COPD, families with a history of asthma and in parents of young children.
Until recently, COPD generally was considered to be a man's disease. However, currently the number women dying from COPD each year is greater than the number of men dying from COPD. More than 61,000 women died from COPD in 2002, compared with 59,000 men (Citation[18]). Mortality rates in white men have remained relatively steady since the early 1980s but have steadily increased among women and African Americans (Citation[4]), with the highest increases in white women. The 2002 mortality rates among white women were almost double those of African-American women 4).
The implications of these numbers for primary care could be extrapolated to suggest that at least 10 to 12% of all of the adults we see have COPD or that as many as 15 to 20% of the adults over 50 have COPD. These patients are smokers and often make physician visits for prolonged colds and respiratory infections. We just need to take the next step and learn to regularly assess these high risk patients for early signs of COPD (Citation[2], Citation[19]).
If COPD is present in 15 to 20% of older adults why are you not seeing them in your office? A major reason is the late stage at which COPD is usually diagnosed (Citation[20]). As the disease progresses and we believe it always does, the symptoms become impossible to ignore and patients finally seek care for breathlessness, dyspnea on exertion and inability to do simple daily activities such as shopping, bathing, walking up a few stairs or engaging in sexual activity due to “exhaustion or breathing problems.” Why would a person wait so long to ask for help with these progressive symptoms? Patients report that they thought the COPD-associated symptoms were from normal aging or just normal smoking symptoms, or from lack of exercise and increasing obesity. Smokers report being concerned about being stigmatized and judged by health care professionals (personal communication Peter Sommerich, July 2006). In a survey mailed to 6000 primary care physicians (personal communication Peter Sommerich, July 2006), physician respondents agreed that many smokers are reluctant to report symptoms and 44% stated that patients report feeling stigmatized by friends, family and co-workers who believe the patient is “getting what they deserve” for smoking. Only 5% of the physicians reported a belief that they or their staff were judgmental.
PATHOLOGY OF COPD
COPD is a combination of the effects of inflammation, oxidation, proteolytic destruction, and genomics (; 16, 17). All areas of the lung are affected, and changes can be seen in the central and peripheral airways, including destruction of the alveolus and bronchioles, excretion of fluid and cells in the airway wall, edema, and changes of goblet and squamous cells in the epithelium (Citation[16], Citation[17]). The peripheral airways also experience narrowing from scar tissue developed during airway remodeling (Citation[16], Citation[17]). The bronchioles, particularly in the upper airways, are often destroyed, but this destruction can be found throughout the lung (Citation[16], Citation[17]). The multiplicity of pathological effects helps explain the use of multi-faceted therapy in dealing with COPD. Unfortunately, we currently have limited pharmacotherapy for certain aspects of COPD such as the mucus excretion and the remodeling ().
Figure 4 COPD pathogenesis. Adapted with permission. Barnes PJ. Chest 2000; 117:10S–14S. © American College of Chest Physicians.
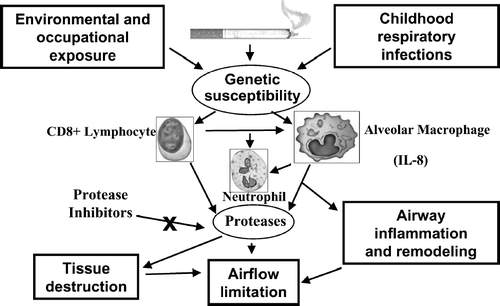
Figure 5 COPD: Pathology. Reproduced with permission. Barnes PJ. N Engl J Med 2000; 343:269–280. © 2000 Massachusetts Medical Society. All rights reserved.
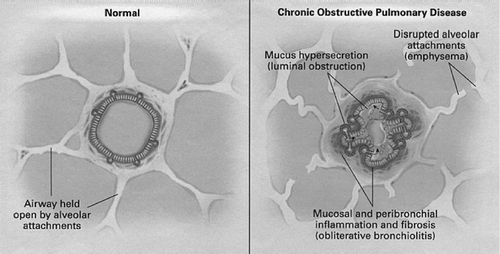
COPD is also associated with oxidative stress (Citation[21]). Oxidants, such as those contained in tobacco smoke, can damage biological molecules (proteins, lipids, nucleic acids), leading to cellular dysfunction or death, damage to the lung's extra cellular matrix as well as inflammation (Citation[16], Citation[17]). The inflammatory process in COPD involves neutrophils, macrophages, and CD8+ lymphocytes (; 16, 17). Studies demonstrate that increases in sputum neutrophil counts correlate with a more rapid decline in forced expiratory volume (FEV; 22). Eosinophils, though present in COPD, are more prominent in exacerbations and appear to play a dominant role in airflow response to corticosteroids (Citation[15], Citation[23]). For primary care physicians, understanding the role of neutrophils and eosinophils in COPD explains why corticosteroids are reserved for more severe stages of COPD when the major goal is to prevent exacerbations.
Table 1 Causes of airflow limitation
Environmental exposures increase the risk of COPD by activating inflammatory cells and induce the release of inflammatory mediators. Cigarette smoke is the single most important risk factor for COPD, with at least 15% to 20% of smokers eventually developing the disease (Citation[16], Citation[17]). Among COPD patients, 85% have a significant history of smoking, and more than 90% of COPD-related deaths can be attributed to smoking (Citation[24]). In COPD, the inflammatory response to cigarette smoke results in mucus hyper secretion, ciliary dysfunction, and airflow limitation, ultimately leading to pulmonary hyperinflation, gas exchange abnormalities, pulmonary hypertension, and core pulmonale.
Tobacco smoke activates macrophages, resulting in recruitment of neutrophils, which leads to the degradation of elastin and collagen in the lung and the airways (Citation[25]). This inflammation continues even after the airways are no longer exposed to cigarette smoke (Citation[26]). Although it cannot reverse pulmonary damage, smoking cessation is the single most effective way to reduce the risk of developing COPD. It is also the only intervention known to slow the progression of COPD (Citation[27]).
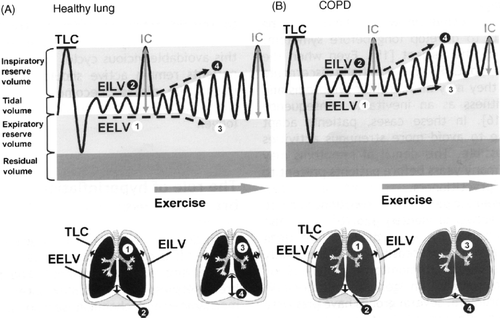
Hyperinflation and dynamic hyperinflation also play a major role in COPD symptoms and exercise intolerance. As the elastic recoil of the lung tissues decrease, the work of exhalation becomes greater and the time required to remove air from the lung becomes longer. As the need for oxygen increases, the respiratory rate increases, the time available for exhalation decreases and lung emptying is less and less complete. The result is hyperinflation with less room for air to be inhaled and greater work of inhalation as the lung tissue and muscle are stretched and moved into the zone of high pressure.
A simple way to understand the dynamic hyperinflation is to empty your lungs completely, take in a very large breath and hold it to a count of five and then without exhaling take in three additional short breaths. By the third breath you are using all your accessory muscles and the work of inhalation is difficult and unpleasant. Price and colleagues have provide a nice illustration of what happens to the space available to increase the tidal volume in COPD and what happens to the physiological lung volume (28; ).
MAKING THE DIAGNOSIS
Deciding who to assess
Far too often the diagnosis of COPD is made when the symptoms have become too bothersome for the patient or their family members to ignore (Citation[29], Citation[30]). In primary care we usually fail to assess for COPD until the patient or family presents a concern about symptoms (Citation[31], Citation[32], Citation[33], Citation[34]). Therefore we need a method to screen patients and determine who is appropriate for further evaluation including lung function testing. Some groups have suggested that we simply use spirometry testing to screen all adults over 50 or all smokers over 45 to 50. A review of the published literature (Citation[2]) points out that such an approach in primary care practices will identify a large group of people without COPD as well as many people who may develop COPD but are currently asymptomatic and will not benefit from any therapy other than smoking cessation which is the standard of care without the need for spirometry. A simple questionnaire of 5 to 7 items that could be completed by the patient has been shown to be of value in other conditions and in early work on COPD screening (Citation[35], Citation[36], Citation[37], Citation[38], Citation[39], Citation[40]).
Price and his colleagues have developed and tested such a questionnaire among primary care patients who smoke (; 41). Some of the information is basic vital status such as age, BMI and smoking status likely to be in every patient's chart. As anticipated the odds ratio for having COPD increases with age from being about 2.3 times as likely in those 50 to 59 compared to those 40 to 49 to 8.5 times as likely in those 60 to 69. A high or increasing BMI decreases the likelihood of late stage COPD since the later stages of COPD are usually associated with loss of muscle mass and weight. The likelihood of COPD also increases with duration and intensity of smoking to over 4 times the likelihood in those with 50 or more pack-year history compared to those with a 14 or less pack-year history.
Table 2 COPD questionnaire
The information that is beyond what most patients already have recorded in their medical records, include the presence of cough that changes with weather, coughing up phlegm without a cold or chronically increased frequency of wheezing without respiratory allergies. From this group of questions it is the presence of phlegm that may help to distinguish between COPD and asthma. No scoring rules for the questions have yet been developed. However, simply asking about chronic cough, cough with phlegm, the affect of weather on cough, presence and frequency of wheezing will identify patients whose symptoms may not yet be severe enough for them to report without prompting, allowing earlier potential diagnosis of COPD.
When a middle aged to elderly smoker presents with severe cough or marked breathlessness and dyspnea on exertion, the differential diagnosis is going to include COPD but is lengthy and includes heart disease, lung disease, endocrine disorders, de-conditioning, obesity and aging (Citation[12]). provides a basis for ruling out some potential etiologies. A combination of the actors in requires that COPD be high in the differential diagnosis.
Table 3 Diagnosis–suggestive features
Table 4 Indicators for COPD diagnosis
Making the diagnosis
The diagnosis of COPD is based on the patient's medical history, a physical exam, and pulmonary function assessment. An early diagnosis of COPD is critical because identifying and treating “at-risk” patients with smoking cessation therapy can help slow or halt disease progression (Citation[42]). Asking about smoking, specifically about “smoker's” cough, what activities they cannot do this year that they did 5 years ago and what they cannot do that they want to do, can open a discussion of respiratory or breathing symptoms.
Recently primary care guidelines have been developed by the International Primary Care Airways Group (Citation[43]). Those guidelines begin with the primary care physician's suspicion of the presence of respiratory symptoms, and then asking patients more in-depth questions. Therefore, it is critical that healthcare professionals ask all patients about their smoking status at every visit (Citation[15]). A history of exposure to other risk factors, such as occupational and environmental toxins, should also be elicited. The more detailed questions must include the frequency, severity, and duration of symptoms such as cough, sputum production, dyspnea and wheezing (; 16, 17).
Table 5 Differential diagnosis questionnaire
In patients with mild COPD, physical signs of airflow limitation usually are not apparent until a decline in lung function has occurred (Citation[16], Citation[17]). During the physical exam, it is important to distinguish between the early stages of COPD, where patients may exhibit only slow expiration and wheezing on forced expiration, and later stages of the disease. During the later stages, hyperinflation of the lungs is evident, anterior posterior diameter of the chest increases, motion of the diaphragm becomes limited, breath sounds are decreased, and heart sounds become distant. At end-stage COPD, patients tend to alter their posture to accommodate difficulty in breathing. The patient may lean forward with arms outstretched and place their weight on their hands. Movements of the muscles of the neck and shoulder girdle are visible and expiration is through pursed lips. Another clinical finding may include an enlarged, tender liver indicating heart failure (Citation[42]). However, it is important to remember that physical signs of airflow limitation are usually not apparent until a marked decline in lung function has occurred; therefore, although physical examination may help in recognizing COPD in the later stages, it is not as essential in targeting patients in the early stages (Citation[16], Citation[17]; ). Both the diagnosis and the staging of COPD depend on lung function assessment.
Table 6 Classifying COPD
Lung function
The most critical diagnostic value in the screening, management, and treatment of COPD is an assessment of lung function via spirometry. In an effort to identify patients earlier in the disease progression, it is recommended that spirometry be performed on any patient with persistent respiratory symptoms (chronic cough, sputum production, dyspnea; 2, 16, 17). Although early intervention will not be able to stop airway deterioration, effective management of COPD may slow disease progression, prevent and control symptoms, improve exercise tolerance and health status, prevent and treat exacerbations and complications, and reduce mortality (Citation[16], Citation[17], Citation[44]).
A diagnosis of COPD is suspected by a prebronchodilator FEV1 of less than 80% of the predicted normal value and an FEV1/FVC ratio of less than 70%. The lack of bronchodilator response confirms that airflow limitation is not fully reversible (Citation[16], Citation[17]), and the lack of a measurable response to bronchodilation is a cardinal characteristic of the irreversible airflow limitation that differentiates COPD from asthma (Citation[16], Citation[17]).
In the elderly patient (70 and older) the normal loss of lung function may result in over diagnosis of COPD, especially in non-smokers (Citation[45]). Therefore, spirometry should not be considered diagnostic in this age group without the accompanying appropriate signs and symptoms.
This lung function testing can be done in family medicine and other primary care offices using small and inexpensive spirometers that have become available in the past 5 years (Citation[46]). Research shows that spirometry is underused, mainly because of a lack of confidence among health professionals in conducting the test and interpreting the results (Citation[47], Citation[48], Citation[49], Citation[50]). This lack of confidence is not surprising given how few medical schools or residency programs teach spriometry technique or interpretation. However, with training and support, family physicians can certainly conduct and correctly interpret spirometric testing (Citation[46]).
Of those without in-office spirometry equipment, at least one-third list each of the following reasons for not having the equipment:
Too expensive
Reimbursement too low
Not sufficiently trained to perform
Insufficient training to interpret results
Physicians commented that they had “too much turnover of office staff to have tests administered accurately in office.” Others said that the “patients were reluctant to get tested so that having the equipment was not cost effective.” A final physician said he had “experienced great patient resistance in spirometry screening” (personal communication, Peter Sommerich, July 2006).
SEPARATING ASTHMA AND COPD
Distinguishing between asthma and COPD in the 40-to 50-year-old may be difficult. Unlike earlier medical “wisdom,” we now know that new onset asthma is possible in people into their 70s and may be especially prominent in women in the peri-menopausal period perhaps due to the decreased use of hormonal replacement therapy in recent years (Citation[51], Citation[52]). The history and symptoms of COPD often mimic those of asthma and accurately distinguishing it from asthma is one of the many challenges facing physicians in diagnosis and management of respiratory problems (Citation[53], Citation[54]).
However, airway hyper-responsiveness in COPD is minimal compared to asthma, and the airflow limitation in COPD does not respond as effectively to bronchodilator use as it does in asthma. Furthermore, changes in disease burden or severity in COPD is generally constantly downward and much slower than the up and down variability often seen in asthma (Citation[13], Citation[53], Citation[55], Citation[56]). and summarize the structural and functional differences between asthma and COPD ( and ).
Table 7 Pathological distinctions between asthma and COPD
Table 8 Structural and functional distinctions between asthma and COPD
Multiple morbidities in people with COPD
To ensure effective treatment and management, it is important to consider the multiple morbidities that are present in many people with COPD such as hypertension, gastro-intestinal reflux, heart disease, and arthritis as they may contribute to lung irritation or the restriction of activity in patients with COPD. Coronary artery disease and left heart failure also contribute to dyspnea and mimic the symptoms of a COPD exacerbation. In primary care, it is common to treat patients with multiple morbidities but also easy to ignore one in favor of another that is easier to treat or appears to be the most symptomatic at the time. Use of problem lists and tools to remind the physician and patient about important data to gather at each visit about each condition may help lessen this burden. In addition, dealing with the multiple specialists that care for a patient's cardiac disease, pulmonary disease or rheumatic condition pose a special challenge for the primary care physician. To date only limited work has been done to smooth the obstacles for co-management of patients between primary and specialty care. This area requires additional elaboration, enumeration and increased reimbursement for the primary care physician who must coordinate, monitor and often explain to the patient the frequent and sometimes conflicting recommendations of a single disease or organ focused specialist.
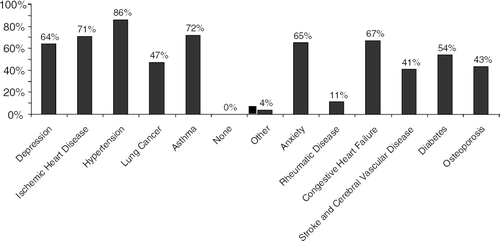
Physicians report a high frequency of comorbid conditions in the patients they treat for COPD (). As many as 76% report that being over or underweight adversely affects the mortality of people with COPD. Chronic use of steroids and inactivity increase the rate of osteoporosis and the risk for osteoporotic fractures in those with COPD. Other co-morbid conditions include sleep disruption seen in 25% of people with one COPD symptom and over 75% of people with two or more COPD symptoms.
DEPRESSION—A COMMON COPD CO-MORBIDITY
Rates of recognized depression among men and women who have COPD vary from 20% to 50% and increase as the severity of COPD increases (Citation[61], Citation[62]). Among patients who receive home oxygen therapy, rates of anxiety and depression may be as high as 62% (Citation[63]). Unfortunately, only one-third of persons who have COPD and are depressed receive therapy for depression (Citation[64]).
Depression and anxiety amplify the adverse affect of COPD, and decrease treatment compliance and quality of life. A 2001 study suggested that up to 18% of the variance in physical functioning among people who have similar levels of COPD severity can be attributed to depressive symptoms. A person who has COPD and who is depressed is more likely to perceive a lack of support from family, friends and the medical community and may refuse all treatment.
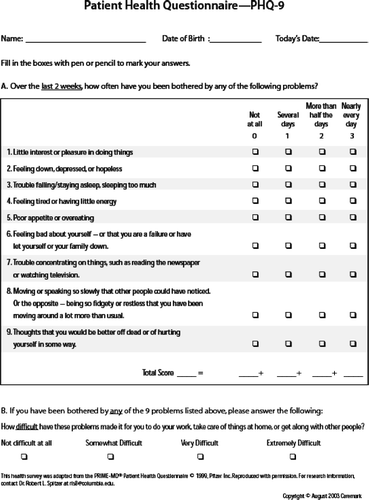
Depression screening using the PHQ-9 <http://www. predictonline.com/phq9.htm () can identify appropriate candidates for treatment that includes pulmonary rehabilitation and medication and counseling for depression. In one study, subjects who completed pulmonary rehabilitation showed improvements in self-concept and decreased depressive symptoms (Citation[65]).
Older anti-depressants, such as nortriptyline, can improve depression and are safe to use in persons who have even severe COPD. Newer anti-depressants have been tested on a very limited basis among persons who have COPD. A single episode of brief cognitive behavioral therapy (as short as 2 hours) may help decrease the sensation of dyspnea, anxiety, and depression, but such resources may not be available in all communities (Citation[66]).
The management of patients with COPD and other co-morbid conditions deserve additional research and additions to the international COPD treatment guidelines. For example, the treatment of COPD with steroids in patients with diabetes has implications for management that are often ignored. Management of co-existing COPD and heart failure or cardiovascular disease is seldom addressed in any guidelines. Having a separate guideline for each of the patient's four or five chronic diseases is of limited value in caring for the whole person and integration of multiple guidelines is time consuming and difficult. This challenge needs to be accepted by large national bodies with varied expertise including that offered by primary care physicians.
Clinical management of COPD
The goals of management of COPD have been summarized in a list developed by the World Health Organization's GOLD guidelines (Citation[16], Citation[17]; See ). Since COPD is a chronic and progressive disease, management is largely focused on slowing or preventing disease progression, and maintaining function and quality of life for the patient. Preventing acute exacerbations is also a key objective since exacerbations are associated with increased morbidity, health care costs and mortality (Citation[16], Citation[17]). Clinical management is generally determined by the stage of the disease and gives an overview of the stepwise approach to adding therapeutics with advancing stages of disease.
Figure 9 Therapy additions with change in stage of disease. *Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2006).
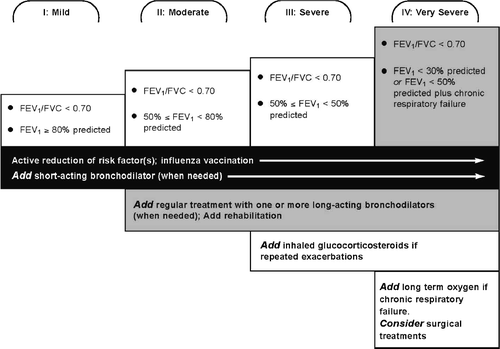
Table 9 GOLD goals of COPD management
PREVENTION STRATEGIES
Since the great majority of COPD in the Western world is caused by smoking the obvious first prevention strategy is to convince the patient to quit smoking. This strategy will be discussed further next. In addition, all persons with a family history of COPD should be advised to avoid exposure to passive smoke and other environmental pollutants. Early reports from the world wide BOLD (Burden Of Lung Disease) study indicate that COPD in non-smokers is much higher than previously appreciated, presumably due to exposure to noxious smoke and pollutants (Citation[67]). The GOLD guidelines also recommend that all persons at risk for COPD, which includes all smokers with or without symptoms, should receive influenza vaccine every year since viral respiratory infections are a common cause of exacerbations. Pneumococcal and Hemophilus Influenzae respiratory infections are 2 of the most common bacterial causes of exacerbations in COPD. To date there is not sufficient evidence to warrant recommending these immunizations in all COPD patients; however, they may be worth considering in a high-risk patient.
Smoking cessation
Perhaps the most challenging aspect to managing COPD is getting patients to successfully quit smoking. Fortunately, there have been significant advances in both behavioral and pharmacological interventions to enhance success. It is clear that primary care physicians are the key to the success of these public health efforts. About 70% of smokers have at least one primary care visit a year and approximately the same number express a desire to quit (Citation[68]). Just the concern and advice of the primary care physician have been shown to increase motivation to quit smoking, and the level of a person's motivation is predictive of their success at quitting (Citation[69], Citation[70]).
The United States Public Health guidelines have developed helpful behavioral strategies (The Five “A's”; see ) to assist with a smoking cessation intervention. In general, an approach that is empathic and supportive rather than confrontational is more successful (Citation[71]). Sometimes a clinical situation (pregnancy, hospitalization, diagnosis of COPD, passive smoking-related problems in family members, e.g., asthma, otitis) creates an opportunity to motivate quitting. When these events arise the physician should take advantage of the chance to advise and assist. ( the Five “A's”) In addition to behavioral strategies several pharmacological aids have been very helpful in enhancing quitting success. lists available agents. Only about 7% of persons attempting to quit on their own will be successful at the end of one year (Citation[72]). Nicotine replacement therapy has been associated with a doubling (about 10–14%) of that success rate (Citation[73]). The addition of extended-release Bupropion, an anti-depressant that reduces smoking drive, has been reported to increase the success of cessation interventions to 20–30% (Citation[74]). Very recently, a large clinical trial using the nicotine receptor agonist, varinicline, demonstrated a 44% quitting success at 3 months and a sustained quitting rate of 22% at one year. In general these agents are most successful when used in conjunction with the behavioral and support strategies listed here.
Table 10 The five “A”s: Strategies for smoking cessation
PHARMACOLOGICAL THERAPIES
GOLD stage I
The GOLD guidelines recommend adding medications to the management based on the stage of severity (). Stage I is usually not associated with significant chronic symptoms or functional limitations so daily maintenance medication is not indicated, but patients can benefit from the availability of a short-acting bronchodilator (e.g., albuterol, ipatropium) on a prn basis. There are many short-acting bronchodilator agents available and can be given by tablet, metered dose inhaler, dry powder inhaler, or nebulizer depending on a patients preference. Inhaled products are quicker acting, so generally preferable for “rescue” use. Side effects of beta agonists can include tremor and tachycardia, but are generally safe, even in patients with a history of cardiovascular disease. Older, short-acting anti-cholinergics often cause dry mouth and may aggravate other problems such as urinary retention, constipation, or narrow angle glaucoma. It may help reduce side effects of inhaled agents if the patient is instructed to rinse out their mouth after inhalation.
GOLD stage II
By the time most patients present to their physician with respiratory complaints related to COPD they have already developed chronic symptoms or functional limitations, or GOLD Stage II. These patients, in addition to the interventions listed previously, will benefit from daily maintenance therapy with a long-acting bronchodilator. The agents available include beta-agonists, anti-cholinergics, and methylxanthines. The advantage of longer-acting agents in maintenance therapy is more than the convenience of dosing. Longer-acting agents avoid the peaks and troughs of shorter-acting agents and have a desirable carryover effect from previous dose to the first morning dose. Inhaled agents are generally preferable since they are delivered directly to the desired site of action and have fewer side effects.
Several of the medications are available in breath-actuated dry power inhalers that might pose a theoretical concern regarding effective dose delivery in a person with severe COPD. However, these devices have been shown to deliver the therapeutic dose of medication in persons with severely limited lung function (FEV1 = 20% predicted). Using a combination of different classes of bronchodilators rather than pushing the dose of a single agent offers the advantage of employing complementary mechanisms of action and reduces the likelihood of side effects. Tiotropium, a long-acting selective anti-cholinergic agent has the major advantage of once a day dosing and generally compares favorably to other bronchodilators in improvement of lung function, reduction in exacerbations and sustained efficacy over time (Citation[75]). Pharmacoeconomic data suggest that the higher cost for tiotropium compared to other broncho-dilators may be more than offset by reduced healthcare utilization costs (Citation[76], Citation[77]).
Since COPD is caused by an abnormal inflammatory reaction in the lungs it makes intuitive sense to use anti-inflammatory medications in therapy. The guidelines discourage the use of systemic corticosteroids for maintenance because the risk to benefit is too great. However, a short course of high-dose systemic corticosteroids (steroid burst) has been shown to be beneficial in the treatment of acute exacerbations. Inhaled corticosteroids (ICS) have been shown to reduce exacerbations, and the current guidelines recommend ICS in persons with Stage II or greater COPD if they have a history of frequent exacerbations (2–3 exacerbations per year; 16, 17). Although some of the earlier clinical trials using ICS in COPD failed to show much benefit, there is gathering evidence that ICS, especially at higher doses, are helpful in reducing exacerbations and hospitalization and are associated with better quality-of-life scores (Citation[78], Citation[79]).
More recently, several large clinical trials have demonstrated that the combination of an ICS with a long acting beta-agonist bronchodilator (fluticasone, 250–500 mcg/salmeterol 50 mcg bid) was more effective than either ICS or bronchodilator alone in reducing hospitalizations and exacerbations and improving lung function and quality of life (Citation[44], Citation[80]). A recent pilot study using fluticasone 500 mcg/salmeterol 50 mcg and tiotropium 18 mcg as combination therapy versus either therapy alone showed that the combination was significantly more effective in improving lung function in very severe COPD (Citation[81]).
GOLD stages III and IV
In general the more severe stages of COPD are managed with the same medications, but with greater use of combinations of therapy to maximize benefit and the addition of supplemental oxygen is beneficial for the very severe patient with evidence of hypoxia. Chronic use of antibiotics is not recommended, but some physicians educate their COPD patients to have antibiotics on hand at home and to start them at the first sign of an exacerbation. The choice of antibiotics should cover the most common pathogens associated with exacerbations (Strep Pneumoniae, Hemophilus Inlfuenzae, and Moraxella), and the newer macrolides and quinolones offer good coverage. Mucolytics may offer some modest benefit in helping clear secretions. Antitussives should be avoided since they may compromise cough clearance of secretions and vasodilators should also be avoided in the severe patient because they can aggravate the ventilation perfusion mismatch in the lungs. Narcotics and other respiratory depressants must be used cautiously, but may be indicated for patient comfort in terminal situations. In addition to medications, some patients with very severe disease may be candidates for lung volume reduction surgery or possibly lung transplant.
Pulmonary rehabilitation
Pulmonary rehabilitation (PR) is defined by the American Thoracic Society as, “a multidisciplinary program of care for persons with a chronic respiratory impairment that is individually tailored and designed to optimize physical and social performance and autonomy”(Citation[82]). There are no specific criteria as to which patients should be referred to a PR program, but any person who has chronic respiratory or functional limitations from their COPD (GOLD stage II or greater) should be considered. Physical reconditioning is an important component of PR and exercise programs have shown outcomes of improved physical function, decreased dyspnea with exertion, improved health status, and decreased healthcare utilization costs (Citation[83]). A comprehensive PR program goes beyond reconditioning, and includes assessing and addressing an individual's specific risks and needs. lists the goals of PR.
Table 11 Goals of pulmonary rehabilitation
The PR program is tailored to the individual's stage of disease, their motivation and their co-morbidities, and the goals target the common areas of risk and need that can benefit from intervention. Long-term benefits of rehabilitation programs are less positive and seem to depend mostly on the individual's ability and motivation to continue the exercises and other recommendations of the program at home (Citation[84]). Rehabilitation programs have been clearly shown to have immediate benefits to health status and patient well-being, even though they do not directly improve lung function (FEV1) or long-term survival (; 85).
Table 12 Pharmacologic agents to aid in smoking cessation
What we say we need
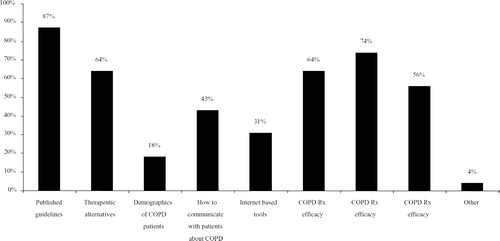
When asked what they need to increase their ability to identify and manage COPD, 867 physicians attending chronic care disease educational workshops in Los Angeles, Raleigh-Durham, and Chicago said they need more information on how to identify high-risk people, how to interpret and incorporate spirometry results in the diagnosis and management of COPD, as well learn more about COPD medication effectiveness and about non-medication therapies such as information about elements of pulmonary rehabilitation that can be done within their community when formal rehabilitation programs are not available. (). We need guidelines like those of the International Primary Care Airways Group (Citation[43]) that are translated into practical steps. In addition, we need better tools to help us communicate with the people and families of people with COPD. COPD needs to receive the same attention that primary care has given asthma recognition, diagnosis and management.
REFERENCES
- World Health Organization. The GOLD global strategy for the management and prevention of COPD. 2001, www.gold-copd.com 2001
- Wilt T J, Niewoehner D, Kim C, Kane R L, Linabery A, Tacklind J, MacDonald R, Rutks I. Use of Spirometry for Case Finding, Diagnosis, and Management of Chronic Obstructive Pulmonary Disease (COPD). Agency for Healthcare Research and Quality, Rockville, MD August, 2005, http://www.ahrq.gov/clinic/epcsums/spirosum.htm Summary, Evidence Report/Technology Assessment: Number 121. AHRQ Publication Number 05-E017-1
- Mannino D, Holguin F. Epidemiology and global impact of chronic obstructive pulmonary disease. Resp Med: COPD Update 2006; 1: 114–120
- National Heart Lung and Blood Institute. Morbidity and Mortality Chartbook. 2004, Available at http://www.nhlbi.nih.gov/resources/docs/cht-book.htm
- Ward M M, Javitz H S, Smith W M, Bakst A. Direct medical cost of chronic obstructive pulmonary disease in the USA. Respir Med 2000; 94: 1123–1129
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: NHLBI/WHO Workshop Report. National Institutes of Health National Heart, Lung, and Blood Institute, Bethesda, MD April, 2001, NIH Publication 2701
- Strassels S A, Smith D H, Sullivan S D, Mahajan P S. The costs of treating COPD in the United States. Chest 2001; 119: 344–352
- Mannino D M. COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest 2002; 121: 121S–126S
- Yawn B P. A US national agenda for COPD in primary care. Airways J 2004; 2(3)168–169
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2004, Executive Summary
- Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ 1977; 1(6077)1645–1648
- Celli B R, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23: 932–946
- Pauwels R A, Buist A S, Ma P, Jenkins C R, Hurd SS, GOLD Scientific Committee. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care 2001; 46(8)798–825
- www.cdc.gov/niosh/userguid.html
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. National Institutes of Health, Bethesda, MD 2003, http://www.goldcopd.com/(NIH publication no. 2701) Accessed June 2005
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, http://www.goldcopd.com/GuidelinesResources.asp?11=2&12=0 Updated 2005. Available at Accessed December 9, 2005
- Global Initiative for Chronic Obstructive Lung Disease. Spirometry for diagnosis of COPD, http//www.goldcopd.com/GuidelinesResources.asp?11=2&12=0 Updated 2005. Available at Accessed December 9, 2005
- American Lung Association. Search Lung USA. Search Lung USA, Available at http://www.lungusa.org/site/apps/s/content.asp?c=dvLUK9O0E&b=34706&ct=67295
- von Hertzen L, Reunanen A, Impivaara O, Malkia E, Aromaa A. Airway obstruction in relation to symptoms in chronic respiratory disease-a nationally representative population study. Respir Med 2000; 94(4)356–363
- http://www.cdc.gov/nchs
- Leigh R, Pizzichini M M, Morris M M, Maltais F, Hargreave F E, Pizzichini E. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J 2006; 27(5)964–971
- Stanescu D, Sanna A, Veriter C, Kostianev S, Calcagni P G, Fabbri L M, Maestrelli P. Airways obstruction, chronic expectoration, and repaid decline of FEV1 in smokers are associated with increased levels of sputum neutrophils. Thorax 1996; 51(3)267–271
- Kitaguchi Y, Fujimoto K, Kubo K, Honda T. Characteristics of COPD phenotypes classified according to the findings of HRCT. Respir Med 2006; 100(10)1742–1752
- Stopping smoking: The benefits and aids to quitting. Action on Smoking and Health. November, 2005, Available at http://www.ash.org.uk/ Action on Smoking and Health Factsheet No. 11
- Churg A, Zay K, Shay S, Xie C, Shapiro S D, Hendricks R, Wright J L. Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice. Am J Respir Cell Mol Biol 2002; 27: 368–374
- Croxton T L, Weinmann G G, Senior R M, Hoidal J R. Further research directions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002; 165: 838–844
- Executive Summary, Global Strategy for the Diagnosis, Management, and Prevention of COPD. 2005, Update. Available at http://www.goldcopd.com/Guidelineitem.asp?11equals;2&12equals;1&intId=1389
- Price D, Freeman D, Kaplan A, Ostrem A, Reid J, van der Molen T. Progressive breathlessness in COPD—the role of hyperinflation and its pharmacological management. Prim Care Respir J 2005; 14: 285–293
- Petty T L. Definitions, causes, course, and prognosis of chronic obstructive pulmonary disease. Respir Care Clin N Am 1998; 4: 345–358
- van den Boom G, Rutten-van Molken M P, Tirimanna P R, van Schayck C P, Folgering H, Van Weel C. Association between health-related quality of life and consultation for respiratory symptoms: results from the DIMCA programme. Eur Respir J 1998; 11: 67–72
- den Otter J J, van Weel C. How to avoid under-diagnosed asthma/chronic obstructive pulmonary disease?. J Asthma 1998; 35: 381–387
- Hurd S. The impact of COPD on lung health worldwide: epidemiology and incidence. Chest 2000; 117: 1S–4S, (2 suppl)
- Ferguson G T, Enright P L, Buist A S, Higgins M W. Office spirometry for lung health assessment in adults: a consensus statement from the National Lung Health Education Program. Chest 2000; 117: 1146–1161
- van Weel C. Underdiagnosis of asthma and COPD: is the general practitioner to blame?. Monaldi Arch Chest Dis 2002; 57: 65–68
- van Schayck C P, Loozen J M, Wagena E, Akkermans R P, Wessling G J. Detecting patients at a high risk of developing chronic obstructive pulmonary disease in general practice: cross sectional case finding study. BMJ 2002; 324: 1370
- Calverley P MC, Nordyke R J, Halbert R J, Isonaka S, Nonikov D. Development of a population-based screening questionnaire for COPD. J COPD 2005; 2: 225–232
- Müllerová H, Wedzicha J, Soriano J V, Vestbo J. Validation of a chronic obstructive pulmonary disease screening questionnaire for population surveys. Respir Med 2004; 98: 78–83
- van Schayck C P, Halbert R J, Nordyke R J, Isonaka S, Maroni J, Nonikov D. Comparison of existing symptoms-based questionnaires for identifying COPD in the general practice setting. Respirology 2005; 10: 323–333
- Kida K, Wakabayashi R, Mizuuchi T, Katsura H, Yamada K, Motegi T, Nishimura N, Omata M, Goto R, Mizuno S. Pre-screening test for chronic obstructive pulmonary disease (COPD) using an 11-item questionnaire for elderly subjects (abstract P1663). 12th Annual Congress of the European Respiratory Society, Stockholm, September, 14–182002
- Freeman D, Nordyke R J, Isonaka S, Nonikov D V, Maroni J M, Price D, Halbert R J. Questions for COPD diagnostic screening in a primary care setting. Respir Med 2005; 99: 1311–1318
- Price D B, Tinkelman D G, Halbert R J, Nordyke R J, Isonaka S, Nonikov D, Juniper E F, Freeman D, Hausen T, Levy M L, Ostrem A, van der Molen T, van Schayck C P. Symptom-based questionnaire for identifying COPD in smokers. Respiration 2006; 73: 285–295
- American Thoracic Society. European Respiratory Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. 2004, Available at http://www-test.thoracic.org/copd/ Accessed December 9, 2005
- International Primary Care Airways Group (IPAG). Chronic Airways Diseases. A Guide for Primary Care Physicians. January, 2005
- Ferguson G T, Calverley P MA, Anderson J A, Celli B, Jenkins C, Jones P WJ. The TORCH (Towards a Revolution in COPD Health) study: salmeterol/fluticasone propionate (SFC) improves survival in COPD over three years. ERJ 2006; 28: 34s–35s, (suppl 50)
- Hardie J A, Buist A S, Vollmer W M, Ellingsen I, Bakke P S, Morkve O. Risk of over-diagnosis of COPD in asymptomatic elderly never-smokers. Eur Respir J 2002; 20: 1117–1122
- Yawn B P, Enright P L, Lemanske R F, Israel E, Pace W, Wollan P, Boushey H. Spirometry may modify family physicians' management of asthma and COPD. Chest, Submitted 2006
- Eaton T, Withy S, Garrett J E, Mercer J, Whitlock R M, Rea H H. Spirometry in Primary Care Practice: the importance of quality assurance and the impact of spirometry workshops. Chest 1999; 116(2)416–423
- den Otter J J, de Bryuyn-Schmidt M A, Wolters M J, van Schayck C P, Folgering H TM, van den Hoogen H JM, van Weel C. Lung function measurement in general practice: general practice measurements compared with laboratory measurements during the DIMCA trial. Fam Pract 2000; 17: 314–316
- Schermer T R, Jacobs J E, Chavannes N H, Hartman J, Folgering H T, Bottema B J, van Weel C. Validity of spirometric testing in a general practice population of patients with chronic obstructive pulmonary disease. Thorax 2003; 58: 861–866
- Jones K P. The role of measuring FEV1 in determining therapeutic changes made in an asthma clinic in general practice. Respir Med 1995; 89(3)171–177
- Troisi R J, Speizer F E, Willett W C, Trichopoulos D, Rosner B. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. Am J Respir Crit Care Med 1995; 152: 1183–1188, (4 Pt 1)
- Asthma in women after menopause. Hormone replacement therapy may explain why many women over 50 develop this disease. Health News 2005; 11(4)4–5, Unauthored
- Sutherland E R. Outpatient treatment of chronic obstructive pulmonary disease: comparisons with asthma. J Allergy Clin Immunol 2004; 114(4)715–724, quiz 725
- Pauwels R A, Lofdahl C G, Laitinen L A, Schouten J P, Postma D S, Pride N B, Ohlsson SV, for the European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med 1999; 340(25)1948–1953
- van Noord J A, Bantje T A, Eland M E, Korducki L, Cornelissen P JG. A randomized controlled comparison of tiotropium and ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study Group. Thorax 2000; 55(4)289–294
- Yawn B P. Exploring factors accounting for asthma variability: achieving optimal symptom control for individual patients. Prim Care Respir J 2006, in press
- National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Expert Panel report 2: guidelines for the diagnosis and management of asthma. National Institutes of Health, Bethesda, MD 2002, Publ. No. 97-4051. Available at www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm
- Fabbri L M, Hurd S S. Global Strategy for the Diagnosis, Management and Prevention of COPD: 2003 update. Eur Respir 2003; 22: 1–2
- Barnes P J. Mechanisms of COPD: differences from asthma. Chest 2000; 117: 10S–14S, (2 suppl)
- National Heart, Lung, and Blood Institute. National Asthma Education and Prevention Expert Panel report 2: guidelines for the diagnosis and management of asthma. National Institutes of Health, Bethesda, MD 1997, Publ. No. 97-4051. Available at www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm
- Yohannes A M. Depression and COPD in older people: a review and discussion. Br J Community Nurs 2005; 10(1)42–46
- Breslin E, van der Schans C, Breukink S, Meek P, Mercer K, Volz W, Louie S. Perception of fatigue and quality of life in patients with COPD. Chest 1998; 114(4)958–964
- Lacasse Y, Rousseau L, Maltais F. Prevalence of depressive symptoms and depression in patients with severe oxygen-dependent obstructive pulmonary disease. J Cardiopulm Rehabil 2001; 21(2)80–86
- Van Ede L, Yzermans C J, Brouwer H J. Prevalence of depression in patients with chronic obstructive pulmonary disease: a systematic review. Thorax 1999; 54(8)688–692
- Garuti G, Cilione C, Dell'orso D, Gorini P, Lorenzi M D, Totaro L, Cirelli G, Clini E. Impact of comprehensive pulmonary rehabilitation on anxiety and depression in hospitalized COPD patients. Monaldi Arch Chest Dis 2003; 59(1)56–61
- Kunik M E, Braun U, Stanley M A. One session cognitive behavioral therapy for elderly patients with chronic obstructive pulmonary disease. Psychol Med 2001; 31(4)717–723
- Buist S. Press release. Using Standardized methods to estimate COPD prevalence worldwide; Early Data from Seven Countries. European Respiratory Society Congress, MunichGermany September, 2006
- The Tobacco Use and Dependence Clinical Practice Guideline Panel, Staff, and Consortium Representatives. A clinical practice guideline for treating tobacco use and dependence. A US public health service report. JAMA 2000; 283: 3244–3254
- Russell M A, Stapleton J A, Feyerabend C, Wiseman S M, Gustavsson G, Sawe U, Connor P. Targeting heavy smokers in general practice: randomized controlled trial of transdermal nicotine patches. BMJ 1993; 306(6888)1308–1312
- Prochaska J O, DiClemente C C, Norcross J C. In search of how people change. Applications to addictive behaviors. Amer Psychol 1992; 47(9)1102–1114
- Miller W R, Rollnick S. Motivational Interviewing. Preparing People to Change Addictive Behavior. The Guildford Press, New York, London 1991
- Hughes J R, Gulliver S B, Fenwick J W, Valliere W A, Cruser K, Pepper S, Shea P, Solomon L J, Flynn B S. Smoking cessation among self-quitters. Health Psychol 1992; 11(5)331–334
- Fiore M C, Smith S S, Jorenby D E, Baker B. The effectiveness of the nicotine patch for smoking cessation. A meta-analysis. JAMA 1994; 271(24)1940–1947
- Hays J T, Hurt R D, Rigotti N A, et al. Sustained-release Bupropion for pharmacologic relapse prevention after smoking cessation. Ann Int Med 2001; 135(6)423–433
- Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium competed with twice daily salmeterol in patients with COPD. Thorax 2003; 58(5)399–404
- D'Souza A O, Smith M J, Miller L A, Kavookjian J. An appraisal of pharmacoeconomic evidence of maintenance therapy for COPD. Chest 2006; 129(6)1693–1708
- Lee K H, Phua J, Lim T K. Evaluating the pharmacoeconomic effect of adding tiotropium bromide to the management of chronic obstructive pulmonary disease patients in Singapore. Respir Med 2006, [Epub ahead of print]
- Burge P S, Calverley P M, Jones P W, Spencer S, Anderson J A, Maslen T K. Randomized, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000; 320(7245)1297–1303
- Sin D D, Tu J V. Inhaled corticosteroids and the risk of mortality and readmission in elderly patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164(4)580–584
- Calverley P M. Combined salmeterol and fluticasone for COPD. Lancet 2003; 361(9369)1652–1653
- Cazzola M, Noschese P, Salzillo A, De Giglio C, D'Amato G, Matera M G. Bronchodilator response to formoterol alter regular tiotropium or to tiotropium alter regular formoterol in COPD patients. Respir Med 2005; 99(5)524–528
- American Thoracic Society. Pulmonary rehabilitation—1999. Am J Respir Crit Care Med 1999; 159(5)1666–1682
- Goldstein R S, Gort E H, Stubbing D, Avendano M A, Guyatt G H. Randomized controlled trial of respiratory rehabilitation. Lancet 1994; 344(8934)1394–1397
- Lacasse Y, Wong E, Guyatt G H, King D, Cook D J, Goldstein R S. Meta-analysis of respiratory rehabilitation in chronic obstructive pulmonary disease. Lancet 1996; 348(9035)1115–1119
- Troosters T, Gosselink R, Decramer M. Short-and long-term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomized trial. Am J Med 2000; 109(3)207–212