Abstract
Patients with chronic obstructive pulmonary disease (COPD) are often caught in a downward spiral that progresses from expiratory flow limitation to poor quality of life and invalidity. Within this downward spiral, exercise tolerance represents a key intermediate outcome. As recently stated by the GOLD initiative, improvement in exercise tolerance is now rec ognized as an important goal of COPD treatment. This objective will be achieved only by a comprehensive understanding of the mechanism of exercise limitation in this disease. The objective of this paper is to review the mechanisms of exercise limitation in COPD and discuss their relative contribution to exercise intolerance in patients suffering from this disease.
Keywords::
INTRODUCTION
Exercise intolerance is a troublesome manifestation of chronic obstructive pulmonary disease (COPD). It is closely linked to impairment/disability and is a stronger predictor of poor quality of life and survival than either spirometry or oxygenation (Citation[1], Citation[2], Citation[3]). The causes and mechanisms of exercise limitation are complex and involve central and peripheral factors. In healthy untrained individuals, exercise capacity is mainly limited by maximal oxygen consumption (![]() O2max)–more specifically by its cardiac output component-and by symptoms of dyspnea, general fatigue and/or leg fatigue. In patients with COPD, exercise intolerance results from a complex interaction between symptoms, ventilatory and respiratory mechanics impairment, gas exchange limitations, and peripheral muscle fatigue. The relative contribution of these exercise-limiting factors varies considerably from patient to patient and seems to differ between different exercise modalities (Citation[4], Citation[5]). The objective of this paper is to review the mechanisms of exercise limitation in COPD and discuss their relative contribution to exercise intolerance in patients suffering from this disease.
O2max)–more specifically by its cardiac output component-and by symptoms of dyspnea, general fatigue and/or leg fatigue. In patients with COPD, exercise intolerance results from a complex interaction between symptoms, ventilatory and respiratory mechanics impairment, gas exchange limitations, and peripheral muscle fatigue. The relative contribution of these exercise-limiting factors varies considerably from patient to patient and seems to differ between different exercise modalities (Citation[4], Citation[5]). The objective of this paper is to review the mechanisms of exercise limitation in COPD and discuss their relative contribution to exercise intolerance in patients suffering from this disease.
PHYSIOLOGICAL DETERMINANTS OF EXERCISE LIMITATION
Exercise capacity is often discussed in terms of maximal ![]() O2 and its determinants, cardiac output and oxygen extraction, as defined by the Fick equation. In elite athletes, a plateau in
O2 and its determinants, cardiac output and oxygen extraction, as defined by the Fick equation. In elite athletes, a plateau in ![]() O2 despite increasing workrate can be observed toward the end of exercise ascertaining that the physiological limits of the system have been reached. The situation is different in patients with COPD in whom exercise termination usually occurs prior to the occurrence of physiological
O2 despite increasing workrate can be observed toward the end of exercise ascertaining that the physiological limits of the system have been reached. The situation is different in patients with COPD in whom exercise termination usually occurs prior to the occurrence of physiological ![]() O2 limitation, and therefore does not reflect the capacity of the cardiovascular and muscular systems to transport and extract oxygen. In fact, greater cardiac output and oxygen extraction can be observed during exercise in patients with COPD when unloading the respiratory systems with heliox (Citation[6]) or oxygen (Citation[7]).
O2 limitation, and therefore does not reflect the capacity of the cardiovascular and muscular systems to transport and extract oxygen. In fact, greater cardiac output and oxygen extraction can be observed during exercise in patients with COPD when unloading the respiratory systems with heliox (Citation[6]) or oxygen (Citation[7]).
It is thus clear that at end-exercise, a physiological reserve is still present in the cardiovascular and muscular systems. Should this observation be interpreted as indicating that patients with COPD do not perform maximal effort when asked to exercise to their peak capacity? Patients with COPD are in fact prematurely limited during exercise as a result of the uncomfortable sensation of exercise. However, peak symptom intensity during exercise testing is comparable between patients with COPD and healthy subjects (Citation[8]), providing an indication that the presence of lung disease does not prevent an individual from performing to his/her peak capacity. It is thus useful to focus on the physiopathology of symptom generation to understand the factors involved in exercise limitation in this population (Citation[9]).
SYMPTOM PERCEPTION DURING EXERCISE
From the patients' perspective, poor functional status can be explained by the discomfort experienced while performing various activities of daily living. Exercise testing in the physiology laboratory offers a unique opportunity to assess the uncomfortable sensations related to physical activity and to understand their origins. The 2 symptoms most commonly cited by COPD patients as the main reason for exercise termination are dyspnea and leg fatigue (Citation[4], Citation[5], Citation[10], Citation[11], Citation[12]). The locus and the intensity of symptom limitation are similar between patients with COPD and other respiratory diseases and matched healthy controls ().
Figure 1 Dyspnea and leg fatigue Borg scores during incremental cycling exercise in healthy individuals and in patients with respiratory diseases. Adapted with permission from Hamilton et al. AJRCCM 1995; 152:2021–2031.
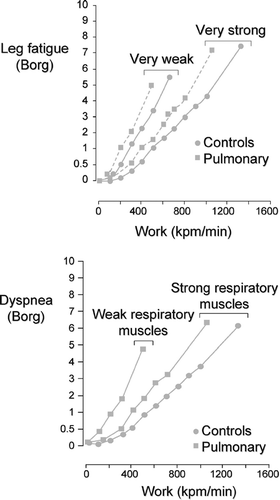
However, limiting symptoms are reached at a lower intensity in COPD patients (Citation[8], Citation[13]) and are described differently than in healthy individuals (Citation[14], Citation[15]). Phrases denoting an “increased work/effort of breathing” and “heaviness of breathing” are commonly used by both healthy and diseased individuals to describe exertional dyspnea, but descriptors of “increased inspiratory difficulty,” “unsatisfied inspiratory effort,” and “shallow breathing” appears to be specific to patients with COPD (Citation[14], Citation[15]).
This qualitatively distinct sensation of exertional dyspnea is believed to be linked to the presence of hyperinflation in these patients (Citation[14]). Indeed, the intensity of exertional dyspnea correlates with the degree of dynamic hyperinflation experienced by COPD patients (Citation[16]). In general, the perception of dyspnea is more intense than that of leg fatigue in patients with moderate-to-severe COPD (Citation[10]), while the reverse is often observed in milder disease. The proportion of patients stopping for one symptom or the other varies according to disease severity, personal characteristics such as training status and individual susceptibility to muscle fatigue (Citation[17]), bronchodilation status (Citation[18]) and exercise modalities used (Citation[4], Citation[5]). The remainder of this paper will discuss the physiological mechanisms underlying both dypsnea and peripheral muscle fatigue during exercise in patients with COPD.
VENTILATORY LIMITATION
Expiratory flow limitation in COPD is the hallmark feature of COPD and it is related to reduced airway calibre and loss of lung elastic recoil (Citation[19], Citation[20]). Ventilatory capacity, which is largely determined by expiratory flow rates (Citation[21]), is therefore reduced in patients with COPD (Citation[22], Citation[23]). In contrast to healthy individuals, ventilation (![]() E) frequently reaches maximum voluntary ventilation as estimated from resting FEV1 in this population (Citation[21], Citation[24]). It is important to keep in mind that this situation is likely worse than expected since maximum voluntary ventilation is likely to be overestimated from resting expiratory flows (Citation[25]). This is because the efficiency of respiratory muscles as pressure generators will further decrease during exercise as a result of the increased velocity of shortening and functionally induced muscle weakness closely linked to the development of dynamic hyperinflation (Citation[25]). Higher dead space ventilation and inefficient gas exchange will be associated with exaggerated
E) frequently reaches maximum voluntary ventilation as estimated from resting FEV1 in this population (Citation[21], Citation[24]). It is important to keep in mind that this situation is likely worse than expected since maximum voluntary ventilation is likely to be overestimated from resting expiratory flows (Citation[25]). This is because the efficiency of respiratory muscles as pressure generators will further decrease during exercise as a result of the increased velocity of shortening and functionally induced muscle weakness closely linked to the development of dynamic hyperinflation (Citation[25]). Higher dead space ventilation and inefficient gas exchange will be associated with exaggerated ![]() E responses during submaximal exercise.
E responses during submaximal exercise.
All these interrelated phenomena contribute to a higher ![]() E/maximum voluntary capacity ratio at a given exercise workrate in COPD compared to normal. Although reduced ventilatory capacity is undoubtedly an important determinant of exercise tolerance (Citation[9]), the wide range of exercise tolerance in patients with comparable ventilatory capacity indicates that other factors are also involved in exercise intolerance in COPD.
E/maximum voluntary capacity ratio at a given exercise workrate in COPD compared to normal. Although reduced ventilatory capacity is undoubtedly an important determinant of exercise tolerance (Citation[9]), the wide range of exercise tolerance in patients with comparable ventilatory capacity indicates that other factors are also involved in exercise intolerance in COPD.
PHYSIOLOGICAL DETERMINANTS OF DYSPNEA
The perception of dyspnea at a given level of exercise is greater in patients with COPD compared to healthy subjects and the situation is further worsened in the presence of weak respiratory muscles (Citation[8]). Dyspnea is a complex phenomenon and the reader is referred to excellent reviews for in-depth discussion (Citation[26], Citation[27]). A key concept is that the intensity of dyspnea is positively correlated with the pressure generated by respiratory muscles during tidal breathing as a function of the maximum pressure available. In COPD, this ratio could be increased by greater airway resistances or alternatively, by intrinsically or functionally weakened respiratory muscles, which would work at a higher proportion of their capacity even if normal efforts are made during tidal breathing. Dynamic hyperinflation and its related consequences on respiratory muscles are involved in the pathogenesis of dyspnea and exercise intolerance in COPD (Citation[28]); this topic is the subject of the following sections.
DYNAMIC HYPERINFLATION
At rest and during exercise, healthy individuals breathe within the maximal envelope of the flow-volume relationship such that inspiratory and expiratory flows can be easily increased to accommodate the ventilatory requirements of exercise. In these individuals, end-expiratory volume remains stable or decreases during exercise (Citation[28], Citation[29]), as reflected by an increased inspiratory capacity with exercise. The physiological benefits of this reduction in end-expiratory lung volume could be to place the diaphragm in a more advantageous position in terms of its length-tension relationship and to store elastic energy in the chest wall during expiration, whose release during inspiration could assist the respiratory muscles (Citation[30]).
In patients with COPD, the ability to increase inspiratory and expiratory flows is compromised because patients are often already breathing on some parts of the maximum flow-volume loop envelope at rest, both in mild to moderate and advanced diseases (Citation[31], Citation[32]) (). This problem can be overcome, at least temporarily, by breathing at higher lung volumes permitting greater expiratory flows to be generated (Citation[31], Citation[32], Citation[33]). In fact, an increase in end-expiratory lung volume is seen in the majority of patients with COPD in patients, translating into a progressive reduction in inspiratory capacity as exercise proceeds (Citation[28]) (). This one positive consequence of dynamic hyperinflation, ie to allow patients to increase ventilation, is not without important disadvantages.
Figure 2 Examples of flow volume loops obtained in one healthy subject and one patient with COPD. Blue lines represent the maximal envelope, black lines depict the flow-volume loops at rest and the orange lines shows the flow-volume loops during exercise. Adapted with permission from O'Donnell DE, Banzett RB, Carrieri-Kohlman V, et al. Pathophysiology of Dyspnea in Chronic Obstructive Pulmonary Disease: A Roundtable. Proc Am Thorac Soc. 2007; 4:145–168. Official Journal of The American Thoracic Society. © American Thoracic Society.
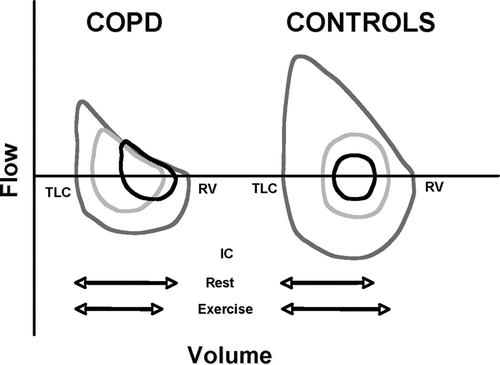
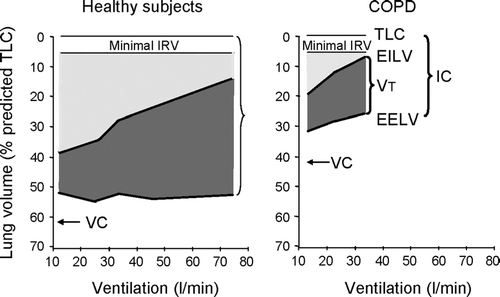
Figure 3 Operational lung volumes as ventilation increases during exercise in COPD patients and in age-matched healthy subjects. Used with permission from O'Donnell et al. AJRCCM 2001; 164:770–777.
Dynamic hyperinflation limits the expansion in tidal volume once end-inspiratory lung volume reaches a critical zone approximating 500 ml of total lung capacity (Citation[34], Citation[35]). At such high end-inspiratory volume, dyspnea rises exponentially leading to rapid exercise termination (Citation[28], Citation[34], Citation[36]). Breathing at high lung volumes also implies that a portion of tidal breathing will occur on the flat portion of the lung volume-pressure relationship thereby increasing work of breathing. An important concept is that dynamic hyperinflation uncouples the relationship between respiratory effort and the resulting tidal volume (Citation[26], Citation[27]). In other words, respiratory efforts will not be rewarded appropriately in terms of the tidal volume generated during the breath, as indicated by a marked increased in esophageal pressure excursion to tidal volume ratio in patients with COPD compared to healthy controls (Citation[26], Citation[27]). This phenomenon, referred to as neuromechanical uncoupling, is a strong determinant of dyspnea (Citation[26], Citation[35], Citation[37]).
Dynamic hyperinflation also places the diaphragm in an unfavorable portion of its length-tension relationship, further compromising its role as the main pressure generator during inspiration. As a result of both the increased work of breathing and the weakened respiratory muscles, a higher fraction of the respiratory muscle strength will be used to generate tidal breathing, another contributor to dyspnea (Citation[8], Citation[26]).
These physiological concepts have been tested in clinical trials evaluating the impact of long acting bronchodilation on exercise tolerance (Citation[34], Citation[38]). In these studies, the impact of bronchodilation on operating lung volumes and dyspnea during exercise as well as on the endurance to constant workrate cycling exercise was assessed. Bronchodilation markedly reduced end-expiratory lung volume at rest allowing patients to tolerate the exercise stimulus for a longer period of time. In these trials, the reduction in operational lung volumes, the decrease in dyspnea and the improvement in exercise tolerance were all tightly interrelated providing a strong clinical validation of physiological concepts supporting the role of dynamic hyperinflation on functional status in COPD.
Despite its clear relevance to exercise intolerance, dynamic hyperinflation is not sufficient in itself to cover all aspects of exercise intolerance in COPD. For instance, the improvement in endurance to submaximal exercise only moderately correlates with the change in inspiratory capacity with bronchodilation (Citation[36]). In one study, approximately 20% of patients did not show improvement in endurance time to constant workrate cycling exercise despite improvement in inspiratory capacity with bronchodilation (Citation[36]). This interesting finding suggests that factors other than impairment in lung function and dynamic hyperinflation must be involved in exercise limitation in COPD.
PERIPHERAL MUSCLE FATIGUE
Definition
Muscle fatigue can be defined as a reversible decrease in the force-generating ability of a muscle resulting from recent activity (Citation[39]). Muscle fatigue can be caused by a dysfunction at any step of the chain linking the central nervous system to the contractile apparatus itself. This means that reductions in the force-generating capacity of a muscle need not to be due to central mechanisms (such as a decline in central nervous system motor drive or failing neuromuscular transmission), but can also stem from peripheral mechanisms attributable solely to changes in muscle contractile properties.
Peripheral muscle fatigue, also called contractile fatigue, is a reversible failure of the working skeletal muscle to maintain a given force in response to neural stimulation. Mechanisms occurring at a number of sites have been implicated in peripheral muscle fatigue. These include the conduction of the action potential along the muscle fiber membrane into the transverse tubule system, the release of calcium into the myoplasm, the binding of calcium to troponin, the interaction between myosin and actin during cross-bridge cycling, and the active reuptake of calcium into the sarcoplasmic reticulum (Citation[40]). Also, there is growing evidence that an impaired excitation-contraction coupling is implicated as a contributing common factor to fatigue induced by diverse forms of exercise (Citation[41], Citation[42], Citation[43]).
Metabolic changes in the muscle, such as lactate accumulation and phosphocreatine depletion (Citation[44]), limitations in muscle energy supply (Citation[45], Citation[46], Citation[47]), and structural and metabolic disorganization of contractile proteins (Citation[41], Citation[48]) can all be involved in the development of contractile muscle fatigue. Other factors, such as redox status (Citation[49], Citation[50]) and muscle inflammation (Citation[51], Citation[52], Citation[53]) must also be considered as potential contributors to muscle fatigue in patients with COPD.
The accumulation of lactic acid occurring during exercise has also been suspected to have a direct effect on contractile fatigue (Citation[54]). Lactic acid is dissociated into lactate and hydrogen ions, leading to a significant decrease in pH. Lactate ions have no effect on muscular contraction (Citation[55]), but the increased concentration of hydrogen ions, which affects the excitation-contraction coupling by decreasing the quantity of calcium released, has been proposed as a contributor to the development of contractile fatigue (Citation[56]). However, a causal relationship between acidosis and development of muscle fatigue has been challenged in recent studies showing that under physiological temperature conditions, muscular pH reduction has little effect on both strength of contraction and muscular fatigue (Citation[48]).
Manifestation of muscle fatigue
Muscular fatigue can be measured in terms of reduced force- (or work-) generating capacity. It is also accompanied by many other measurable changes, such as a shift in the electromyogram (EMG) power spectrum, a slowing muscle conduction velocity and contractile speed, and an accumulation of hydrogen ions, lactate, or others metabolites whose measurement can also reflect the occurrence of muscle fatigue.
Fatigue in patients with COPD
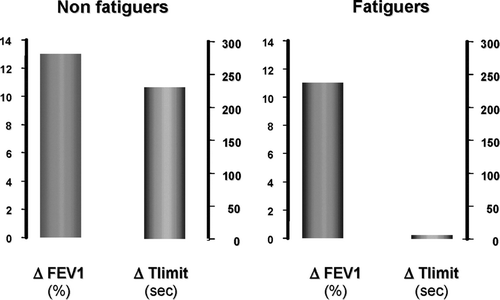
As mentioned previously, leg fatigue is a symptom commonly cited by COPD patients as the main limiting factor to exercise (Citation[10]) Recently, studies using magnetic stimulation, a ensitive test for the identification of quadriceps muscle fatigue (Citation[4]), have confirmed that contractile fatigue of the quadriceps occurred during exercise in patients with COPD (Citation[4], Citation[57], Citation[58], Citation[59]). In line with this, the impact of leg fatigue on the exercise response to acute bronchodilation in patients with COPD was recently evaluated by Saey and colleagues (Citation[60]). In this study, the improvement in airflow obstruction following acute bronchodilation did not translate into greater exercise capacity in patients with higher susceptibility to leg fatigue (), providing direct evidence for the role of peripheral muscle dysfunction in exercise intolerance in COPD.
Figure 4 Changes in endurance time to constant-load cycling (Δ Tlimit) in response to bronchodilation according to the presence (fatiguers) or not (non-fatiguers) of contractile leg fatigue. Adapted with permission from Saey et al. AJRCCM 2003; 168:425.
Results of this physiological study are further supported by a pooled analysis of the two large, multicentre, randomized clinical trials evaluating the efficacy of tiotropium during cycling exercise in patients with COPD (Citation[18], Citation[34], Citation[38]). In this combined analysis, the magnitude of the improvement in the endurance time to constant workrate cycling exercise was smaller in patients whose main exercise limiting symptom was leg fatigue compared to those reporting dyspnea as their primary limitation to exercise (Citation[61]). An important clinical message arises from those studies: The occurrence of leg fatigue will prevent patients with COPD from obtaining full advantage of bronchodilation. In these patients, treatment of the peripheral muscles in combination with pharmacological interventions should be incorporated into the management plan.
Muscle fatigue in COPD has been linked to certain peripheral muscle alterations, such as poor oxidative capacity (Citation[62]), muscle atrophy (Citation[63]) and muscle weakness (Citation[64]), which are commonly observed in this disease and which increase susceptibility to contractile fatigue. The perception of leg fatigue is influenced by muscle strength (Citation[8]). In a large sample of healthy subjects and patients with lung diseases, most of whom had COPD, the perception of leg fatigue for a given power output was greater in weak compared to strong individuals; this was true for both the healthy controls and patients with COPD (Citation[8]). In the same study, muscle strength was found to be an independent determinant of exercise capacity, highlighting the role of peripheral muscle impairment as an exercise-limiting factor. Changes in muscle enzymatic profile and capillarization leading to a greater reliance on glycolytic metabolism during exercise are also associated with contractile fatigue and poor skeletal muscle endurance in patients with COPD (Citation[65], Citation[66]).
Surface electromyographic signals (sEMG) can also provide useful information relating to motor unit recruitment and central response to decreasing muscle contractile properties during exercise. Muscle fatigue in patients with COPD is associated with a decrease in electromyographic median frequency and an increase in root mean square voltage (Citation[17], Citation[66], Citation[67], Citation[68], Citation[69], Citation[70]). These electromyographic adjustments were also found in conditions of hypoxemia (Citation[71], Citation[72], Citation[73]). This must be interpreted as an adaptation of the central nervous system to muscle fatigue, confirming the peripheral origin of muscle fatigue and the relatively preserved central neural drive in this disease.
Interaction between peripheral muscle fatigue and the respiratory system
An appealing concept is that the peripheral and central components of exercise limitation may interact with each other to further reduce exercise tolerance. As of now, only indirect evidence exists to support this notion in patients with COPD and research is this field is needed. One obvious possible mechanism for this interaction between the peripheral muscles and the respiratory system is that metabolic changes occurring in the fatiguing muscles lead to early acidosis (Citation[74], Citation[75]) and likely contribute to the increase ventilatory requirement during exercise (Citation[76]). This imposes an additional burden on the respiratory muscles already facing increased impedance to breathing.
Moreover, there could be a steal phenomenon of blood from the peripheral muscles toward the respiratory muscles that would leave both muscle groups with insufficient perfusion and oxygenation during exercise. This competition for blood flow between the respiratory and contracting peripheral muscles has been described elegantly in athletes in whom unloading the respiratory muscles using non-invasive ventilatory support improved blood flow and oxygen transport to the contracting locomotor muscles (Citation[77], Citation[78]) while reducing quadriceps fatigability (Citation[79]). Only indirect proofs of this phenomenon are currently available in patients with COPD. Richardson and colleagues recently evaluated the effects of unloading the respiratory muscles by having patients with COPD breathe an helium-oxygen mixture during whole body cycling exercise (high ventilatory requirement) and single-knee extension exercise (low ventilatory requirement) (Citation[6]).
Consistent with the concept of blood flow redistribution, they found that breathing the helium-oxygen mixture was associated with higher peak ![]() O2 only during whole body cycling exercise. Breathing a helium mixture during single-knee extension exercise did not improve peak
O2 only during whole body cycling exercise. Breathing a helium mixture during single-knee extension exercise did not improve peak ![]() O2 presumably because the maximum ventilation and the demand placed upon the respiratory muscles were lower during this exercise modality therefore reducing the potential for redistributing blood flow from the respiratory to the knee-extensor muscles. In one small study, a subgroup of patients with COPD showed a plateau in lower limb
O2 presumably because the maximum ventilation and the demand placed upon the respiratory muscles were lower during this exercise modality therefore reducing the potential for redistributing blood flow from the respiratory to the knee-extensor muscles. In one small study, a subgroup of patients with COPD showed a plateau in lower limb ![]() O2 during cycling exercise despite the progression of total body
O2 during cycling exercise despite the progression of total body ![]() O2 and external workrate (Citation[80]).
O2 and external workrate (Citation[80]).
This increased in total body ![]() O2 while lower limb
O2 while lower limb ![]() O2 was plateauing could only be explained by an increase metabolic activity outside the legs. This observation would also be consistent with the redistribution of blood (and oxygen) flow and from the exercising lower limb muscles to another group of muscles, perhaps the respiratory muscles, also supporting this concept of a competition for blood flow an oxygen between the peripheral and respiratory muscles.
O2 was plateauing could only be explained by an increase metabolic activity outside the legs. This observation would also be consistent with the redistribution of blood (and oxygen) flow and from the exercising lower limb muscles to another group of muscles, perhaps the respiratory muscles, also supporting this concept of a competition for blood flow an oxygen between the peripheral and respiratory muscles.
The cardiovascular and respiratory adjustments induced by exercise are linked to the working muscles themselves. Indeed, muscle-fatiguing exercise may significantly contribute to the increase in the perception of dyspnea and ventilatory requirements during exercise through the stimulation of metaboreceptors within the muscles (Citation[81]). These receptors are sensitive to exercise-induced changes in muscle metabolism and to the accumulation of several chemical products in the extra-cellular environment. During a fatiguing exercise, a cardiovascular and respiratory reflex activation has been partly attributed not only to the metabo-sensitive afferent fibers (group II and III) (Citation[82], Citation[83]), but also to the direct or indirect humoral pathway sensitive to the metabolites released by the working muscle (Citation[84]). This metabo-system pathway is involved in the regulation of respiratory and cardiovascular centers and contributes to increase heart rate, arterial blood pressure, myocardial contractility and ventilation in response to muscle activity.
Thus, there are several interrelated mechanisms by which muscle fatigue can contribute to exercise limitation in COPD patients. Some act directly on the muscle contraction process and others through their effects on both the cardiorespiratory and nervous systems.
RELATIVE CONTRIBUTION OF EXERCISE-LIMITING FACTORS
The relative contribution of ventilatory mechanics and peripheral muscle fatigue as limiting factors to exercise tolerance varies considerably according to individual factors. As described earlier, the locus of symptom limitation is not uniform among patients with COPD. Likewise, the physiological response to exercise is not homogeneous in this disease. Although dynamic hyperinflation may occur in as many as 80% of patients with moderate-to-severe airflow obstruction (Citation[28]), the increase in end-expiratory lung volumes during exercise varies considerably in magnitude from patient to patient (Citation[85]). Interestingly, the degree of dynamic hyperinflation during exercise positively correlates with resting inspiratory capacity, such that patients with greater volume constraint at rest (i.e., with smaller inspiratory capacity) tend not to further worsen hyperinflation during exercise (Citation[28]).
Contractile leg fatigue occurs in approximately 60% of COPD patients after cycling exercise (Citation[5], Citation[58], Citation[65]). Peripheral muscle fatiguers and non-fatiguers have been found to be similar in terms of age, body mass index, physical activity level, resting lung function, mid-thigh muscle cross-sectional area, maximal voluntary contraction, and resting arterial gases (Citation[65]). However, fatiguers could be differentiated from non-fatiguers based on certain muscle and metabolic features such as reduced muscle capillarization, increased LDH activity and higher blood lactate during exercise (Citation[65]).
Apart from the individual factors, the relative contribution of peripheral and central factors of exercise limitation is also influenced by the exercise modality used for testing (Citation[4], Citation[5], Citation[12]). Leg fatigue, alone or in combination with dyspnea, is predominant during both incremental and constant-load cycling exercise (Citation[5], Citation[13]), while dyspnea outweighs leg fatigue as a limiting symptom during walking protocols (Citation[5], Citation[12]). illustrates the relative contribution of both symptoms as the main limiting factor in a study comparing the constant-load cycling test to the endurance shuttle walk in patients with COPD (Citation[5]). Contractile fatigue of the quadriceps, evaluated via magnetic stimulation, is also more frequent and more pronounced after cycling than after walking (Citation[4], Citation[5]). This observation that symptom intensity varies according to the exercise testing modality that is being used is highly relevant for the design of studies to assess the response to bronchodilation in patients with COPD. Because of the greater frequency of leg fatigue as the limiting symptom during cycling than walking, the cycling endurance test may be less responsive to bronchodilation than the endurance shuttle walk (Citation[5]) ().
Figure 5 Locus of symptom limitation during the constant-load cycling test and the endurance shuttle walk (left panel) and the comparative improvement in exercise tolerance obtained with bronchodilation (ipratropium bromide) during constant workrate cycling exercise and endurance shuttle walking. Adapted with permission from Pepin et al. AJRCCM 2005; 172:1517–1522.
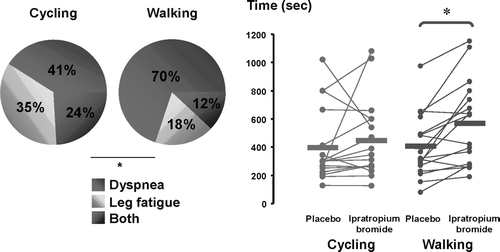
Another factor to consider when assessing the relative contribution of peripheral and central factors to exercise intolerance is the bronchodilation status of the patients. A significant change in the locus of symptom limitation has been nicely illustrated in a large clinical trial looking at the impact of long-acting bronchodilation on exercise tolerance in COPD (Citation[18]). In that study, 18% of the patients reported leg fatigue as the most important symptom at peak exercise while this symptom was mentioned by 30% of the patients when on optimal bronchodilation. Presumably, alleviating dyspnea allowed more patients to sufficiently activate their peripheral muscles to manifest leg fatigue during exercise.
CONCLUSION
The causes and mechanisms of exercise intolerance in patients with COPD are complex and involve symptoms, ventilatory and respiratory mechanics impairment, gas exchange limitations, and peripheral muscle impairment. The mechanisms of exercise limitation are heterogeneous within the COPD population, highlighting the importance of comprehensive exercise testing (assessing cardiopulmonary and muscular contributions to exercise limitation) in this patient population. The current knowledge also indicates that the mechanisms of exercise limitation are influenced by a number of factors such as baseline respiratory function, individual susceptibility to muscle fatigue, exercise modality and bronchodilation status. All these factors should therefore be considered when assessing exercise intolerance in COPD.
REFERENCES
- ATS/ACCP. Statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med 2003; 167: 211–277
- Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med 2003; 167: 544–549
- Martinez F J, Foster G, Curtis J L, Criner G, Weinmann G, Fishman A, De Camp M M, Benditt J, Sciurba F, Make B, Mohsenifar Z, Diaz P, Hoffman E, Wise R, for the NETT Research Group. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006; 173: 1326–1334
- Man W D, Soliman M G, Gearing J, Radford S G, Rafferty G F, Gray B J, Polkey M I, Moxham J. Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 168: 562–567
- Pepin V, Saey D, Whittom F, Leblanc P, Maltais F. Walking versus cycling: sensitivity to bronchodilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 1517–1522
- Richardson R S, Sheldon J, Poole D C, Hopkins S R, Ries A L, Wagner P D. Evidence of skeletal muscle metabolic reserve during whole body exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159: 881–885
- Maltais F, Simon M, Jobin J, Desmeules M, Sullivan M J, Belanger M, Leblanc P. Effects of oxygen on lower limb blood flow and O2 uptake during exercise in COPD. Med Sci Sports Exerc 2001; 33: 916–922
- Hamilton A L, Killian K J, Summers E, Jones N L. Muscle strength, symptom intensity, and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med 1995; 152: 2021–2031
- Jones N L, Killian K J. Limitation of exercise in chronic airway obstruction. Chronic Obstructive Pulmonary Disease, 1st ed., N S Cherniack. W.B. Saunders, Philadelphia 1991; 196–206
- Killian K J, Leblanc P, Martin D H, Summers E, Jones N L, Campbell E JM. Exercise capacity and ventilatory, circulatory, and symptom limitation in patients with airflow limitation. Am Rev Respir Dis 1992; 146: 935–940
- Hamilton A L, Killian K J, Summers E, Jones N L. Symptom intensity and subjective limitation to exercise in patients with cardiorespiratory disorders. Chest 1996; 110: 1255–1263
- Palange P, Forte S, Onorati P, Manfredi F, Serra P, Carlone S. Ventilatory and metabolic adaptations to walking and cycling in patients with COPD. J Appl Physiol 2000; 88: 1715–1720
- Killian K J, Summers E, Jones N L, Campbell E J. Dyspnea and leg effort during incremental cycle ergometry. Am Rev Respir Dis 1992; 145: 1339–1345
- O'Donnell D E, Bertley J C, Chau L K, Webb K A. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med 1997; 155: 109–115
- Mahler D A, Harver A, Lentine T, Scott J A, Beck K, Schwartzstein R M. Descriptors of breathlessness in cardiorespiratory diseases. Am J Respir Crit Care Med 1996; 154: 1357–1363
- O'Donnell D E, Webb K A. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. Am Rev Respir Dis 1993; 148: 1351–1357
- Saey D, Côté C H, Mador M J, Laviolette L, Leblanc P, Jobin J, Maltais F. Assessment of muscle fatigue during exercise in chronic obstructive pulmonary disease. Muscle Nerve 2006; 34: 62–71
- Maltais F, Hamilton A, Marciniuk D, Hernandez P, Sciurba F C, Richter K, Kesten S, O'Donnell D. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest 2005; 128: 1168–1178
- Pauwels R A, Buist A S, Calverley P M, Jenkins C R, Hurd S S. GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop Summary. Am J Respir Crit Care Med 2001; 163: 1256–1276
- Petty T L, Silvers G W, Stanford R E, Baird M D, Mitchell R S. Small airway pathology is related to increased closing capacity and abnormal slope of phase III in excised human lungs. Am Rev Respir Dis 1980; 121: 449–456
- Clark T J, Freedman S, Campbell E J, Winn R R. The ventilatory capacity of patients with chronic airways obstruction. Clin Sci 1969; 36: 307–316
- Gallagher C G. Exercise limitation and clinical exercise testing in chronic obstructive pulmonary disease. Clin Chest Med 1994; 15: 305–326
- Marciniuk D D, Gallagher C G. Clinical exercise testing in chronic airflow limitation. Med Clin North Am 1996; 80: 565–587
- Potter W A, Olafsson S, Hyatt R E. Ventilatory mechanics and expiratory flow limitation during exercise in patients with obstructive lung disease. J Clin Invest 1971; 50: 910–919
- Leblanc P, Summers E, Inman M D, Jones N L, Campbell E JM, Killian K J. Inspiratory muscles during exercise: a problem of supply and demand. J Appl Physiol 1988; 64: 2482–2489
- O'Donnell D E, Webb K. Physiological basis of dyspnoea. Pulmonary rehabilitation, C F Donner, N Ambrosino, R S Goldstein. Hodder Arnold, London 2005
- Killian K J, Jones N L. Mechanisms of exertional dyspnea. Clin Chest Med 1994; 15: 247–257
- O'Donnell D E, Revill S M, Webb K A. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164: 770–777
- Babb T G, Rodarte J R. Exercise capacity and breathing mechanics in patients with airflow limitation. Med Sci Sports Exerc 1992; 24: 967–974
- Dodd D S, Brancatisano T, Engel L A. Chest wall mechanics during exercise in patients with severe chronic air-flow obstruction. Am Rev Respir Dis 1984; 129: 33–38
- Grimby G, Stiksa J. Flow-volume curves and breathing patterns during exercise in patients with obstructive lung disease. Scand J Clin Lab Invest 1970; 25: 303–313
- Stubbing D G, Pengelly L D, Morse J L, Jones N L. Pulmonary mechanics during exercise in subjects with chronic airflow obstruction. J Appl Physiol 1980; 49: 511–515
- Babb T G, Viggiano R, Hurley B, Staats B, Rodarte J R. Effect of mild-to-moderate airflow limitation on exercise capacity. J Appl Physiol 1991; 70: 223–230
- O'Donnell D E, Voduc N, Fitzpatrick M, Webb K A. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. European Respir J 2004; 24: 86–94
- O'Donnell D, Hamilton A L, Webb K A. Sensory-Mechanical Relationships during High Intensity, Constant Work Rate Exercise in COPD. J Appl Physiol 2006; 101: 1025–1035
- O'Donnell D E, Lam M, Webb K A. Spirometric Correlates of Improvement in Exercise Performance after Anticholinergic Therapy in Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 1999; 160: 542–549
- O'Donnell D E. Hyperinflation, dyspnea, and exercise intolerance in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2006; 3: 180–184
- O'Donnell D E, Fluge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23: 832–840
- Bigland-Ritchie B, Woods J J. Changes in muscle contractile properties and neural control during human muscular fatigue. Muscle Nerve 1984; 7: 691–699
- Westerblad H, Lee J A, Lannergren J, Allen D G. Cellular mechanisms of fatigue in skeletal muscle. Am J Physiol 1991; 261: C195–C209
- Westerblad H, Allen D G. Recent advances in the understanding of skeletal muscle fatigue. Curr Opin Rheumatol 2002; 14: 648–652
- Fujimoto T, Nishizono H. Involvement of membrane excitation failure in fatigue induced by intermittent submaximal voluntary contraction of the first dorsal interosseous muscle. J Sports Med Phys Fitness 1993; 33: 107–117
- Edwards R H, Hill D K, Jones D A, Merton P A. Fatigue of long duration in human skeletal muscle after exercise. J Physiol 1977; 272: 769–778
- Sahlin K. Metabolic factors in fatigue. Sports Med 1992; 13: 99–107
- Sahlin K. Muscle fatigue and lactic acid accumulation. Acta Physiol Scand 1986; 128: 83–91
- Sahlin K, Tonkonogi M, Soderlund K. Energy supply and muscle fatigue in humans. Acta Physiol Scand 1998; 162: 261–266
- Sahlin K, Soderlund K, Tonkonogi M, Hirakoba K. Phosphocreatine content in single fibers of human muscle after sustained submaximal exercise. Am J Physiol 1997; 273: C172–C178
- Westerblad H, Allen D G, Lannergren J. Muscle fatigue: lactic acid or inorganic phosphate the major cause?. News Physiol Sci 2002; 17: 17–21
- Reid M B. Invited review: redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol 2001; 90: 724–731
- Reid M B. Muscle fatigue: mechanisms and regulation. Handbook of Oxidants and Antioxydants in Exercise, C K Sen, L Packer, O Hanninen. Elsevier Science, Amsterdam, Netherlands 2000; 599–629
- Wilcox P G, Hards J M, Bockhold K, Bressler B, Pardy R L. Pathologic changes and contractile properties of the diaphragm in corticosteroid myopathy in hamsters: comparison to peripheral muscle. Am J Respir Cell Mol Biol 1989; 1: 191–199
- Tracey K J, Lowry S F, Beutler B, Cerami A, Albert J D, Shires G T. Cachectin/tumor necrosis factor mediates changes of skeletal muscle plasma membrane potential. J Exp Med 1986; 164: 1368–1373
- Reid M B, Lannergren J, Westerblad H. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med 2002; 166: 479–484
- Mainwood G W, Renaud J M. The effect of acid-base balance on fatigue of skeletal muscle. Can J Physiol Pharmacol 1985; 63: 403–416
- Posterino G S, Dutka T L, Lamb G D. L(+)-lactate does not affect twitch and tetanic responses in mechanically skinned mammalian muscle fibres. Pflugers Arch 2001; 442: 197–203
- Hermansen L, Osnes J B. Blood and muscle pH after maximal exercise in man. J Appl Physiol 1972; 32: 304–308
- Polkey M I, Kyroussis D, Hamnegard C H, Mills G H, Green M, Moxham J. Quadriceps strength and fatigue assessed by magnetic stimulation of the femoral nerve in man. Muscle Nerve 1996; 19: 549–555
- Mador M J, Kufel T J, Pineda L. Quadriceps fatigue after cycle exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161: 447–453
- Mador M J, Bozkanat E, Kufel T J. Quadriceps fatigue after cycle exercise in patients with COPD compared with healthy control subjects. Chest 2003; 123: 1104–1111
- Saey D, Debigaré R, Le Blanc P, Mador M J, Côté C H, Jobin J, Maltais F. Contractile leg fatigue after cycle exercise: A factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2003; 168: 425–430
- O'Donnell D E, Hamilton A, Kesten S. Influence of the Exercise-limiting symptom on the relationship between lung hyperinflation and endurance time in COPD patients treated with tiotropium. Am J Respir Crit Care Med 2005; 171: A314
- Maltais F, Simard A A, Simard C, Jobin J, Desgagnés P, Leblanc P. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med 1996; 153: 288–293
- Bernard S, Leblanc P, Whittom F, Carrier G, Jobin J, Belleau R, Maltais F. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158: 629–634
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996; 153: 976–980
- Saey D, Michaud A, Couillard A, Côté C H, Mador M J, Leblanc P, Jobin J, Maltais F. Contractile fatigue, muscle morphometry, and blood lactate in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171: 1109–1115
- Allaire J, Maltais F, Doyon J F, Noel M, Leblanc P, Carrier G, Simard C, Jobin J. Peripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPD. Thorax 2004; 59: 673–678
- van' tHul A, Harlaar J, Gosselink R, Hollander P, Postmus P, Kwakkel G. Quadriceps muscle endurance in patients with chronic obstructive pulmonary disease. Muscle Nerve 2004; 29: 267–274
- Gosselin N, Matecki S, Poulain M, Ramonatxo M, Ceugniet F, Prefaut C, Varray A. Electrophysiologic changes during exercise testing in patients with chronic obstructive pulmonary disease. Muscle Nerve 2003; 27: 170–179
- Gosselin N, Lambert K, Poulain M, Martin A, Prefaut C, Varray A. Endurance training improves skeletal muscle electrical activity in active COPD patients. Muscle Nerve 2003; 28: 744–753
- Spruit M A, Troosters T, Trappenburg J C, Decramer M, Gosselink R. Exercise training during rehabilitation of patients with COPD: a current perspective. Patient Educ Couns 2004; 52: 243–248
- Dousset E, Steinberg J G, Balon N, Jammes Y. Effects of acute hypoxemia on force and surface EMG during sustained handgrip. Muscle Nerve 2001; 24: 364–371
- Dousset E, Decherchi P, Grelot L, Jammes Y. Effects of chronic hypoxemia on the afferent nerve activities from skeletal muscle. Am J Respir Crit Care Med 2001; 164: 1476–1480
- Gosselin N, Durand F, Poulain M, Lambert K, Ceugniet F, Prefaut C, Varray A. Effect of acute hyperoxia during exercise on quadriceps electrical activity in active COPD patients. Acta Physiol Scand 2004; 181: 333–343
- Maltais F, Jobin J, Sullivan M J, Bernard S, Whittom F, Killian K J, Desmeules M, Bélanger M, Leblanc P. Metabolic and hemodynamic responses of the lower limb during exercise in patients with COPD. J Appl Physiol 1998; 84: 1573–1580
- Sala E, Roca J, Marrades R M, Alonso J, Gonzalez de Suso J M, Moreno A, Barbera J A, Nadal J, de Jover L, Rodriguez-Roisin R, Wagner P D. Effects of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159: 1726–1734
- Casaburi R, Patessio A, Ioli F, Zanaboni S, Donner C F, Wasserman K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis 1991; 143: 9–18
- Harms C A, Wetter T J, McClaran S R, Pegelow D F, Nickele G A, Nelson W B, Hanson P, Dempsey J A. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol 1998; 85: 609–618
- Harms C A, Babcock M A, McClaran S R, Pegelow D F, Nickele G A, Nelson W B, Dempsey J A. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 1997; 82: 1573–1583
- Romer L M, Lovering A T, Haverkamp H C, Pegelow D F, Dempsey J A. Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol (Lond) 2006; 571: 425–439
- Simon M, Le Blanc P, Jobin J, Desmeules M, Sullivan M J, Maltais F. Limitation of lower limb VO2 during cycling exercise in COPD patients. J Appl Physiol 2001; 90: 1013–1019
- Scott A C, Davies L C, Coats A J, Piepoli M. Relationship of skeletal muscle metaboreceptors in the upper and lower limbs with the respiratory control in patients with heart failure. Clin Sci (Lond) 2002; 102: 23–30
- McCloskey D I, Mitchell J H. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol 1972; 224: 173–186
- Hayward L, Wesselmann U, Rymer W Z. Effects of muscle fatigue on mechanically sensitive afferents of slow conduction velocity in the cat triceps surae. J Neurophysiol 1991; 65: 360–370
- Adams L, Frankel H, Garlick J, Guz A, Murphy K, Semple S J. The role of spinal cord transmission in the ventilatory response to exercise in man. J Physiol 1984; 355: 85–97
- Calverley P M. Dynamic hyperinflation: is it worth measuring?. Proc Am Thorac Soc 2006; 3: 239–244