Abstract
Limitation of activity and impaired quality of life are important outcomes of COPD. There is an association between measures of self-reported physical activity and overall health status, and they appear to change together spontaneously over time and in response to treatment. The relationship between symptoms and activity limitation is complex, because activity can be limited entirely by symptoms, or impaired by symptoms so that it requires greater effort or causes discomfort. The patient has the choice of whether to restrict their activity or maintain it at the cost of having symptoms. In theory, this may make it difficult to produce reliable standardized assessments of activity limitation because it may not be clear exactly what is being measured. Analysis of items in the St George's Respiratory Questionnaire (SGRQ) concerned with activities that are either not possible due to breathlessness, or are a cause of breathlessness, show that they contribute to a unidimensional model of activity limitation in daily life and a unidimensional model of overall COPD-related health status. The items lie distributed evenly along the same severity continuum, from very mild to very severe, along with other items concerned with symptoms and the psycho-social impact of the disease. This suggests that self-reported limitation of activity may form a reliable construct, and may also provide a good surrogate marker of health status in COPD.
Keywords::
INTRODUCTON
Markers of clinical outcome
Restriction to daily activity and impaired quality of life are important outcomes of COPD (Citation[1]), but the impact of poor health on the quality of life of an individual will be determined by factors that are unique to him or her. These will include their personality and their environment—physical, financial, social and emotional. For example the degree to which a person's quality of life is impaired by an inability to walk faster than 2 miles per hour (3 km/hr) for a sustained period will be determined by their social and recreational need for walking faster, and their wish to do so. In a population of patients with COPD, this degree of physical limitation will, on average, be associated with moderate impairment of their health-related quality of life, equivalent to a SGRQ score of ≈ 50 units (calculated from data reported in (Citation[2])), but it will have a much greater effect on some than on others.
Scientific study usually requires standardized measurement, so the impact of disease on a patient's self-reported daily physical activity must be assessed using methods that assess each patient in the same way. This means that each patient is asked the same standard questions–as if he or she were a standard or typical patient.
Questionnaires are population derived, being developed and validated in groups of patients. For this reason, their scores should be viewed as standardized markers of clinical outcomes, rather than direct measurements of the outcome of interest in each patient (Citation[1]). For example, health status questionnaire scores provide a marker of a patient's health-related quality of life, and activity or functional performance questionnaires provide a marker of the level of activity limitation experienced by the patient as he or she goes about daily life.
Activity limitation in COPD
COPD is a disease of airways obstruction diagnosed using the FEV1, but its characteristic symptoms are breathlessness during activity and sputum production. Other than through screening measurements of FEV1 in adults who smoke, the diagnosis is usually made following an exacerbation or the progression of breathlessness during routine daily activity to the point at which the patient seeks medical attention. Interestingly, patients usually report their symptom of breathlessness, rather than the activity limitation it causes, even though many have quite marked impairment at first presentation.
An international telephone survey of COPD patients reported that, on average, they had a mean MRC (Medical Research Council) dyspnoea grade of 3 (“I walk slower than people of the same age on the level”) in males, and 4 (“I stop for breath after walking about 100 yards or after one minute in the level”) in females (Citation[3]). The significance of activity restriction in COPD is also apparent from a recent report that approximately 30% of moderate-severe patients were effectively housebound due to their disease when stable, rising to nearly half during an exacerbation (Citation[4]).
Measurement of activity limitation in daily life
In theory, activity restriction in daily life can be measured through direct observation by a trained observer, quantification of movement through motion detection devices, and patient self-report. Movement detection techniques are becoming more reliable and can quantify and classify patterns of daily movement, but provide no context for the movements that are taking place. Quantification through direct observation is possible for activities that are confined to the home, but this is very time consuming, requires good inter-rater reliability and is limited to basic daily functions. The majority of stable COPD patients are active outside their home, so for most purposes it is only practicable to measure the full range of restriction of daily activities through questionnaires that rely on patient self-report, rather than by direct observation.
At this point, it may be worth classifying activity limitation in COPD into two types:
Involuntary limitation—an activity that can't be done at all because of breathlessness or fatigue
Voluntary limitation—an activity that causes symptoms, but the patient can choose to avoid or minimize their symptoms by doing the activity more slowly or not at all.
The reason for suggesting this is that there are 3 principal patterns in the relationship between symptoms and activity limitation in COPD:
Activities not possible due to symptoms
Activities that are possible but at a cost of greater symptoms or effort
Activities carried out more slowly to avoid or minimise symptoms
The last two of these can be thought of as being two solutions to an accounting rule that relates the price of activity limitation to the cost of symptoms. In one, symptoms are minimized at the cost of limiting activity. In the other, activity limitation is minimized, but at the cost of greater symptoms. In practice, it is likely that most patients do not use one solution for all types of activity and may not use the same solution for the same activity all of the time. For example, the need to catch a bus that is about to depart may cause a switch from a symptom-minimization solution to a ‘maximize activity’ solution. For this reason it may be very difficult to design a measure of self-reported activity that captures all of the nuances of the relationship between activity and symptoms.
The design and content of some of the questionnaires that attempt to measure activity limitation reflect the complex interaction between activity and breathlessness. For example the MRC Dyspnea Scale contains an item that addresses two different aspects of activity and breathlessness in the same severity grade: “I walk slower than people of the same age on the level because of breathlessness, or I have to stop for breath when walking at my own pace on the level.” The Dyspnea component of the CRQ is clearer in this respect and asks about activities that cause breathlessness (Citation[5]). The Physical Function domain of the generic questionnaire, the SF-36, asks directly about the degree of limitation of specific daily activities, but without attribution (Citation[6]). A Physical Component Summary (PCS) score can also be calculated from the SF-36 by aggregating responses to a number of items that are related in some way or other to physical activity, not just specific activities.
Another widely used pair of measures of breathlessness in daily life, the Baseline and Transition Dyspnea Indices, address activity limitation due to breathlessness differently from the other measures by asking separately about functional impairment, the magnitude of the task and the magnitude of effort (Citation[7]). Responses to these assessments are then combined into a composite score. A somewhat similar approach is used in the Activity component of the SGRQ which produces a score for disturbance of activity, calculated from responses to items that ask about activities that are affected by dyspnea and another set of items that enquire about activities that cause breathlessness (Citation[2]).
Relationship between activity limitation and health status
The most widely used measurement of activity limitation in COPD is the MRC Dyspnea Scale. It correlates well with exercise tolerance and health status (Citation[8]). The relationship between MRC grade and disease specific health status measured using the St. George's Respiratory Questionnaire (SGRQ) and functional performance measured with a generic instrument, the Sickness Impact Profile (SIP) is illustrated in . Correlations between MRC grade and the total and physical activity components of these two questionnaires are very similar, and similar to the correlation with the 6-minute walking distance ().
Figure 1 Pearson correlations between MRC Dyspnoea grade and 6-minute walking distance (6MWD), and Sickness Impact Profile (SIP) and St George's Respiratory Questionnaire (SGRQ) scores. Data pooled from Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure for chronic airflow limitation–the St George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145:1321–1327.
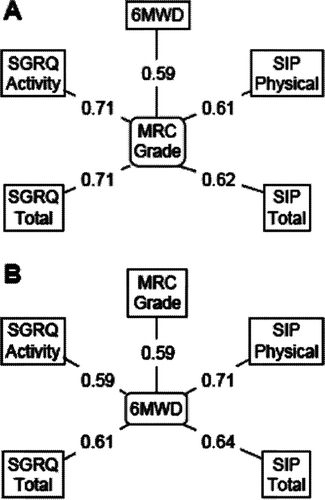
In both of these questionnaires, the items that make up the physical activity component also contribute to the total score, 30% in the case of the SGRQ and 36% with the SIP. For this reason, there will be an automatic correlation between physical activity and total scores of both instruments, but the strength of the measured correlation was surprisingly high: SIP r = 0.87, SGRQ r = 0.89 (Citation[2]). Expressed another way, 75–80% of variance in the total scores was attributable to variance in the physical activity scores. This observation led to a deeper exploration of the relationship between the Activity component of the SGRQ and its Total score.
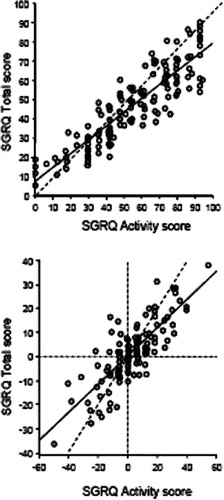
When plotted, the good correlation is apparent (), but at higher scores the distribution around the regression line gets a little greater and the Activity score becomes proportionally a little more severe than the Total score. Conversely at the mildest end, at the intercept where the regression line crosses the point at which the Activity score is zero, the Total score is 7 units. This intercept value for the Total score is significantly greater than zero (p < 0.0001). Changes in SGRQ Activity and Total scores over 1 year in a large subset of the same patients are shown in . Again, the correlation is high. The change in Activity score is greater than the change in Total score, but the slope of the regression passes through the intercept—so there is no step difference in the change in scores over time. These two observations suggest that the Activity component of the SGRQ is a good surrogate marker of the Total score for the patients in middle of the severity range, but less good at the extremes.
Figure 2 Pearson correlations between SGRQ Activity and Total scores at baseline (upper panel), r = 0.89 and within-patient change in score over 1 year (r = 0.80). The intercept on the y axis is 7 units, p < 0.0001. The solid line is the regression slope and the dotted line is the line of identity. The intercept value was -0.4 units and not significantly different from zero. Data pooled from Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure for chronic airflow limitation–the St George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145:1321–1327.
The Impacts component of the SGRQ, which addresses the psycho-social impact of COPD, shares no items with Activity component, so auto-correlation is not a problem. The cross-sectional correlation between these components was r = 0.75, and the longitudinal within-patient correlation was r = 0.53. These analyses all provide further support for the hypothesis that the ability to carry out daily activity is an important determinant of health-related quality of life.
Changes in activity and health status
Pulmonary rehabilitation improves activity and the physical components of health status, whether measured using the dyspnea score of the CRQ, Activity score of the SGRQ or Physical Function score from the SF-36 (Citation[9]). It is usually not possible to make a direct comparison of the size of change in score between questionnaires, or even domains within the same questionnaire, because the scales may not be the same. Standardization comparisons can be made by calculating the effect size, which is the change in score divided by standard deviation of the differences between patients at baseline. It is possible to calculate effect sizes for a number of measures using published data from a large pulmonary rehabilitation study (Citation[9]). After 1 year these were: SGRQ Activity 0.33; SGRQ Total 0.27; CRQ Dyspnea 0.53; SF-36 Physical Function 0.35, SF-36 PCS score 0.12. For comparison, the effect size for the 6-minute walk was 0.09.
Much of rehabilitation is directed at the muscles through physical training, so the greater sensitivity of the physical activity scores in this study is not surprising. A meta-analysis of the change in CRQ score with pulmonary rehabilitation (Citation[10]) provides further insight into the responsiveness of activity questionnaire items versus other components of health-related quality of life. The mean improvement in the dyspnea component per item (expressed as the difference between rehabilitation and control arms in the change from baseline) was 1.0 (95% Confidence Interval (CI) 0.8–1.2) CRQ units. For the other components the changes were: Fatigue 0.9 (95% CI 0.7–1.0) units, Emotional Function 0.7 (95% CI 0.4–1.0) units, and Mastery 0.9 (95% CI 0.7–1.2) units. Unfortunately, no total score was computed for the CRQ, but this analysis shows that the size of change was similar across its different domains, and the confidence intervals overlapped widely.
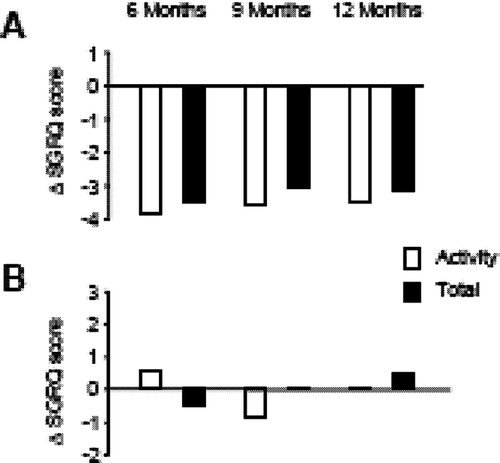
There are few data in the public domain that permit an analysis of the relative sensitivity of questionnaires to pharmacological therapy directed to the lungs. In one study, the long-acting bronchodilator tiotropium produced improvement in SGRQ Activity and Total scores 6–12 months after the start of therapy, but patients in the placebo arm did not improve (Citation[11]). The changes are shown in , and show that the Activity component is clearly responsive. Unfortunately the standard deviation for the scores at baseline are not available, so it is not possible to calculate an effect size; however coupling this analysis with that presented in suggests that the relative magnitude of change in Activity score was similar to that in the Total score.
Figure 3 Change in SGRQ Activity and Total scores from baseline in patients treated with tiotropium (A) or placebo (B). The differences in scores between tiotropium and placebo were all significant at p < 0.05. Modified from Casaburi R, Mahler DA, Jones PW, Wanner A, San Pedro G, ZuWallack RL, et al. A long-term evaluation of once daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002; 19:217–224. Used with permission.
In another study, a significant reduction in the rate of deterioration in the health status of COPD patients over 3 years was seen with fluticasone compared with placebo in the Activity component of the SGRQ and the Physical Function score of the SF-36, as well as the Total SGRQ score (Citation[12]). To obtain an estimate of the relative sensitivity of these measures to the effects of disease progression, the annual rate of deterioration was divided by the standard deviation of the baseline scores, in a manner analogous to calculating the effect size. For the SGRQ this ratio was: Total score 0.17, Activity score 0.19; SF-36: Physical Function score 0.12, Physical Component Summary score 0.13.
In summary, published data show that measures of self-reported physical activity and overall disease-specific health status appear to have similar responsiveness to changes over time and with therapy.
A proposed methodology for developing activity scales
The preceding sections suggested a strong link between physical activity and overall health impairment. It produced an argument for measurement of daily activity as a core marker of disease severity in COPD. The following section uses the activity items in the SGRQ as the starting point for an exploration of the potential to develop a reliable self-reported measure of activity limitation in daily life.
The SGRQ was developed using classical psychometric techniques, but over recent years probabilistic approaches such as item-response techniques, first used in education, have been applied to clinical questionnaires. The method used most widely is the one-parameter model advocated by Rasch (Citation[13]). This starts from the assumption that the construct under test is unidimensional—i.e., all the items contained in the questionnaire reflect different aspects of the same unifying concept. This does not exclude the possibility that the construct may be made up of different domains—for example, health may have mental and physical domains, but the items can still behave as if they are just different reflections of the same underlying overall construct.
Rasch methodology tests each item in a questionnaire to establish whether it conforms to a unidimensional model when combined with all the other constituent items (Citation[13]). A questionnaire that meets Rasch model requirements will have true interval scaling properties–just like a ruler. This has important implications since it means that severity scaling remains constant over the questionnaire's measurement range. Any given distance between two points will indicate the same difference in severity, regardless of where this occurs along the scale. This is a valuable property for a number of reasons, not least because the minimum clinically important difference will be valid across the scaling range.
Rasch methodology contains several powerful tools that can be used in the development and testing of questionnaires, and recently we have analysed the SGRQ using this approach (Citation[14]). When all 50 items were combined, the Person Separation Index (PSI), which is the Rasch equivalent of Cronbach's alpha and a measure of internal reliability, was 0.93 (judged to be “excellent”). The Activity component was not quite so reliable with a PSI of 0.84, but this is still good. This is an important observation, since this component of the SGRQ is made up two groups of items that address very similar disturbances to mobility and daily activity, but from two different perspectives either: “Activities that make you breathless” or “Activities that might be affected by your breathing.”
Principal Components Analysis (PCA) carried out when the questionnaire was first developed showed that the activity-related items all grouped together as a single factor–which is why this component was created. The later Rasch analysis confirmed this structure, but it also suggested that items addressing activities limited by dyspnea or causing dyspnea can form a unidimensional construct. Rasch methodology, combined with classical techniques such as PCA, provide the methodologies by which to explore the concepts underlying activity limitation in COPD; furthermore, their application can permit the development of an activity scale with true interval scaling properties.
Rasch analysis of the SGRQ suggested that activity-related items can form a definable unidimensional domain within COPD-related health status (as defined by the SGRQ), but it also showed that they provide a reliable reflection of the overall severity of health impairment–as evidenced by the fact that the individual activity items fitted quite well in a model containing all 50 SGRQ items. This methodology also provided another useful insight, because it can place all the questionnaire items on the same severity scale, regardless of the domain to which they belong. This scale is usually called an item map, and the item map for the SGRQ is shown in . The unit of the scale is the logit, which is the log odds of a patient having a 50:50 chance of answering affirmatively to that item. By convention, the item scale is centered on zero, a negative logit indicating milder disease, a positive logit more severe disease.
Figure 4 Rasch item map for the SGRQ. The units are logits (see text). The location along the map shows the level of severity of COPD indicated by each item. The items are divided into their composite SGRQ domains, but their location on this map is drawn from a model that incorporated all 50 items together in a single model.
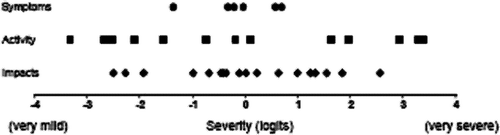
shows that the severity location of the activity items are distributed evenly across the range, furthermore they cover the entire range from mildest to most severe. This explains why the Activity and Total scores were found to be so closely correlated in the earlier analyses.
There is an important inference to be drawn from this observation. The item map in shows that the three domains of the SGRQ are made up of items that can be combined to form a single continuous severity scale. In fact, a questionnaire for overall COPD health status could be made up of a selection from the 50 SGRQ items, regardless of their domain, as long as each of them reflected the underlying unidimensional construct. Indeed it could be made up of just the activity items. This has considerable significance since it leads to the conclusion that a properly developed activity scale could provide a reliable surrogate marker of the severity of overall health status impairment, not just activity limitation.
SUMMARY
This review started by examining the relationship between activity and quality of life. It concludes by suggesting that activity limitation may be a central determinant of impaired quality of life due to poor health, and indeed may act as a reliable proxy marker of poor health. Health status questionnaires are standardized markers of impaired health-related quality of life. Any activity questionnaire that is developed will similarly be a marker of the true activity limitation experienced by each patient in his or her daily life. It will assess each patient in terms of the common activity-related problems that COPD patients experience in their daily lives, so it will not provide a precise estimate in individuals, but would provide a reliable standardized measurement in groups of patients. Use of modern methodology will allow many of the important measurement issues concerning the relationship between activity and breathlessness to be resolved and it will be possible to develop a self-reported instrument with fundamental reliable measurement properties.
REFERENCES
- Jones P W, Augusti A A. Outcomes and markers in the assessment of COPD. Eur Respir J 2006; 27: 822–832
- Jones P W, Quirk F H, Baveystock C M, Littlejohns P. A self-complete measure for chronic airflow limitation–the St. George's Respiratory Questionnaire. Am Rev Respir Dis 1992; 145: 1321–1327
- Rennard S, De Cramer M, Calverley P MA, Pride N B, Soriano J B, Vermeire P A, Vestbo J. Impact of COPD in North America and Europe in 2000: subjects' perspective of Confronting COPD International Survey. Eur Respir J 2002; 20: 799–805
- Donaldson G C, Wilkinson T MA, Hurst J R, Perera W R, Wedzicha J A. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Resp Crit Care Med 2005; 171: 446–452
- Guyatt G H, Berman L B, Townsend M, Pugsley S O, Chambers L W. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987; 42: 773–778
- Ware J E, Gandeck B. Overview of the SF-36 health survey and the International Quality of Life Assessment (IQOLA) Project. J Clin Epidemiol 1998; 51: 903–912
- Mahler D A, Weinberg D H, Wells C K, Feinstein A R. Measurements of dyspnea. Contents, interobserver correlates of two new clinical indices. Chest 1984; 85: 751–758
- Bestall J C, Paul E A, Garrod R, Garnham R, Jones P W, Wedzicha J A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54(7)581–586
- Griffiths T L, Burr M L, Campbell I A, Lewis-Jenkins V, Mullins J, Shiels K, Turner-Lawlor P J, Payne N, Newcombe R G, Ionescu A A, Thomas J, Tunbridge J. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet 2000; 355(9201)362–368
- Lacasse Y, Brosseau L, Milne S, Martin S, Wong E, Guyatt G H. Pulmonary Rehabilitation for Chronic Obstructive Pulmonary Disease (Cochrane Review). John Wiley & Sons, Ltd, ChichesterUK 2003
- Casaburi R, Mahler D A, Jones P W, Wanner A, San Pedro G, ZuWallack R L, Menjoge S S, Serby C W, Witek T, Jr. A long-term evaluation of once daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J 2002; 19: 217–224
- Spencer S, Calverley P MA, Burge P S, Jones P W. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163: 122–128
- Andrich D. Rasch Models for Measurement. Sage Publications, London 1988
- Meguro M, Barley E A, Spencer S, Jones P W. Development and validation of an improved COPD-specific version of the St. George's Respiratory Questionnaire. Chest 2006, (Accepted for Publication)