INTRODUCTION
Proton MRI of the lung is a major challenge as the lung is probably the most difficult organ to be studied by MRI. This is caused by the low amount of tissue which relates to a small number of protons leading to low signal, countless air-tissue interfaces that cause substantial susceptibility artifacts, as well as respiratory and cardiac motion. In several lung diseases, such as tumors, the amount of protons or the blood volume is actually increased and motion is reduced, which provides better pre-conditions for MRI (Citation[1]). In emphysema, however, there are no facilitating disease-related effects as there is loss of tissue and reduced blood volume due to hypoxic vasoconstriction and the degree of hyperinflation has a negative correlation with the MR signal (Citation[2]). The depiction of airway dimensions and size of the airway walls by MRI is certainly limited to the central bronchi. Fortunately, MRI has significant potential beyond the mere visualization of structure by providing comprehensive information about “function,” i.e., perfusion, hemodynamics, ventilation, and respiratory mechanics. In this review the current knowledge about the potential of proton MRI in COPD and emphysema is presented.
MORPHOLOGY
In contrast to MRI, CT is well established as the imaging modality of choice for high-resolution imaging (HRCT) of pulmonary structure. CT routinely depicts airways down to the 8th generation. Lumen diameter and wall thickness can be easily measured. Highly accurate evaluations perpendicular to the centre line of the bronchial lumen are feasible using sophisticated software. CT also allows for quantification of emphysematous destruction using mean lung density, emphysema index, low-end percentiles, and other measures (Citation[3], Citation[4], Citation[5], Citation[6]). Modern multi-detector CT scanners allow for contiguous acquisition of 1 mm thick slices. From these 3D datasets deeper insights into local hyperinflation and expiratory obstruction can be generated (Citation[7]). However, CT reaches its limits when it comes to the functional impact or interpretation of such measurements related to airway obstruction, hyperinflation, perfusion, and dyspnea.
The attempt of using MRI for depiction of pulmonary structure in COPD will always be compared to CT. By physics, MRI will never outperform CT when it comes to visualization of either bronchial dilation, wall thickening, or emphysematous destruction. This is probably the reason why there are only a few publications on this topic. People have started to use MRI for cystic fibrosis, which—when it comes to morphology of the airways—could be regarded as a very advanced and specific form of COPD. When there is obvious disease, MRI performs as well as CT (Citation[8]). The performance of MRI in early, subtle disease has not been investigated yet. Measurements of the bronchial diameter and wall thickness are limited by the spatial resolution of MRI, which normally is in the range of 4 mm. Only a few sequences provide three-dimensional datasets with 1 mm section thickness and interpolation. The most frequently used sequences are acquired in a breath-hold. The T2-weighted HASTE sequence in coronal and/or axial orientation allows for the depiction of inflammatory bronchial wall thickening and mucus collections (). T1-weighted 3D gradient echo sequences, such as VIBE, are suitable for the assessment of the mediastinum and common nodular lesions. The intravenous application of contrast material markedly improves the diagnostic yield of these sequences by a clearer depiction of vessels, hilar structures, and solid pathologies (). A major goal is to use such T1-and T2-weighted images as well as contrast enhancement to differentiate inflammation within the wall from muscular hypertrophy, edema, and mucus collection as this cannot be achieved by CT. Additionally, CT and MRI have never been used in exacerbation. Since the use of CT in and after exacerbation might be limited by the risks associated with radiation exposure by repeated examinations, MRI could be an attractive means to search for mucus collections or small bronchocentric infiltrates. By T2-and T2-weighted sequences MRI has unique capabilities to detect and also quantify fluid collections. Such techniques might also be used to measure edema in airway inflammation. Proton signals from lung parenchyma provide information on pulmonary function in models of airway diseases in rats (Citation[9]). Initial measurements carried out in artificially ventilated control rats revealed a highly significant negative correlation between the parenchymal signal and the partial pressure of oxygen (pO2) in the blood for different amounts of oxygen administered. Experiments carried out in spontaneously breathing animals challenged with allergen or endotoxin revealed parenchymal signal changes that reflected the oxygenation status of the lungs and were consistent with airway remodeling or hyporesponsiveness. The same group also demonstrated the potential of MRI to detect mucus hypersecretion and inflammation in rats after intratracheal administration of endotoxin (lipopolysaccharide (LPS)). The signal had two components: one of diffuse appearance and higher intensity, which was particularly prominent up to 48 h after LPS, probably reflecting generalized granulocytic inflammation; the second showing an irregular appearance and weaker intensity, which was predominant later, probably reflecting mucus (Citation[10]).
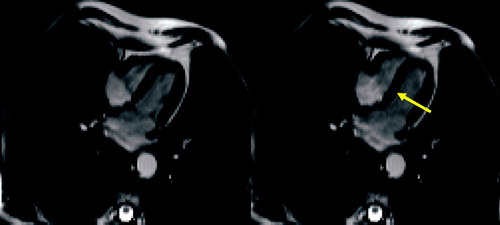
Figure 1 T2 weighted axial MR images (HASTE) showing bronchial wall thickening of left upper lobe and lower lobes (arrow).
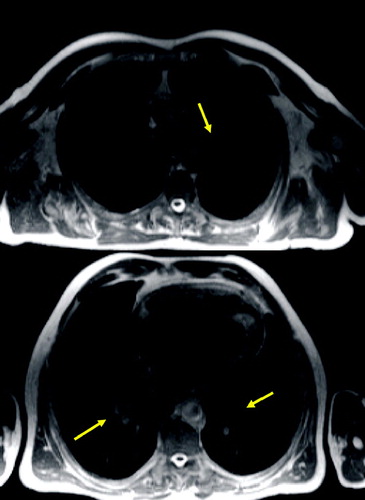
Figure 2 T1 weighted axial GRE (VIBE): small nodule in the right middle lobe (arrow) and dystelectatic changes in the right lower lobe.
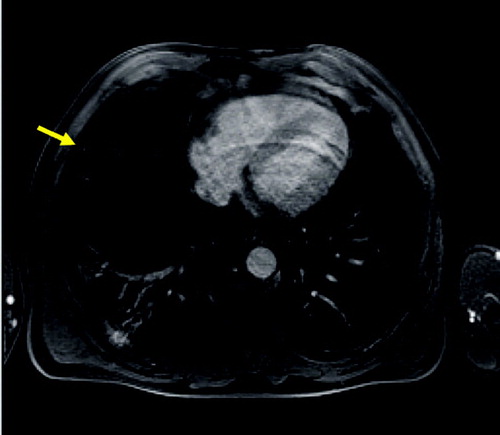
Emphysematous destruction can hardly be diagnosed by a loss of signal in the periphery ( and ). It is much easier to detect consecutive hyperinflation just by the size or the volume of the thorax as well as the reduced blood volume in the severely affected areas by perfusion MRI.
Figure 3 Axial T2 weighted MR (HASTE) image (A) and corresponding HRCT (B) showing severe panlobular emphysema and a large bulla (arrow). The rarefication of the pulmonary arteries and reduction of parenchyma are reflected by a general signal loss on MRI.
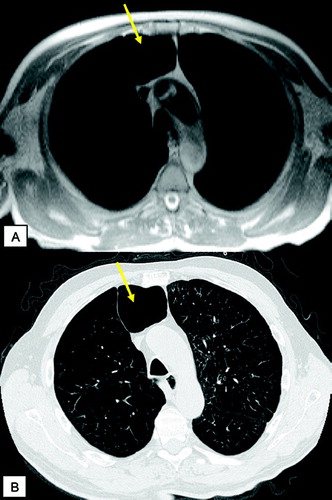
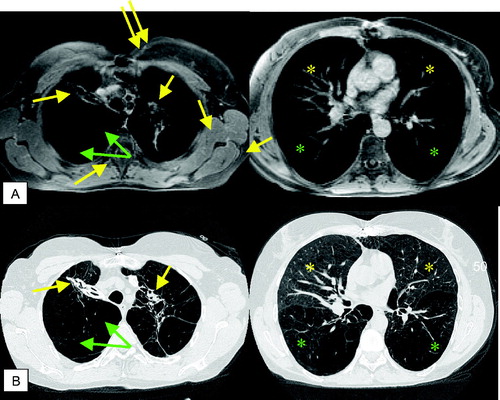
Figure 4 T1 weighted GRE (VIBE) post contrast (A) and corresponding HRCT (B): scar tissue (yellow arrows) and emphysematous bullae (green arrows) in the apical regions; centrilobular changes in the middle lobe and lingula (yellow asterisks), severe panlobular emphysema (green asterisks) in the lower lobes. Note the nice delineation between the centrilobular and panlobular changes by signal intensity (A, right image) on MRI.
PERFUSION
Gas exchange in the lungs is maintained by a balance between ventilation and perfusion. In patients with COPD and emphysema, ventilation is impaired due to airway obstruction and parenchymal destruction. The structural loss of lung parenchyma leads to a significant increase of closed volume and a decreased ventilation of those regions (Citation[11]) with subsequent hypoxic vasoconstriction, (Citation[12]) including a local reduction in pulmonary blood flow (Citation[13], Citation[14]). The loss of pulmonary arteries is related to the severity of parenchymal destruction and the mechanical compression of pulmonary arteries in case of hyperinflation (Citation[15], Citation[16]). However, it is known that the distribution of perfusion does not necessarily match morphological, emphysematous parenchymal changes (Citation[17]).
Conventional radionuclide perfusion scintigraphy can be used to assess these abnormalities, but it has substantial limitations with respect to spatial and temporal resolution (Citation[18]). A superior technique is SPECT (Citation[19]), which is rarely used as it is rather time-consuming and not routinely applied for lung imaging.
Compared with nuclear medicine studies, MR perfusion offers higher temporal and spatial resolution without ionizing radiation. The basic principle of contrast-enhanced MR perfusion is a dynamic image acquisition following an intravenous bolus injection of a paramagnetic contrast agent. New MR techniques are an attractive alternative to provide volumetric perfusion weighted 3D datasets with a high spatial resolution and the possibility for multiplanar reformation (Citation[20], Citation[21], Citation[22]). As COPD and emphysema are heterogeneous diseases affecting all parts of the lung with variable severity, the evaluation of the whole lung is important ( and ). Due to high spatial resolution analysis of pulmonary perfusion and precise anatomical localization of the perfusion defects can be achieved, thereby allowing for lobar and segmental analysis.
Figure 5 MR perfusion (10 mm MIP) of COPD patient showing a reduced perfusion of the lower lobes and normal perfusion of the upper lobes.
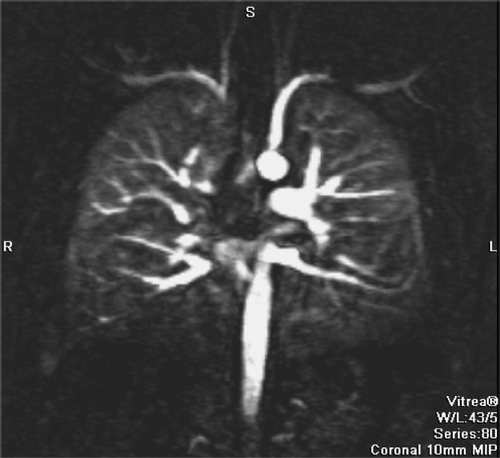
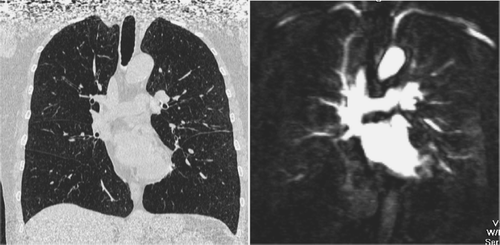
Figure 6 Coronal reformatted CT image and MR perfusion (10 mm MIP) of the patient suffering from severe emphysema showing a severe reduction of pulmonary perfusion and only marginal perfusion of the right basal and left subpleural regions.
MR perfusion allows for a high diagnostic accuracy in detecting perfusion abnormalities (Citation[21], Citation[23]). Furthermore, MR perfusion ratios correlate well with radionuclide perfusion scintigraphy ratios (Citation[24], Citation[25]). Pulmonary perfusion during breath-hold depends on the inspiratory level () being higher at expiration (Citation[26]).
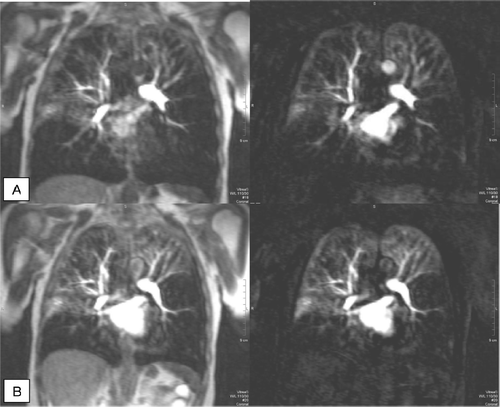
Figure 7 Original and subtracted images of MR perfusion during inspiratory (A) and expiratory (B) breath-hold: loss of perfusion in lower lung regions and moderate perfusion of the upper regions with an increase in perfusion after expiration.
The perfusion abnormalities in COPD differ clearly from those caused by vascular obstruction. While in embolic obstruction wedge-shaped perfusion defects occur, a generally low degree of contrast enhancement is found in COPD with emphysema (Citation[27], Citation[28]). Furthermore the peak signal intensity is reduced. These features allow for easy visual differentiation.
3D MR perfusion datasets allow for quantitative assessment of regional pulmonary perfusion parameters (Citation[29]). Quantitative values for pulmonary perfusion can be obtained by applying the principles of indicator dilution techniques (Citation[30]). Efficient gas exchange depends on the close matching of regional pulmonary blood flow (PBF) to ventilation. In a study with quantitative evaluation of 3D perfusion in patients with COPD, mean PBF, mean transit time (MTT), and pulmonary blood volume (PBV) were diffusely decreased and the changes were heterogeneous. Calculated mean PBF and PBV were significantly decreased, and MTT was significantly shortened (Citation[29]).
It was discussed that patients with emphysema have hypoxia as well as destruction of lung parenchyma and fewer alveolar capillaries. This causes increased pulmonary arterial resistance and, secondarily, pressure. Therefore, PBF was decreased. In addition, the heterogeneous destruction of lung parenchyma caused the decrease in PBV. MTT is determined by the ratio between PBV and PBF. Therefore, the results suggested that MTT is significantly decreased, reflecting a larger degree of decrease in PBV compared with PBF, and the heterogeneous changes of regional PBV and MTT were larger than those of PBF.
Therefore, accurate quantitative measurements of these changes in the regional pattern of PBF are important to understand physiology and pathophysiology of the lung ().
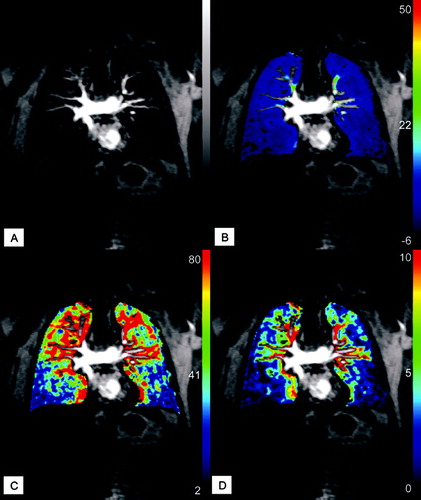
Figure 8 Parameter maps of quantitative analysis of MR perfusion acquired during inspiratory breath-hold: original image of time-resolved MR perfusion (A) showing severe perfusion defects in the lower lobes and normal perfusion in the right upper lobe; mean concentration (B); pulmonary blood flow (C) and pulmonary blood volume (D).
HEMODYNAMICS
Most studies devoted to the investigation of pulmonary hemodynamics and right ventricular function in chronic lung diseases have dealt with patients suffering from COPD. In COPD, pulmonary hypertension is closely linked to the presence of chronic alveolar hypoxia. On the one hand, alveolar hypoxemia acts directly on the walls of the pulmonary arterioles and thus determines vasoconstriction. The effect of chronic hypoxia on the pulmonary circulation appears to be caused by structural and functional alterations of the vascular endothelium. From a functional point of view, the pulmonary vessels in patients affected by COPD show reduced or no capacity for vessel dilatation due to a defect in synthesis and/or release of nitric oxide. On the other hand, it favors pulmonary hypertension via an indirect mechanism such as compensating polycythemia. Blood viscosity increases in the pulmonary circulation and, together with the hypovolemia and tachycardia induced by hypoxemia, induces an increase of the flow rate, causing hypertension. Pulmonary hypertension is most often mild to moderate (mean pulmonary artery pressure (PAP) in the range of 20–35 mmHg), but it may worsen markedly during acute exacerbations of the disease, sleep, and exercise. Long-term oxygen therapy is the logical treatment for hypoxic pulmonary hypertension. Over time, pulmonary hypertension leads to right ventricular enlargement, which includes hypertrophy and dilatation. This is a beneficial adaptation, allowing the right ventricle to cope with an increased afterload and to maintain a normal cardiac output. The right ventricular function, and in particular the right ventricular contractility, are generally preserved in patients with advanced COPD. “True” right ventricular failure can be observed during acute exacerbations of the disease, when worsening of hypoxemia induces a marked increase in afterload (PAP and pulmonary vascular resistance) (Citation[31]). Severe pulmonary hypertension is observed in a minority of COPD patients who present with some physiologic particularities, i.e., moderate bronchial obstruction contrasting with a marked hypoxemia and normocapnia or hypocapnia. Different patterns of hemodynamic abnormalities have been described in COPD. Patients with a severe obstructive ventilatory impairment but with relatively normal arterial blood gases usually do not demonstrate pulmonary hypertension at rest.
Patients with hypoxemia typically demonstrate pulmonary hypertension at rest accompanied by clinical and ECG evidence of right ventricular hypertrophy. Postmortem findings provide further evidence of the relation between hypoxemia and the development of right ventricular hypertrophy. Given these findings, it can be hypothesized that the adaptation mechanism of the right ventricle is different in COPD patients without compared to those with hypoxemia. Studies in large groups of patients with COPD and hypoxemia demonstrated increased right ventricular volumes, decreased right ventricular function, and impaired left ventricular diastolic function (Citation[32]).
Detection of severe pulmonary hypertension in COPD patients is necessary for at least two reasons: (Citation[1]) such patients have a poor prognosis; and (Citation[2]) they need adequate treatment that includes pulmonary vasodilators. It has been demonstrated by several studies that the level of pulmonary hypertension has a prognostic impact in COPD patients. In one of these studies, the 5-year survival rates were 50% in patients with mild (20 to 30 mmHg), 30% in those with moderate-to-severe (30 to 50 mmHg), and 0% in the small group (n = 15) of patients with very severe pulmonary hypertension (> 50 mmHg). Thus, severe pulmonary hypertension bears a poor prognosis, and this also has been observed in COPD patients receiving long-term oxygen therapy (Citation[33]).
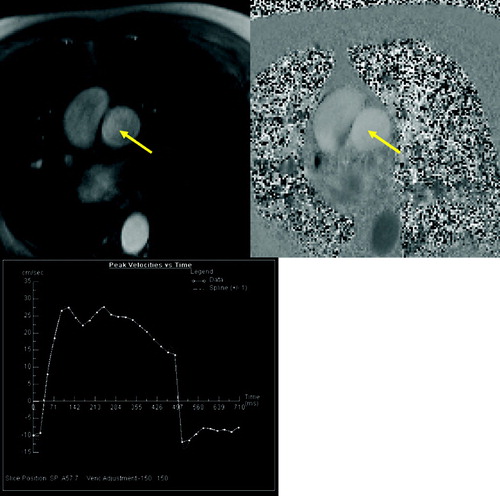
Prior to the onset of clinical symptoms, patients exhibit signs of vascular bed obstruction and elevated pulmonary artery pressure, including pulmonary artery dilatation; right ventricular performance is usually maintained. Evaluation of the right ventricle and pulmonary blood flow by echocardiography is difficult in patients with emphysema as the acoustic window is limited. Therefore, MRI has been used for imaging the right ventricle for quite a long time, and a loose correlation between increased right ventricular mass and the severity of emphysema was found (Citation[34]). Assessment of right ventricular function can be done either by flow measurements in the pulmonary trunk () or by short axis cine-acquisition of the right ventricle. By use of phase contrast flow measurements in the pulmonary trunk it is possible to characterize the hemodynamics of the blood flow. Laffon et al. were able to calculate the pressure wave velocity allowing to non-invasively assessing pulmonary arterial hypertension (Citation[35]). However, this method has not yet been applied to COPD patients. For cine imaging of the whole cardiac cycle ECG gated gradient-echo pulse sequences are applied. By covering the complete heart by short axis cine images, it is possible to accurately measure early changes of the complex geometry of the right ventricular wall and chamber volume (). In a study by Vonk-Nordegraaf et al. the right ventricular mass and ejection fraction in 25 clinically stable, normoxic COPD patients were analyzed. It was found that the position of the heart is rotated and shifted to a more vertical position in the thoracic cavity due to hyperinflation of the lungs, increasing the retrosternal space. The right ventricular wall mass was significantly higher (68 g) in the patient group compared to healthy volunteers (59 g). The right ventricular ejection fraction was not changed (53%) (Citation[36]). In another study from the same group, structural and functional cardiac changes in COPD patients with normal PaO2 and without signs of right ventricular failure were evaluated. Compared to healthy volunteers there were no indications of pulmonary hypertension. The end-systolic and end-diastolic volumes of the right ventricle were significantly reduced while the ejection fraction was not changed. The right ventricular mass was significantly elevated while the left ventricular myocardial mass was not changed. The authors conclude that concentric right ventricular hypertrophy is the earliest sign of right ventricular pressure overload in patients with COPD. This structural adaptation of the heart does not alter right and left ventricular systolic function (Citation[37]).
Figure 9 Quantitative flow measurement of pulmonary blood flow. Magnitude (A) and velocity encoded (B) image of pulmonary trunk (arrow). Results of peak velocity over time (C) show the normal increase of velocity at the beginning of systolic phase following by abnormal plateau and regurgitation during diastole.
As this is the only study so far in patients with mild emphysema, no strong conclusions can be drawn from the early adaptation mechanisms of the right ventricle in patients with normoxemia or mild hypoxemia and the consequences of any structural changes on right and left ventricular function.
VENTILATION
As sufficient gas exchange depends on matched perfusion and ventilation, assessment of regional ventilation in human lungs is important for the diagnosis and evaluation of various pulmonary disorders, including pulmonary emphysema. Currently, the most established method for imaging regional lung ventilation are nuclear medicine studies using krypton-81m (Kr-81m), xenon-133 (Xe-133), radiolabeled aerosol (Technegas), and technetium-99m (Tc-99m)-labeled diethylentriaminepentaacetic acid (DTPA) (Citation[38], Citation[39]). The utility of nuclear medicine in pulmonary diseases has been well documented; however, poor spatial resolution and the necessity of inhalation of a radioactive substance remain major limitations of these methods. Over the past decade hyperpolarized noble gas MRI using He-3 and Xe-129 were developed to improve imaging of pulmonary ventilation (Citation[40]). He-3 was used in several studies showing a high sensitivity to airflow obstruction in asthmatics and impairment of ventilation in emphysematous patients (Citation[41], Citation[42]). He-3 proved also to be a valuable tool for quantification of ventilation and correlated well with parenchymal destruction in emphysema (Citation[11], Citation[43]). Overall, the high cost of the laser polarizer and noble gases and the need for non-proton imaging remain the major drawbacks of these methods, preventing it from broader clinical applications.
Recently, several investigators reported that oxygen-enhanced MRI could demonstrate regional ventilation (Citation[44], Citation[45], Citation[46], Citation[47]). The paramagnetic effect of molecular oxygen promotes longitudinal relaxation of nearby protons. As oxygen is rapidly taken up into the blood, the exact location of the detected signal increase and the subsequent interpretation of its changes are still unclear. It seems obvious that the ventilatory component to the measured signal is actually rather small, while the contribution of dissolved oxygen in the interstitium and especially the blood seems to be much more predominant. Recent works also suggest that the simple administration of pure oxygen induces the pulmonary arteries to dilate, resulting in an increase of pulmonary blood volume and a consecutive increase in signal intensity.
The technique of oxygen-enhanced MRI has been successfully applied in volunteers; the translation into clinical examination, however, is difficult. Only a few studies have successfully applied oxygen-enhanced MRI to patients with pulmonary diseases in a clinical setting and revealed the pathophysiologic significance of oxygen enhancement (Citation[48]).
In some basic measurements it was shown that the T1 times of the lung parenchyma are significantly shorter in patients with emphysema than in volunteers (Citation[49]). In a preliminary study (including only two emphysema patients), an inhomogeneous and weak signal intensity increase after application of oxygen was observed, compared to healthy volunteers (Citation[50]).
In studies including a larger patient population, Ohno et al. found that oxygen-enhanced MR ventilation imaging showed regional changes in ventilation reflecting regional lung function. They reported that the maximum mean relative enhancement ratio was excellently correlated with the diffusion capacity for carbon monoxide. The mean slope of relative enhancement was strongly correlated with the forced expiratory volume in 1 sec, and the maximum mean relative enhancement had a good correlation with the high-resolution CT emphysema score (Citation[51], Citation[52]).
It can be concluded that oxygen-enhanced MRI demonstrates significant heterogeneities in the distribution of ventilation and perfusion including their matching as well as the vasodilator response of the pulmonary vasculature in COPD and emphysema. However, the use of high oxygen concentrations in severe COPD patients may be risky.
RESPIRATORY DYNAMICS
Hyperinflation severely reduces the mechanical advantage of the diaphragm with respect to the rib cage to respiratory motion, while it commonly increases the contribution of the rib cage itself. The mechanical disadvantage of the diaphragm is presumably primarily the result of the length changes. Further contributing factors include a reduction in the appositional component of diaphragmatic action (through reduction in the zone of apposition) and the insertional component (through a shift in the alignment of the diaphragmatic fibers from axial to radial). In chronic hyperinflation, the diaphragm adapts to the chronically hyperinflated state. This adaptation to chronic foreshortening is similar to the adaptation occurring in the skeletal muscle. It is caused by a dropout of sarcomeres in series along the muscle fibers. It restores the force-generating capacity of the muscle, in part, but it reduces the capacity of the muscle to undergo length changes. Finally, hyperinflation commonly results in additional recruitment of expiratory muscles (Citation[53]).
This complex interaction between chest wall and diaphragm motion can be visualized by fluoroscopy. However, this technique is limited by the use of x-ray and the fact being a projection technique. These days it is possible to image the chest wall and diaphragmatic motion by 2D or 3D dynamic MR techniques (Citation[54], Citation[55], Citation[56]). For data acquisition time resolved techniques are used based on FLASH or trueFISP sequences (). This allows for a temporal resolution of 100 ms per frame (Citation[57]).
Figure 11 Coronal TrueFISP sequence with temporal resolution of 100 ms for assessment of the thoracic motion. Fast forced expiration (FEV1 manouver) starting from the maximum of inspiration (A) and ending at the maximum of expiration (B). Note the marginal difference of both images in the patient suffering from severe emphysema.
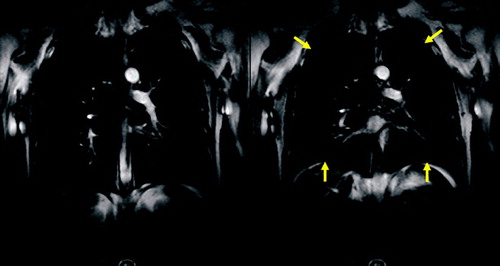
In contrast to normal subjects with regular, synchronous diaphragm and chest wall motion, patients with emphysema frequently showed reduced, irregular, or asynchronous motion, with a significant decrease in the maximum amplitude and the length of apposition of the diaphragm (Citation[58]). Parts of the diaphragm moved paradoxically, relative to the change in lung area. The paradoxical diaphragmatic motion correlated with hyperinflation, although severe hyperinflation tended to restrict both normal and paradoxical diaphragmatic motion (Citation[59]). In some patients the ventral portion of the hemidiaphragm moved downward while the dorsal part moved upward like a seesaw (Citation[60]). Also, the abnormal rib cage motion can be revealed by MRI. In some cases the ventral rib cage moved anteriorly while the hemidiaphragm moved upward (Citation[60]). After lung volume reduction surgery, patients showed improvements in diaphragm and chest wall configuration and mobility (Citation[58]).
CONCLUSION
Proton MRI is a promising modality to assess the different aspects of COPD. While the spatial resolution is lower than at HRCT, it is possible to image the characteristic changes of the lung parenchyma. Beyond all other techniques, MRI allows for functional imaging regarding perfusion and assessment of right ventricular function and cor pulmonale. Furthermore, time resolved imaging of diaphragmatic motion tops off the possibilities of MR by providing an assessment of the impaired respiratory mechanics in COPD.
REFERENCES
- Kauczor H U, Kreitner K F. MRI of the pulmonary parenchyma. Eur Radiol 1999; 9: 1755–1764
- Bankier A A, O'Donnell C R, Mai V M, et al. Impact of lung volume on MR signal intensity changes of the lung parenchyma. J Magn Reson Imaging 2004; 20: 961–966
- Gevenois P A, de Maertelaer V, De Vuyst P, et al. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1995; 152: 653–657
- Gevenois P A, De Vuyst P, de Maertelaer V, et al. Comparison of computed density and microscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1996; 154: 187–192
- Baldi S, Miniati M, Bellina C R, et al. Relationship between extent of pulmonary emphysema by high-resolution computed tomography and lung elastic recoil in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 585–589
- Madani A, Zanen J, de Maertelaer V, et al. Pulmonary emphysema: objective quantification at multi-detector row CT—comparison with macroscopic and microscopic morphometry. Radiology 2006; 238: 036–1043
- Zaporozhan J, Ley S, Eberhardt R, et al. Paired inspiratory/expiratory volumetric thin-slice CT scan for emphysema analysis: comparison of different quantitative evaluations and pulmonary function test. Chest 2005; 128: 3212–3220
- Puderbach M, Eichinger M, Gahr J, et al, Proton MRI appearance of cystic fibrosis: Comparison to CT. Eur Radiol 2006. Epub ahead of print; DOI 10.1007/s00330-006-0373-4
- Beckmann N, Cannet C, Zurbruegg S, et al. Proton MRI of lung parenchyma reflects allergen-induced airway remodeling and endotoxin-aroused hyporesponsiveness: a step toward ventilation studies in spontaneously breathing rats. Magn Reson Med 2004; 52: 258–268
- Beckmann N, Tigani B, Sugar R, et al. Noninvasive detection of endotoxin-induced mucus hypersecretion in rat lung by MRI. Am J Physiol Lung Cell Mol Physiol 2002; 283: L22–L30
- Zaporozhan J, Ley S, Gast K K, et al. Functional analysis in single-lung transplant recipients: a comparative study of high-resolution CT, (3)He-MRI, and pulmonary function tests. Chest 2004; 125: 173–181
- Euler U S, Liljestrand G. Observation on the pulmonary arterial blood pressuer in the cat. Acta Physiol Scand 1946; 12: 301–320
- Morrison N J, Abboud R T, Muller N L, et al. Pulmonary capillary blood volume in emphysema. Am Rev Respir Dis 1990; 141: 53–61
- Cederlund K, Hogberg S, Jorfeldt L, et al. Lung perfusion scintigraphy prior to lung volume reduction surgery. Acta Radiol 2003; 44: 246–251
- Bishop J M. Cardiovascular complications of chronic bronchitis and emphysema. Med Clin North Am 1973; 57: 771–780
- Thabut G, Dauriat G, Stern J B, et al. Pulmonary hemodynamics in advanced COPD candidates for lung volume reduction surgery or lung transplantation. Chest 2005; 127: 1531–1536
- Sandek K, Bratel T, Lagerstrand L, et al. Relationship between lung function, ventilation-perfusion inequality and extent of emphysema as assessed by high-resolution computed tomography. Respir Med 2002; 96: 934–943
- Brudin L H, Rhodes C G, Valind S O. Regional structure-function correlations in chronic obstructive lung disease measured with positron emission tomography. Thorax 1992; 47: 914–921
- Fujita E, Nagasaka Y, Kozuka T. Correlation among the indices of high-resolution computed tomography, pulmonary function tests, pulmonary perfusion scans and exercise tolerance in cases of chronic pulmonary emphysema. Respiration 2002; 69: 30–37
- Ley S, Fink C, Puderbach M. [Contrast-enhanced 3D MR perfusion of the lung: application of parallel imaging technique in healthy subjects]. Fortschr Röntgenstr 2004; 176: 330–334
- Fink C, Puderbach M, Bock M. Regional lung perfusion: assessment with partially parallel three-dimensional MR imaging. Radiology 2004; 231: 175–184
- Fink C, Ley S, Kroeker R. Time-resolved contrast-enhanced three-dimensional magnetic resonance angiography of the chest: combination of parallel imaging with view sharing (TREAT). Invest Radiol 2005; 40: 40–48
- Sergiacomi G, Sodani G, Fabiano S. MRI lung perfusion 2D dynamic breath-hold technique in patients with severe emphysema. In Vivo 2003; 17: 319–324
- Ohno Y, Hatabu H, Higashino T. Dynamic perfusion MRI versus perfusion scintigraphy: prediction of postoperative lung function in patients with lung cancer. AJR Am J Roentgenol 2004; 182: 73–78
- Molinari F, Fink C, Risse F. Assessment of differential pulmonary blood flow using perfusion magnetic resonance imaging: comparison with radionuclide perfusion scintigraphy. Invest Radiol 2006; 41: 624–630
- Fink C, Ley S, Risse F. Effect of inspiratory and expiratory breathhold on pulmonary perfusion: assessment by pulmonary perfusion magnetic resonance imaging. Invest Radiol 2005; 40: 72–79
- Amundsen T, Torheim G, Kvistad K A. Perfusion abnormalities in pulmonary embolism studied with perfusion MRI and ventilation-perfusion scintigraphy: an intra-modality and inter-modality agreement study. J Magn Reson Imaging 2002; 15: 386–394
- Morino S, Toba T, Araki M. Noninvasive assessment of pulmonary emphysema using dynamic contrast-enhanced magnetic resonance imaging. Exp Lung Res 2006; 32: 55–67
- Ohno Y, Hatabu H, Murase K. Quantitative assessment of regional pulmonary perfusion in the entire lung using three-dimensional ultrafast dynamic contrast-enhanced magnetic resonance imaging: preliminary experience in 40 subjects. J Magn Reson Imaging 2004; 20: 353–365
- Levin D L, Hatabu H. MR evaluation of pulmonary blood flow. J Thorac Imaging 2004; 19: 241–249
- Weitzenblum E. The pulmonary circulation and the heart in chronic lung disease. Monaldi Arch Chest Dis 1994; 49: 231–234
- Budev M M, Arroliga A C, Wiedemann H P. Cor pulmonale: an overview. Semin Respir Crit Care Med 2003; 24: 233–244
- Weitzenblum E, Chaouat A. Severe pulmonary hypertension in COPD: is it a distinct disease?. Chest 2005; 127: 1480–1482
- Boxt L M. MR imaging of pulmonary hypertension and right ventricular dysfunction. Magn Reson Imaging Clin N Am 1996; 4: 307–325
- Laffon E, Laurent F, Bernard V. Noninvasive assessment of pulmonary arterial hypertension by MR phase-mapping method. J Appl Physiol 2001; 90: 2197–2202
- Vonk Noordegraaf A, Marcus J T, Roseboom B. The effect of right ventricular hypertrophy on left ventricular ejection fraction in pulmonary emphysema. Chest 1997; 112: 640–645
- Vonk-Noordegraaf A, Marcus J T, Holverda S. Early changes of cardiac structure and function in COPD patients with mild hypoxemia. Chest 2005; 127: 1898–1903
- Miller T R, Biello D R, Lee J I. Ventilation imaging with Kr-81m. A comparison with Xe-133. Eur J Nucl Med 1981; 6: 11–16
- Alderson P O, Line B R. Scintigraphic evaluation of regional pulmonary ventilation. Semin Nucl Med 1980; 10: 218–242
- Kauczor H U, Hanke A, Van Beek E J. Assessment of lung ventilation by MR imaging: current status and future perspectives. Eur Radiol 2002; 12: 1962–1970
- Altes T A, Powers P L, Knight-Scott J. Hyperpolarized 3He MR lung ventilation imaging in asthmatics: preliminary findings. J Magn Reson Imaging 2001; 13: 378–384
- Ley S, Zaporozhan J, Morbach A. Functional evaluation of emphysema using diffusion-weighted 3Helium-magnetic resonance imaging, high-resolution computed tomography, and lung function tests. Invest Radiol 2004; 39: 427–434
- Swift A J, Wild J M, Fichele S. Emphysematous changes and normal variation in smokers and COPD patients using diffusion 3He MRI. Eur J Radiol 2005; 54: 352–358
- Edelman R R, Hatabu H, Tadamura E. Noninvasive assessment of regional ventilation in the human lung using oxygen-enhanced magnetic resonance imaging. Nat Med 1996; 2: 1236–1239
- Loffler R, Muller C J, Peller M. Optimization and evaluation of the signal intensity change in multisection oxygen-enhanced MR lung imaging. Magn Reson Med 2000; 43: 860–866
- Ohno Y, Chen Q, Hatabu H. Oxygen-enhanced magnetic resonance ventilation imaging of lung. Eur J Radiol 2001; 37: 164–171
- Hatabu H, Tadamura E, Chen Q. Pulmonary ventilation: dynamic MRI with inhalation of molecular oxygen. Eur J Radiol 2001; 37: 172–178
- Ohno Y, Sugimura K, Hatabu H. Clinical oxygen-enhanced magnetic resonance imaging of the lung. Top Magn Reson Imaging 2003; 14: 237–243
- Stadler A, Stiebellehner L, Jakob P M, et al. T1 maps and O(2)-enhanced MRT of the diseased lung Emphysema, fibrosis, mucoviscidosis. Radiologe 2006
- Muller C J, Schwaiblmair M, Scheidler J. Pulmonary diffusing capacity: assessment with oxygen-enhanced lung MR imaging preliminary findings. Radiology 2002; 222: 499–506
- Ohno Y, Hatabu H, Takenaka D. Oxygen-enhanced MR ventilation imaging of the lung: preliminary clinical experience in 25 subjects. AJR Am J Roentgenol 2001; 177: 185–194
- Ohno Y, Hatabu H, Takenaka D. Dynamic oxygen-enhanced MRI reflects diffusing capacity of the lung. Magn Reson Med 2002; 47: 1139–1144
- Decramer M. Hyperinflation and respiratory muscle interaction. Eur Respir J 1997; 10: 934–941
- Cluzel P, Similowski T, Chartrand-Lefebvre C. Diaphragm and chest wall: assessment of the inspiratory pump with MR imaging-preliminary observations. Radiology 2000; 215: 574–583
- Plathow C, Fink C, Ley S. Measurement of diaphragmatic length during the breathing cycle by dynamic MRI: comparison between healthy adults and patients with an intrathoracic tumor. Eur Radiol 2004; 14: 1392–1399
- Plathow C, Schoebinger M, Fink C. Evaluation of lung volumetry using dynamic three-dimensional magnetic resonance imaging. Invest Radiol 2005; 40: 173–179
- Plathow C, Klopp M, Fink C. Quantitative analysis of lung and tumour mobility: comparison of two time-resolved MRI sequences. Br J Radiol 2005; 78: 836–840
- Suga K, Tsukuda T, Awaya H. Impaired respiratory mechanics in pulmonary emphysema: evaluation with dynamic breathing MRI. J Magn Reson Imaging 1999; 10: 510–520
- Iwasawa T, Kagei S, Gotoh T. Magnetic resonance analysis of abnormal diaphragmatic motion in patients with emphysema. Eur Respir J 2002; 19: 225–231
- Iwasawa T, Yoshiike Y, Saito K. Paradoxical motion of the hemidiaphragm in patients with emphysema. J Thorac Imaging 2000; 15: 191–195