Abstract
Bronchodilators, including long-acting β2-adrenoceptor agonists and anticholinergic bronchodilators, are effective in the treatment of chronic obstructive pulmonary disease. Evidence suggests that the addition of a long-acting β2-agonist to an inhaled corticosteroid is associated with a reduced rate of exacerbations compared with either treatment alone or placebo. However, it is not known whether a long-acting β2-agonist/inhaled corticosteroid combination is more effective than an anticholinergic bronchodilator alone in reducing exacerbations. The Investigating New Standards for Prophylaxis In Reduction of Exacerbations (INSPIRE) trial will study salmeterol (a long-acting β2-agonist) in combination with fluticasone propionate (an inhaled corticosteroid) compared with tiotropium bromide (an anticholinergic bronchodilator) in patients with moderate-to-severe chronic obstructive pulmonary disease. The INSPIRE study is a multicentre, randomised, double-blind, double dummy, parallel group study conducted over 104 weeks. This is the first study to use two parallel definitions of an exacerbation; an event-based exacerbation is defined as one that requires use of healthcare resources, including additional treatment and hospitalization, whereas a symptom-based exacerbation is defined as one that satisfies the 1987 Anthonisen criteria. It is also the first study to compare the long-term effects of salmeterol/fluticasone propionate with tiotropium bromide on the rate of event-based exacerbations. Endpoints include rate of exacerbations (primary endpoint), time to first exacerbation, and duration of exacerbations. Health outcomes will be assessed via the St George's Respiratory Questionnaire. If the innovative methodology of utilizing 2 definitions of exacerbation proves successful, it will set the benchmark for future studies in chronic obstructive pulmonary disease.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a multi-component disease driven by inflammation of the airways. It is characterized by the presence of airway obstruction, airway structural changes and mucociliary dysfunction combined with an important systemic component (Citation[1], Citation[2]). COPD is defined by airflow limitation that is not fully reversible (Citation[2]). The airflow limitation is usually progressive and is associated with an abnormal inflammatory response of the lungs to noxious particles or gases, primarily caused by cigarette smoking (Citation[2]). Current evidence suggests that inflammation is central to the pathophysiology and symptoms of COPD (Citation[3]).
In patients with COPD, an acute worsening of respiratory symptoms is often described as an exacerbation. Although the causes of exacerbations in COPD are probably multifactorial, airway inflammation is thought to play an important role in the pathogenesis of worsening airflow obstruction seen during acute exacerbations, demonstrated by increases in inflammatory markers and mediators during exacerbations (Citation[4]). Systemic inflammation, as demonstrated by concurrent increases in systemic inflammatory factors, also occurs during exacerbations (Citation[5], Citation[6]). Therefore, therapeutic interventions that target inflammation may help prevent COPD exacerbations.
The heterogeneity of exacerbations and variation from patient to patient means that there is a lack of consensus concerning the best way to define exacerbations. Many published trials have assessed the effect of interventions on COPD exacerbations via the occurrence of events such as hospitalizations or the increased need for medication (Citation[7], Citation[8], Citation[9], Citation[10], Citation[11], Citation[12], Citation[13]), since these are objective, easily measured parameters. Exacerbations may be assessed alternatively by symptom-based criteria, such as those proposed by Anthonisen in 1987 (Citation[14]).
The current study, Investigating New Standards for Prophylaxis In Reduction of Exacerbations (INSPIRE), utilizes a novel approach as both healthcare-utilization and symptom-based definitions for measuring exacerbations will be compared. The study will compare the effects of the combination of salmeterol/fluticasone propionate (SFC) 50/500 mcg twice daily with tiotropium bromide 18 mcg once daily in patients with moderate-to-severe COPD (stage III and IV) over 104 weeks. Previous data have shown that bronchodilators including tiotropium bromide and salmeterol have some beneficial effects on exacerbations (Citation[15], Citation[16], Citation[17]). Combination therapy with inhaled corticosteroids (ICS) and long-acting β2-agonists (LABA), which have both anti-inflammatory and bronchodilator effects, have also been shown to reduce the exacerbation rate in COPD (Citation[7], Citation[9], Citation[10], Citation[18]), with SFC demonstrating greater effects in reducing exacerbations in moderate-to-severe COPD populations than salmeterol alone (Citation[9], Citation[19]).
INSPIRE is innovative in its use of 2 separate measurements of exacerbations and will be the longest study to date specifically investigating the effects of pharmacotherapy on exacerbations in COPD as a primary outcome. This paper summarizes the methodology of the study, in advance of the publication of the results.
METHODS
Study design
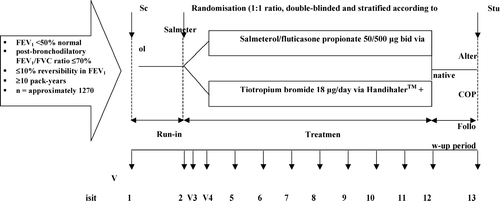
The INSPIRE study is a 104-week, multicentre, international, randomized, double-blind, double-dummy, parallel group study. It aims to recruit 1270 patients from 20 countries with moderate-to-severe COPD as defined by the 2006 Global Initiative for Chronic Obstructive Lung Disease (GOLD) definition (Citation[20]). According to this definition, moderate patients were defined as having an FEV1 of ≥50 to < 80% predicted (stage II), ≥ 30 to < 50% predicted (stage III) and severe COPD (stage IV), < 30% predicted. After a 2-week run-in period, during which all patients receive salmeterol 50 μ g b.i.d., and prednisolone 30 mg/day, patients are randomized to receive SFC 50/500 μ g b.i.d. delivered via the Diskus/Accuhaler (GlaxoSmithKline R&D, Greenford, UK) inhalation device, or tiotropium bromide 18 μg/day delivered via the Handihaler inhalation device (Boehringer Ingelheim Pharma GmbH & Co. KG Ingelheim, Germany), for 104 weeks. Previous data has shown that this dose of 50/500 mcg of SFC is effective in reducing exacerbations (Citation[9]). Within the double-dummy study design (), patients in both groups also receive placebo medication via a matched-placebo inhalation device. The use of other COPD medication is not permitted, with the exception of mucolytics that have been started pre-study and albuterol relief medication, which can be used on an as-required basis throughout the study.
Patient participation
Patients are aged 40–80 years, with a current or former smoking history of ≥ 10 pack-years; a history of COPD exacerbations; a post-bronchodilatory forced expiratory volume in one second (FEV1) of < 50% of the predicted normal value; a post-bronchodilator FEV1/forced vital capacity (FVC) ratio of ≤ 70%; poor reversibility in FEV1 (defined as a change in FEV1 of ≤10% of the predicted normal FEV1 value 30 minutes after inhalation of 400 μg salbutamol); and a minimum score of ≥ 2 on the Modified Medical Research Council Dyspnoea Scale. Exclusion criteria for entry to the run-in period include experience of an exacerbation of COPD in the six weeks before the run-in period, a medical diagnosis of asthma or a respiratory disorder other than COPD, lung transplantation and/or lung volume reduction, and a requirement for regular oxygen therapy. Patients who experience an exacerbation of COPD during the run-in are suspended from the study. They are then allowed to repeat the run-in at least six weeks after finishing treatment for the exacerbation.
Randomization is remote and concealed from centers, is stratified according to smoking status, and uses a 1:1 allocation ratio. The primary population for analysis is the intent-to-treat (ITT) population, which will include all patients randomized to treatment who have received at least one dose of trial medication. A per-protocol (PP) population consisting of all patients in the ITT population not identified as protocol violators will be used for secondary analysis of the primary endpoint.
The study is being conducted in accordance with ICH-GCP and the 1996 version of the Declaration of Helsinki. All patients must provide a written informed consent form prior to participation. The ethics and review boards of all participating institutions have approved the protocol.
Efficacy and health-outcome assessments
Eleven clinic visits are scheduled during the double-blind treatment period (). The primary efficacy endpoint is the rate of moderate and/or severe COPD health care exacerbations. Two different definitions of an exacerbation will be used:
An event-based exacerbation is defined as one requiring healthcare utilization reported during the treatment period. Thus, a mild exacerbation was defined as an acute worsening of COPD symptoms that is self-managed by the subject and/or by increasing the use of relief albuterol. A moderate exacerbation is defined by the requirement for treatment with oral corticosteroids and/or antibiotics, while a severe exacerbation is defined by the requirement for admission to hospital. Information on exacerbations is collected during clinic visits, and any patient experiencing worsening COPD symptoms is instructed to contact the investigator immediately and report to the study clinic as soon as possible if symptoms deteriorate further.
A symptom-based exacerbation is defined as the worsening of 2 or more major symptoms (dyspnoea, sputum volume or sputum purulence) for at least 2 consecutive days, or any one major symptom together with any one minor symptom (sore throat, colds, fever without other cause, increased cough or increased wheeze). Daily diary record cards (DRCs) are filled in every morning by patients to provide information on these symptoms over the previous 24 hours. Details of the information recorded on the DRC are found in .
Table 1 Daily Record Card (DRC) COPD symptom scores
The investigator will assess the severity of COPD exacerbations by reviewing the DRC entries, as well as specific questioning on adverse events, physical examination and collection and examination of expectorated sputum as appropriate. Exacerbations requiring oral corticosteroids will be treated with a standardised course of prednisolone tablets, 30 mg/day, for 14 days or methylprednisolone tablets 24 mg/day for 14 days. Any course of oral corticosteroids started within one week of finishing a previous course will be considered to be treatment for that previous exacerbation.
If there is evidence of infection during an exacerbation as indicated by a change in sputum colour to purulent and/or an increase in sputum volume antibiotics a 7-day course of oral amoxycillin ≤1500 mg/day (or equivalent, expressed as penicillin dose), or clarithromycin ≤1000 mg/day if subjects are allergic to penicillin, and then reviewed. If, after 7 days, the sputum remains purulent as judged by its visual appearance, and antibiotic sensitivity testing does not show evidence of resistance, then the subject will be given a further 7 days' therapy before further review.
Secondary and other efficacy endpoints are listed in .
Table 2 A list of endpoints used in the INSPIRE study
Assessment schedule
Post-randomization, patients will be reviewed at weeks 2 and 8, and every 12 weeks thereafter to record details of COPD exacerbations, any unscheduled healthcare visits, and any adverse events. Post-dose FEV1 and other respiratory parameters will be measured at weeks 2 and 8, and every 24 weeks thereafter. To obtain information on health outcomes, the St George's Respiratory Health Questionnaire (Citation[21]) is administered at baseline and weeks 32, 56, 80 and 104. In addition, information on unscheduled healthcare contacts, unscheduled/emergency visits, hospitalizations and days lost from work or other activities are also recorded.
Exploratory parameters
At all visits post randomization, measurements of fat-free (lean) muscle mass are taken at selected centres to assess muscle wasting. At baseline and 104 weeks (or at withdrawal if earlier), levels of C-reactive protein (CRP) will be assessed to provide data on systemic inflammation.
Clinical safety assessments
Safety will be assessed at each clinic visit by documenting all adverse events (recorded by the investigator and assessed according to clinical judgment as mild, moderate or severe), laboratory screens, vital signs, ECGs, oropharyngeal examinations, and details of skin bruising and fractures.
Statistical analysis
The primary endpoint is the rate of healthcare utilization exacerbations. The expected rate of healthcare use exacerbations in the tiotropium bromide group was estimated to be 1.7 per subject per year. A priori, it was expected that a reduction in the number of exacerbations of at least 15% would be observed in the SFC group; this is equivalent to 1.445 exacerbations per subject per year. With a study duration of 2 years, a total of 508 subjects per treatment group was estimated as being required to detect this difference with 90% power, based on a 2-sided α level of 0.05. Allowing for a 20% withdrawal rate, a total of 1270 subjects (635 per treatment group) are to be recruited, with the aim of having complete 2-year data in 1016 subjects.
Rates of healthcare use exacerbations will be compared between the 2 groups using a negative binomial model, with covariates of baseline smoking status, disease severity (percentage of predicted FEV1 at baseline), body mass index (BMI), number of exacerbations reported in the 12 months prior to screening, age, sex and country. Additionally, the covariates of BMI and the number of exacerbations reported in the 12 months prior to screening will be included for the time to first healthcare utilisation exacerbation and the time to first symptom-defined exacerbation analyses. The negative binomial model allows for a different Poisson rate for each subject and assumes that these rates as a set are distributed across subjects according to a gamma distribution. Adjusted mean rates per year, treatment ratios with associated p-values and confidence intervals will be calculated. This analysis will also be preformed for the secondary endpoint of rate of symptom defined exacerbations.
Time to first healthcare utilization exacerbation and first symptom-defined exacerbation, and time to withdrawal, will be summarized by treatment group using Kaplan–Meier estimates. The hazard ratio for the treatment comparison will be derived using a Cox's proportional hazards model with covariates of baseline smoking status, disease severity (percentage of predicted FEV1 at baseline), age, sex and country. For time to withdrawal, there is potential for differential effects during the early phase of treatment and so treatments will also be compared using the Wilcoxon test.
Post-dose FEV1 will be compared between treatments at each visit using a mixed model repeated measures analysis and including covariates of baseline smoking status, baseline value, disease severity (percentage of predicted FEV1 at baseline), age, sex and country. A similar analysis will be used for the St George's Respiratory Questionnaire scores at each visit. Safety parameters will be assessed by treatment group. The number of deaths occurring will be summarized and compared between treatment groups using Fisher's exact test.
Organizational committees
This study is being guided by a steering committee consisting of external clinical experts and representatives from GlaxoSmithKline. An independent data monitoring committee has been established to ensure the safety of patients in the study. This is because of the high risk of morbidity and mortality associated with moderate-to-severe COPD and because of the extended duration of the protocol. The committee will independently monitor all deaths that occur during the course of the study.
DISCUSSION
Exacerbations of COPD are associated with significant sequelae, including hospitalization (Citation[22], Citation[23]), healthcare costs (Citation[24]), lost productivity (Citation[25]) and reduction in health status (Citation[26]). Respiratory symptoms of exacerbations may include increased shortness of breath, increased volume and purulence of sputum and increased cough (Citation[27]). Exacerbations may also have systemic effects including increased body temperature, increased pulse/heart rate, impaired mental status (Citation[27], Citation[28]), and risk of myocardial infarction (Citation[29]).
Research suggests that treatments that reduce the frequency of exacerbations of COPD could have a significant impact on symptoms, health status (Citation[30]), mortality (Citation[31]), and the economic consequences of the disease (Citation[32]). Hence, reducing the number of exacerbations is key to effective COPD management, as proposed by GOLD (Citation[2]). Current medical interventions used alone appear to have only a modest effect on the intensity, duration, and mortality of COPD exacerbations. Since COPD exacerbations are caused by different mechanisms, it is a challenge for any one therapy class to address them adequately. Therefore, new approaches for preventing and treating exacerbations are needed to improve survival and increase quality of life for COPD patients and to reduce the overall cost of their care and changing long-term outcomes.
A number of trials have successfully employed event-based definitions of exacerbations of COPD (Citation[7], Citation[8], Citation[9], Citation[10], Citation[11], Citation[12], Citation[13]) using objective, easily measurable parameters such as number of hospitalizations or increased need for medication. Yet there are a number of confounders that may affect event-based assessment. For example, comorbidities or the lack of social support may contribute to increased levels of hospital admission. Furthermore, studies have shown that as many as 50% of exacerbations may not be reported by patients, since they are often accustomed to frequent symptom changes and may not regard these changes as exacerbations (Citation[33]). It is clear, therefore, that assessment of health utilization may not capture all exacerbations.
Exacerbations may be assessed alternatively by symptom-based criteria, such as those proposed by Anthonisen in 1987, which list three major symptoms by which exacerbations can be defined: worsening dyspnoea, increased sputum volume and increased sputum purulence (Citation[14]). In addition, less severe exacerbations may be defined by a combination of one major symptom with one minor symptom, such as sore throat, colds, fever, increased cough or increased wheeze (Citation[14]). More recently, an alternative symptom-based definition, the Aspen Consensus, has been proposed. This defines exacerbations as a sustained worsening of the patient's condition, from the stable state and beyond normal day-to-day variation, that is acute in onset and necessitates a change in regular medication in a patient with underlying COPD (Citation[27]).
There is evidence that the recording of symptoms by patients in DRCs may identify the substantial proportion of exacerbations that go unreported to physicians (Citation[33]). This evidence has shown that unreported exacerbations are frequently of similar severity to those that are reported, although changes in minor symptoms such as cough are seen more frequently in reported exacerbations (Citation[33]). Thus, the inclusion of symptom-based criteria within a clinical trial assessment may allow the occurrence of exacerbations and the efficacy of medication to be captured more completely than with an events-based assessment alone. By way of example, in the East London COPD cohort study, DRCs have been used effectively to allow patients to note the appearance or increase in intensity of major and minor symptoms, with the diagnosis of an exacerbation confirmed by observation (Citation[33], Citation[34]). However, symptom-based definitions used alone may be limited by the fact that they rely on daily patient compliance with a DRC system.
The relationship between exacerbations defined symptomatically as occurs when a patient visits a doctor and those defined by the resulting change in health care, i.e., prescription of antibiotics and/or corticosteroids, is not as simple as initially assumed. Many patients report increased symptoms but take no action. Furthermore, using DRCs adapted from the asthma literature led to a significant disagreement in the timing of events in individuals, but not in the whole study population (Citation[35]). A feature of the INSPIRE study is that it uses the carefully validated criteria developed in the East London observational cohort to a clinical trial setting and will provide a direct comparison of exacerbations defined in different ways. It is likely that more events will be identified using the DRC approach. Using already established data card analyses, it will be possible to determine whether certain types of events are influenced more by anti-inflammatory or bronchodilator therapy. This should offer insight into the mechanisms driving exacerbations and the perception of exacerbations in a community setting.
Sin and colleagues have demonstrated that levels of CRP, a marker of systemic inflammation, are reduced by inhaled steroid treatment over 10 weeks (Citation[36]). Systemic inflammation, along with increased rates of exacerbation, has been associated with a faster decline in lung function and increased COPD severity (Citation[37], Citation[38], Citation[39]), and CRP has also been related to fat mass index (Citation[40]). However, it is not known whether tiotropium bromide has any effects on systemic inflammation or fat mass. Therefore, the INSPIRE study will extend the work of Sin et al. by investigating the potential links between systemic inflammation and body mass, examining both fat-free mass (lean muscle mass) and CRP levels during the study and assessing the relationship between these parameters over 2 years.
A further distinguishing feature of the INSPIRE study is that patients are to receive salmeterol in combination with prednisolone during the 2-week run-in period. Research suggests that this stabilization approach may have benefits for trials compared with withdrawing maintenance therapy during run-in. In particular, it may improve detection of clinically important changes in health status, and reduce the proportion of withdrawals during run-in (Citation[41], Citation[42], Citation[43]).
Both SFC and tiotropium bromide have been shown to reduce the frequency of exacerbations in COPD (Citation[15], Citation[16], Citation[17]). However, no study has yet provided a direct comparison between SFC and tiotropium bromide in patients with COPD, although separate placebo controlled studies suggest that SFC is more effective in reducing exacerbations than tiotropium bromide (Citation[9], Citation[15], Citation[44]). Since these 2 treatments are often used in similar patients, and because exacerbations have such a profound effect on patients well-being and quality of life, a direct comparison is particularly apposite. A 52-week study, the Canadian Optimal Therapy of COPD Trial is currently ongoing (Citation[12]). However, a 1-year study may not be sufficiently long to assess adequately the long-term effects of SFC or tiotropium on the timing and frequency of exacerbations. For these reasons, the INSPIRE study has been designed with a duration of 2 years, which will ensure adequate assessment of these parameters, as well as long-term effects on the rate of exacerbations.
In summary, the INSPIRE study is the first large-scale clinical trial to combine the 2 most common methods for the assessment of exacerbations, using event-based definitions and symptom-based definitions. If this combination of different methodologies proves successful, it will become the benchmark methodology for future research in exacerbations of COPD.
This study was funded by GlaxoSmithKline Ltd. (study number: SCO40036)
REFERENCES
- Agusti A G, Noguera A, Sauleda J, Sala E, Pons J, Busquets X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J 2003; 21(2)347–360
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease—updated 2005. Modena: Global Initiative for Chronic Obstructive Lung Disease. 2005
- Agusti A G. COPD, a multicomponent disease: implications for management. Respir Med 2005; 99(6)670–682
- Gompertz S, Bayley D L, Hill S L, Stockley R A. Relationship between airway inflammation and the frequency of exacerbations in patients with smoking related COPD. Thorax 2001; 56(1)36–41
- Seemungal T, Harper-Owen R, Bhowmik A, Moric I, Sanderson G, Message S, Maccallum P, Meade T W, Jeffries D J, Johnston S L, Wedzicha J A. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164(9)1618–1623
- Hurst J R, Perera W R, Wilkinson T M, Donaldson G C, Wedzicha J A. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173(1)71–78
- Burge P S, Calverley P M, Jones P W, Spencer S, Anderson J A, Maslen T K. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. Br J Med 2000; 320(7245)1297–1303
- Pauwels R A, Lofdahl C G, Laitinen L A, Schouten J P, Postma D S, Pride N B, Ohlsson S V. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N J Engl Med 1999; 340(25)1948–1953
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, Anderson J, Maden C. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003; 361(9356)449–456
- Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, Peterson S, Olsson H. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J 2003; 21(1)74–81
- Vestbo J. The TORCH (TOwards a Revolution in COPD Health) survival study protocol. Eur Respir J 2004; 24(2)206–210
- Aaron S D, Vandemheen K, Fergusson D, Fitzgerald M, Maltais F, Bourbeau J, Goldstein R, McIvor A, Balter M, O'Donnell D. The Canadian Optimal Therapy of COPD Trial: design, organization and patient recruitment. Can J Respir 2004; 11(8)581–585
- Decramer M, Celli B, Tashkin D P, Pauwels R A, Burkhart D, Cassino C, Kesten S. Clinical trial design considerations in assessing long-term functional impacts of tiotropium in COPD: The Uplift trial. J Chron Obstruct Pulmon Dis 2004; 1(2)303–312
- Anthonisen N R, Manfreda J, Warren C P, Hershfield E S, Harding G K, Nelson N A. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106(2)196–204
- Vincken W, van Noord J A, Greefhorst A P, Bantje T A, Kesten S, Korducki L, Cornelissen P J. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J 2002; 19(2)209–216
- Niewoehner D E, Rice K, Cote C, Paulson D, Cooper J A, Jr., Korducki L, Cassino C, Kesten S. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomised trial. Ann Intern Med 2005; 143(5)317–326
- Mahler D A, Donohue J F, Barbee R A, Goldman M D, Gross N J, Wisniewski M E, Yancey S W, Zakes B A, Rickard K A, Anderson W H. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest 1999; 115(4)957–965
- Calverley P M, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J 2003; 22(6)912–919
- Kardos P, Wencker M, Glaab T, Vogelmeier C. Combination therapy with salmeterol and fluticasone propionate (SFC) is more effective than salmeterol (SAL) alone in reducing exacerbations of COPD. Eur Respir J 2005; 26: 292S, (Suppl 49)
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. 2006, Executive Summary. NHLBI/WHO Workshop Report
- Jones P W, Quirk F H, Baveystock C M. The St George's Respiratory Questionnaire. Respir Med 1991; 85: 25–31, (Suppl B)
- Andersson F, Borg S, Jansson S A, Jonsson A C, Ericsson A, Prutz C, Ronmark E, Lundback B. The costs of exacerbations in chronic obstructive pulmonary disease (COPD). Respir Med 2002; 96(9)700–708
- Soler-Cataluna J J, Martinez-Garcia M A, Roman S P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60(11)925–931
- Price M J, Hurrell C, Efthimiou J, Medley H V. Health care costs of treating exacerbations of chronic obstructive pulmonary disease (COPD). Eur Respir J 2005; 14: 30S, (Suppl)
- European Respiratory Society & European Lung Foundation. European lung white book. The first comprehensive survey on respiratory health in Europe. 2003
- Spencer S, Calverley P M, Sherwood B P, Jones P W. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163(1)122–128
- Rodriguez-Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000; 117: 398S–401S, (5 Suppl 2)
- Pauwels R, Calverley P, Buist A S, Rennard S, Fukuchi Y, Stahl E, Lofdahl C G. COPD exacerbations: the importance of a standard definition. Respir Med 2004; 98(2)99–107
- Wedzicha J A, Seemungal T A, MacCallum P K, Paul E A, Donaldson G C, Bhowmik A, Jeffries D J, Meade T W. Acute exacerbations of chronic obstructive pulmonary disease are accompanied by elevations of plasma fibrinogen and serum IL-6 levels. Thromb Haemost 2000; 84(2)210–215
- Spencer S, Jones P W. Time course of recovery of health status following an infective exacerbation of chronic bronchitis. Thorax 2003; 58(7)589–593
- Sin D D, Wu L, Anderson J A, Anthonisen N R, Buist A S, Burge P S, Calverley P M, Connett J E, Lindmark B, Pauwels R A, Postma D S, Soriano J B, Szafranski W, Vestbo J. Inhaled corticosteroids and mortality in chronic obstructive pulmonary disease. Thorax 2005; 60(12)992–997
- Wouters E F. Economic analysis of the Confronting COPD survey: an overview of results. Respir Med 2003; 97: S3–14, (Suppl C)
- Seemungal T A, Donaldson G C, Paul E A, Bestall J C, Jeffries D J, Wedzicha J A. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157: 1418–1422, (5 Pt 1)
- Seemungal T A, Donaldson G C, Bhowmik A, Jeffries D J, Wedzicha J A. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161(5)1608–1613
- Calverley P, Pauwels D R, Lofdahl C G, Svensson K, Higenbottam T, Carlsson L G, Stahl E. Relationship between respiratory symptoms and medical treatment in exacerbations of COPD. Eur Respir J 2005; 26(3)406–413
- Sin D D, Lacy P, York E, Man S F. Effects of fluticasone on systemic markers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 170(7)760–765
- de Torres J P, Cordoba-Lanus E, Lopez-Aguilar C, de Fuentes M M, de Garcini A M, Aguirre-Jaime A, Celli B R, Casanova C. C-reactive protein levels and clinically important predictive outcomes in stable COPD patients. Eur Respir J 2006, Feb 2: [Epub ahead of print]
- Donaldson G C, Seemungal T A, Patel I S, Bhowmik A, Wilkinson T M, Hurst J R, MacCallum P K, Wedzicha J A. Airway and systemic inflammation and decline in lung function in patients with COPD. Chest 2005; 128(4)1995–2004
- Kanner R E, Anthonisen N R, Connett J E. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med 2001; 164(3)358–364
- Broekhuizen R, Wouters E F, Creutzberg E C, Schols A M. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax 2006; 61(1)17–22
- Jones P W, Stahl E. Does short-term treatment intensification influence long-term treatment effects on health status in COPD?. Eur Respir J 2005; 26: 293S, (Suppl 49)
- Jarad N A, Wedzicha J A, Burge P S, Calverley P M. An observational study of inhaled corticosteroid withdrawal in stable chronic obstructive pulmonary disease. ISOLDE Study Group. Respir Med 1999; 93(3)161–166
- Stockley R A, Chopra N, Rice L. Addition of salmeterol to existing treatment in patients with COPD: a 12 month study. Thorax 2006; 61(2)122–128
- Casaburi R, Mahler D A, Jones P W, Wanner A, San P G, Wallack Zu RL, Menjoge S S, Serby C W, Witek T, Jr. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002; 19(2)217–224