Abstract
The benefits of long-term oxygen therapy (LTOT) on mood in Chronic Obstructive Pulmonary Disease (COPD) are unproven. Longitudinal studies are affected by disease progression, the increased package of care (with LTOT) and may not control for known confounders on mood. We compared the point prevalence and severity of mood disturbance in patients with severe COPD, not on LTOT (the −LTOT group) to those with COPD on LTOT (the +LTOT group). We mailed the Hospital Anxiety and Depression (HAD) Score to 182 consecutive patients with severe COPD, identified from respiratory case notes in three UK Hospitals. We compared 57 patients not prescribed LTOT to 57 patients on LTOT, and used stratified sampling to match the groups as far as possible for age, gender, lung function and other possible confounders on mood. Or these, 25% of patients in both groups scored in the ‘definite’ case range for anxiety (HAD score ≥ 11). 37% of the −LTOT group and 33% of the +LTOT group scored in the ‘definite’ range for depression (HAD score ≥ 11) (p = N.S). In both groups, only 11% of responders were prescribed anxiolytics and/or antidepressants. Further multiregression analysis confirmed that socio-demographic variables (e.g., lives alone, feels isolated or recent life events) were stronger predictors of mood than the prescription of LTOT or other traditionally accepted factors such as co-morbidity or the use of antidepressants or anxiolytics. High levels of anxiety and depression are present in severe COPD and appear under-treated. The +LTOT and −LTOT patients had a similar high prevalence of anxiety and depression.
Key words: :
INTRODUCTION
High rates of pathological anxiety and depression have been recognised in many chronic medical illnesses (Citation[1]). Chronic obstructive pulmonary disease (COPD) is an irreversible condition, so our main goal is to alleviate symptoms. Although early work on emotional disturbance in COPD was limited by small sample sizes and lack of control groups, most experts now agree that anxiety and depression are common but often underestimated and/or under-treated (Citation[2], Citation[3], Citation[4]).
Long-term oxygen therapy (LTOT) is prescribed for arterial hypoxaemia in an attempt to prevent or postpone the derangements of long-standing arterial hypoxaemia with regard to end organ damage in chronic lung disease. Despite established improvements in life expectancy and exercise capacity in COPD, the role of LTOT in relieving anxiety and depression remains uncertain. By relieving breathlessness or improving exercise tolerance, LTOT may perhaps relieve anxiety and depression. However, this is unproven and the relationship is complex. Importantly, poor levels of compliance with LTOT occur in up to a third of patients (Citation[5]). The reasons for this reluctance may involve fears of dependency on oxygen and fears about limiting mobility. These fears may even lead to higher anxiety and depression levels, which could reduce any symptomatic benefit that the oxygen may have on mood by relieving breathlessness.
The use of a disease-independent, self-report questionnaire to screen for the disorders of mood, valid for COPD and in particular those on LTOT, would be very useful clinically and would be of great benefit to the field. We have previously compared Hospital Anxiety and Depression (HAD) Scores (Citation[6]) in patients with severe COPD and not on LTOT (−LTOT), to those with severe COPD on LTOT (+LTOT). The +LTOT group had similar scores for the depression domain but significantly lower anxiety scores to the −LTOT group. Moreover, both groups had higher anxiety and depression scores than a smaller (unmatched) group of mainly spouses from the same geographical area (Citation[7]). Our analysis was limited by sample size and limited information regarding the healthy (spouse) controls. More importantly, the +LTOT patients were not individually matched to −LTOT patients, so pair-wise comparisons could not be drawn.
The aim of this study was to compare the proportions of severe COPD patients who score as depressed or anxious, in +LTOT vs. −LTOT who are otherwise matched as a group, for several important confounders on mood. A secondary aim was to consider what other factors might influence anxiety and depression in the two groups.
MATERIALS AND METHODS
This was a cross-sectional, case-controlled study with loco-regional ethical approval. (Reference: WMW/02/0124)
Patients
We wrote to a consecutive sample of 182 patients (identified as still living on our hospital database). Subjects were of any age with a clinical diagnosis of severe COPD, according to standard criteria. All patients had at least a 10 pack-year history of cigarette smoking and an FEV1/FVC ratio of less than 70% predicted with FEV1 ≤ 50% predicted, and < 15% reversibility to inhaled salbutamol (Citation[8]). These patients were identified via respiratory clinic case-records, from 3 hospitals in the same geographical area in the United Kingdom.
All had had lung function performed within 6 months, while stable and when previously attending the hospital. They were based in the community but still attending respiratory outpatients and/or oxygen clinics intermittently. LTOT was prescribed for arterial hypoxaemia by respiratory specialists, according to standard guidelines (Citation[9]) but we did not have access to the original blood gases in many cases. Most of the +LTOT patients (55 from 57) had been on LTOT for over 6 months (range 4 months to 4 years) at the time they completed the study questionnaires. Exclusion criteria included those prescribed LTOT for less than 3 months (still adjusting) or for conditions other than COPD, any who remained hypoxic on treatment (PaO2 < 56 mmHg on last assessment), those currently hospitalized, or unable to give informed consent.
Our primary outcome was to compare prevalence, but we were also interested in mean anxiety and depression scores, as these have also been reported in medical outpatient groups and in COPD. Based on our pilot study (Citation[7]), we estimated we would need 52 patients in each group to show a significant difference in both depression and anxiety scores, assuming a standard deviation of 1.8 units (HAD), an alpha error of 0.05 and power of 0.80. The response rate of our pilot study suggested we needed to send at least 160 questionnaires to obtain 104 responses. Following the initial response to the postal questionnaires, we used stratified sampling to ensure that both groups were matched as far as possible for age, sex and lung function (see later).
Questionnaires
Subjects were mailed an Introductory letter/Patient Information Sheet that explained the aim of the study and were asked to complete the Hospital Anxiety and Depression Score (HAD) without prompts from spouses. The HAD was developed from general medical outpatients (Citation[6]). It is a quick questionnaire that is simple to complete and has been widely used in COPD research. It is available via the publishing firm National Foundation for Educational Research (http://www.nfer-nelson.co.uk). When considering numbers of patients falling into each category of the HAD score, we applied the standard definitions of ‘non-cases’ (scoring 0–7), ‘borderline cases’ (scoring 8–10) and‘definite’ cases (scoring 11–21) for both the anxiety and depression sub-scales. We also asked patients to provide information on oxygen therapy, smoking habits, current medication and also sociodemographic details e.g., whether they felt themselves to be socially isolated, housebound or had had recent major life events. This additional, open-ended questionnaire has not been validated elsewhere but is mainly factual (tick-box)–see Appendix 1. Based on clinical experience, we considered these sociodemographic variables to be important but hitherto un-researched potential confounders on mood.
Subjects were given up to 3 months to return the questionnaires together with a signed Consent Form in a prepaid envelope. We used a 3-month deadline for staff logistical reasons. No additional questionnaires were received after this deadline. No follow-up letters or phone-calls were made. Other data regarding patients' age, spirometry, confirmation of diagnosis, co-morbidity and prescribed medication were crosschecked from hospital records.
Statistical analysis
SPSS (version 13.0) was used for all analyses. Tests for normality were applied.
Primary outcome
Comparison of the proportion of +LTOT and –LTOT patients in the ‘definite’ HAD range for anxiety and depression.
Secondary outcomes
Comparison of the mean anxiety and mean depression scores of the +LTOT and –LTOT patients.
Comparison of the proportion of +LTOT and –LTOT patients currently prescribed anxiolytics and anti-depressants
To determine what combination of physical and socio-demographic factors best predicted anxiety and depression scores.
We used non-paired t-tests (2-tailed) with 95% confidence intervals and Mann–Whitney tests to compare continuous data, and Pearson's chi-square for categorical data. Multiple regression procedures were employed to examine the role of 4 blocks of (independent) variables in predicting anxiety and depression scores (dependent variable). We selected variables that were deemed clinically important and that previous studies had suggested were risk factors for mood disturbance. The blocks were entered (cumulatively) into the model in four stages to see what additional predictive power they could contribute. The 4 blocks were:
Prescription of antidepressants, prescription of anxiolytics, presence of co-morbidity.
Age, sex.
Lives alone, feels isolated, housebound.
Prescribed LTOT (yes, no).
Throughout, p < 0.05 was considered statistically significant.
RESULTS
We had a 59% (n = 107 from 182) response rate initially, from 57 -LTOT and 50 +LTOT patients. To improve matching for age, sex and lung function, we mailed questionnaires to 7 consecutive females, with FEV1 less than 50% predicted, once they had been established on LTOT for 3 months. All 7 responded giving an overall response rate of 63%. The data regarding age and HAD scores satisfied tests for normality but FEV1 was significantly non-normal (p = 0.01).
confirms that the groups did not differ significantly in any of the 11 possible confounding factors on mood and had similar prevalences of all (sub-group) ranges of anxiety and depression symptoms.
Table 1 Comparison of −LTOT and +LTOT groups
The mean (SD) anxiety score for the –LTOT group was 9.8 (4.0) and for the +LTOT group was 8.8 (3.6). There was no significant difference (t (112) = 1.35, p = 0.18, 95% CI for difference –0.4 to +2.4. The mean (SD) depression score for −LTOT group was 8.4 (3.5); and for the +LTOT was 8.4 (3.4). There was no significant difference, t (112) = −0.27, p = 0.98,95% CI for difference –1.3 to +1.3.
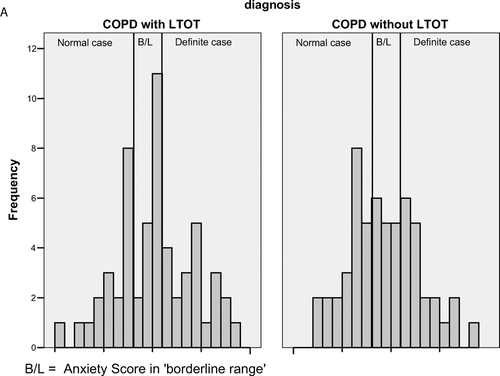
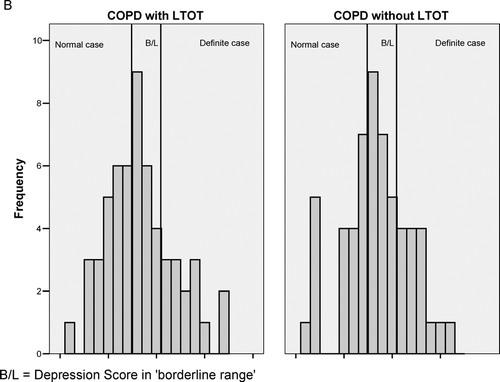
and illustrate the distributions of HAD scores for both groups with super-imposed clinical groupings for HAD ranges. All means are in the “borderline” range for both anxiety and depression and show similar distributions (statistically and clinically).
shows the magnitude of the R2 changes for Anxiety Score at each stage and the significance of these changes. Significant changes in R2 are produced by the addition of the second block (age, sex) with R2 change = 0.149, (p < 0.001), and by the addition of the third block (lives alone, feels isolated, housebound) with R2 change = 0.195, (p < 0.001). Note that the final addition of LTOT does not add significantly to the predictive power of the model.
Table 2 Multi-linear regression for anxiety score for all patients
As can be seen from , using the Depression Score for all responders as the dependent variable, only the introduction of the socio-demographic variables ‘lives alone’, ‘feels isolated’ and ‘housebound’ produces a significant change in R2 (R2 change = 0.202, p < 0.001). Adding LTOT had no effect on the predictive power of the model (R2 change = 0.001, p = N.S).
Table 3 Multi-linear regression for depression score for all patients
DISCUSSION
Our results suggest that patients with severe COPD on LTOT do not differ in prevalence of symptoms of anxiety and depression from those with severe COPD who do not fulfill the criteria for LTOT. We have showed evidence of severe mood disturbance in both patient groups with mean scores for both anxiety and depression in the ‘borderline’ range and higher than the mean scores for general medical outpatients (Citation[6]). Furthermore, a high proportion (about a third) of our patients scored as ‘definite cases’ for both anxiety and a third scored in the ‘definite case’ range for depression. Only 1 in 10 of our patients were prescribed pharmacological treatment for anxiety or depression (including homeopathic remedies such as St. John's wort). We did not ask about psychological support or counseling.
Our multiple regression modeling suggested that sociodemographic variables are more important than age and the traditionally expected medical variables of co-morbidity and LTOT prescription in predicting anxiety and depression. However, we still only explained 37% of anxiety score variance and performed even less well for predicting depression, accounting for 22% of variance. Many other factors not included in this study are also influencing these scores.
Our prevalence of pathological anxiety and depression in COPD of 30–40% is similar to most others, but prevalence rates have ranged from 4–96% (Citation[10]). Why is there is such mood disturbance in COPD? Depression itself may be an indirect cause of COPD by being independently associated with early nicotine dependence (Citation[11]) and continued smoking (Citation[12]). It is possible that a genetic predisposition underlies both depression and nicotine dependence and smoking may even exert an antidepressant effect, possibly by inhibiting monoamine oxidase A (Citation[13]). This suggests that depressed people smoke to feel better and therefore will be at greater risk of developing COPD than non-depressed people. However smokers have more microvascular lesions and these together with mild intermittent hypoxia may result in longer-term defects in neurotransmitters that may themselves cause depression (Citation[14], Citation[15]). The disability resulting from severe COPD leads to impairment in daily function and loss of role model. The restriction in activities and interpersonal relationships can also lead to depression (Citation[14]).
The relationship between anxiety and COPD is also likely to be bidirectional. Excessive nicotine use for its anxiolytic effect in those with anxiety disorders may again explain the link with developing later COPD. However, the direct association between anxiety and COPD is not surprising as dyspnea, chest tightness and palpitations are core symptoms of both. Cognitive behavioural models suggest that in individuals with very low thresholds in chemoreceptors or mechanoreceptors, misinterpretation of often small episodes of hypoxia or hypercarbia are seen as potential ‘suffocation’– which trigger an anxiety state. Finally, anxiety in COPD can be caused by anxiogenic medications including glucocorticoids, beta-agonists, methylxanthines and anticholinergics (Citation[10]). Our groups had similar prescription rates for these drugs but we did not have data regarding the use of intermittent corticosteroids for non-hospitalized exacerbations.
Mood disturbance in COPD is important. As well as personal morbidity (Citation[2], Citation[3], Citation[4], Citation[10], Citation[14], Citation[17]), major depression hinders smoking cessation attempts (Citation[18]) is independently associated with a higher drop out rate in pulmonary rehabilitation (Citation[19]), higher utilization of health resources (Citation[20]), higher admission and readmission rates (Citation[20]), and higher overall mortality (Citation[21]). With such undesirable associations, many have noted the importance of adequately treating mood disturbance in COPD (Citation[3], Citation[4], Citation[8], Citation[9], Citation[10], Citation[20], Citation[21]) but there have been few intervention trials addressing them specifically. A meta-analysis of only psychological support shows trials of mixed quality (Citation[22]) and the evidence of benefit when used alone is not strong. Different anxiolytics and anti-depressants have been tried with varying success (and side-effects) and the trials are also of varying size and quality.
Current guidelines suggest standard pharmacotherapy should be used but tailored to patients needs (Citation[9]). However, medication alone is unlikely to be the answer in such complex relationships, so medication in conjunction with other support is probably best. Pulmonary rehabilitation has been shown to improve anxiety and depression, as measured by generic and specific psychological questionnaires and disease-specific quality-of-life questionnaires (Citation[8], Citation[9], Citation[23]) suggesting mood disturbance mainly follows functional impairment. Longer-term studies are needed to corroborate this and we believe it is very important to note current smoking status in any intervention.
Potential criticisms of our study are that this is not an interventional trial and that hypoxaemic patients needing LTOT may have had even worse HAD scores prior to starting LTOT (Citation[24], Citation[25]) and that these could have improved to be on par with those with severe COPD not requiring LTOT yet. Even if this is so, the prevalence of mood disturbance remains very high.
Any postal survey is open to selection, recall and reporting bias but these considerations must be balanced against ease of administration, low cost and the avoidance of interviewer bias. In severely disabled patients, travelling for interview itself may lead to stress or depression and we did not want to survey patients while they were actually attending our clinics, for similar reasons.
Our high response rate of 63% suggests that we obtained a reasonably representative sample of our patients with severe COPD. We do not have up-to-date information on the non-responders (e.g., recent life events, medication, etc.) nor ethical permission to contact them, their families or primary care physicians, for further details. We cannot therefore fully compare our responders to non-responders but our responders are similar in physical descriptions and sociodemographics to those of other studies (Citation[2], Citation[3], Citation[4], Citation[24], Citation[25], Citation[26]). Although LTOT was prescribed according to standard guidelines (Citation[8], Citation[9]), we were unable to find baseline arterial blood gas results on a significant number and did not have ethical permission to reassess blood gases at the time of HAD questionnaires. Since arterial hypoxaemia (and thereby LTOT prescription) is correlated to airway function, but not closely, it was important that we at least matched patients with regards to lung function and we could determine that any differences could not be attributable to lung function alone.
We cannot guarantee that all hypoxia was identified or corrected at the time of the HAD assessment although all hypoxia appeared adequately treated at their previous clinical assessments. At the time of this study, none of our patients had undergone pulmonary rehabilitation but the long-term effects of pulmonary rehabilitation on mood are still not proven. All our patients were otherwise receiving standard clinical follow-up at one of three Respiratory Units, covering a population of over 400,000 people, representing a range of sociodemographic groups. We believe that our findings could be applied to most other settings in developed countries.
Another criticism is that the HAD is not sensitive enough to pick up small improvements in mood or specific enough for those with COPD. It is possible that disease-specific questionnaires perform better (Citation[27]).
Interventional studies examining changes before and after LTOT have been shown conflicting results. Borak et al. (Citation[26]) reported that emotional status (assessed by psychological questionnaires) improved significantly in 90 patients after 1 year of LTOT. However, they acknowledged the difficulty faced in separating the effects of LTOT from other aspects of an increased care package, because of patients' feelings of increased security. Eaton et al. (Citation[24]) reported clinically important improvements in Chronic Respiratory Questionnaire Scores after 2 and 6 months of LTOT. However, the type of follow-up package was also likely to be different to their group not receiving LTOT. In our patients, LTOT was not associated with additional health care delivery as all patients were already receiving home visits and clinic appointments.
Others have found no difference in mood from interventional studies of LTOT in COPD when measured by generic or disease specific questionnaires. (Citation[27], Citation[28]). The lack of effect of oxygen on mood could be explained by poor compliance, and lower self-esteem. Any psychological dependence on oxygen can also negate the well-being from the relief of dyspnea. There are plausible biological reasons why LTOT will not help mood. It may be that we are prescribing LTOT too late in the disease continuum and the structural/functional brain changes are too established, or patients are so functionally impaired by the time they receive LTOT that they cannot make meaningful psychological as well as physical gains.
Known confounders on mood have not always been well reported. In our pilot study, mood disturbance was more prevalent in females, those who were housebound, felt socially isolated, and who had experienced recent life events (Citation[7]). Being housebound, extensive co-morbidity (Citation[29]) and current smoking status (Citation[10], Citation[11], Citation[12], Citation[13], Citation[14], Citation[15], Citation[16], Citation[17], Citation[18]) are important independent predictors of anxiety and depression in COPD. Moreover, their effects will magnify in longitudinal studies, as they are likely to progress. We believe this is the first study to control for all these other variables.
In summary, mood is vital for quality of life, is intimately associated with symptom relief and important clinical outcomes in COPD. This study provides more evidence for the severe mood disturbance in severe COPD. It also demonstrates the importance of certain confounders on mood and controls for them in two well-defined and matched patient groups. By accounting for these variables, we have shown that those patients with severe COPD on LTOT do not have lower levels of anxiety or lower depression compared with those not on LTOT.
Intervention trials using oxygen earlier in COPD, before permanent structural/physiological changes occur may be considered but, until then, specific strategies to improve the recognition and treatment of mood disturbance should be our priority.
APPENDIX 1
REFERENCES
- Muller J E, Koen L, Stein D J. Anxiety and medical disorders. Curr Psychiatry Rep 2005; 7(4)245–251
- Roundy K, Cully J A, Stanley M A, Veazey C, Souchek J, Wray N P, Kunik M E. Are anxiety and depression addressed in primary care patients with Chronic Obstructive Pulmonary Disease? A chart review. Prim Care Companion J Clin Psych 2005; 7(5)213–218
- Elkington H, White P, Addington-Hall J, Higgs R, Pettinari C. The last year of life of COPD: a qualitative study of symptoms and services. Respir Med 2004; 98(5)439–445
- Gore J M, Brophy C J, Greenstone M A. How well do we care for patients with end stage chronic obstructive pulmonary disease (COPD)? A comparison of palliative care and quality of life in COPD and lung cancer. Thorax 2000; 55(12)1000–1006
- Ringbaek T, Lange P, Viskum K. Compliance with LTOT and consumption of mobile oxygen. Respir Med 1999; 93(5)333–337
- Snaith R P, Zigmond A S. The hospital anxiety and depression scale. Br Med J (Clin Res Ed) 1986; 292(6516)344
- Lewis K E, Annandale J A, Hurlin C, Osbourne S, Harrison N K. Mood disturbance in severe COPD: long-term oxygen therapy helps anxiety but not depression. Am J Respir Crit Care Med 2003; 167(7)A90
- Pauwels R A, Buist A S, Ma P, Jenkins C R, Hurd S S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care 2001; 46(8)798–825
- National Clinical Guideline. On management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004; 59: 1–232, (supplement1) doi: 10.1136/thx.2004. 022707
- Smoller J W, Pollack M H, Otto M W, Rosenbaum J F, Kradin R L. Panic anxiety, dyspnea, and respiratory disease. Theoretical and clinical considerations. Am J Respir Crit Care Med 1996; 154(1)6–17
- Fergusson D M, Lynskey M T, Horwood L J. Comorbidity between depressive disorders and nicotine dependence in a cohort of 16-year-olds. Arch Gen Psych 1996; 53(11)1043–1047
- Glassman A H, Helzer J E, Covey L S, Cottler L B, Stetner F, Tipp J E, Johnson J. Smoking, smoking cessation, and major depression. JAMA 1990; 264(12)1546–1549
- Fowler J S, Volkow N D, Wang G J, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schlyer D, Wolf A P, Warner D, Zezulkova I, Cilento R. Inhibition of monoamine oxidase B in the brains of smokers. Nature 1996; 379(6567)733–736
- Borson S, Claypoole K, McDonald G J. Depression and Chronic Obstructive Pulmonary Disease: Treatment trials. Semin Clin Neuropsych 1998; 3(2)115–130
- Gibson G E, Pulsinelli W, Blass J P, Duffy T E. Brain dysfunction in mild to moderate hypoxia. Am J Med 1981; 70(6)1247–1254
- Pollack M H, Kradin R, Otto M W, Worthington J, Gould R, Sabatino S A, Rosenbaum J F. Prevalence of panic in patients referred for pulmonary function testing at a major medical center. Am J Psych 1996; 153(1)110–113
- Porzelius J, Vest M, Nochomovitz M. Respiratory function, cognitions, and panic in chronic obstructive pulmonary patients. Behav Res Ther 1992; 30(1)75–77
- Glassman A H, Covey L S, Stetner F, Rivelli S. Smoking cessation and the course of major depression: a follow-up study. Lancet 2001; 357(9272)1929–1932
- Garrod R, Marshall J, Barley E, Jones P W. Predictors of success and failure in pulmonary rehabilitation. Eur Respir J 2006; 27(4)788–794
- Soler J, Sanchez L, Roman P, Martinez M A, Perpina M. Risk factors of emergency care and admissions in COPD patients with high consumption of health resources. Respir Med 2004; 98(4)318–329
- Dahlen I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with obstructive pulmonary disease. Chest 2002; 122(5)1633–1637
- Rose C, Wallace L, Dickson R, Ayres J, Lehman R, Searle Y, Burge P S. 2002. The most effective psychologically-based treatments to reduce anxiety and panic in patients with chronic obstructive pulmonary disease (COPD): a systematic review. Patient Educ Couns 2002; 47(4)311–318
- Withers N, Rudkin S T, White R J. Anxiety and depression in severe chronic obstructive pulmonary disease: the effects of pulmonary rehabilitation. J Cardiopulm Rehabil 1999; 19(6)362–528
- Eaton T, Lewis C, Young P, Kennedy Y, Garrett J E, Kolbe J. Long-term oxygen therapy improves health-related quality of life. Respir Med 2004; 98(4)285–293
- Okubadejo A A, Paul E A, Jones P W, Wedzicha J A. Does long-term oxygen therapy affect quality of life in patients with chronic obstructive pulmonary disease and severe hypoxaemia?. Eur Respir J 1996; 9(11)2335–2339
- Borak J, Sliwinski P, Tobiasz M, Gorecka D, Zielinski J. Psychological status of COPD patients before and after one year of long-term oxygen therapy. Monaldi Arch Chest Dis 1996; 51(1)7–11
- Okubadejo A A, Jones P W, Wedzicha J A. Quality of life in patients with chronic obstructive pulmonary disease and severe hypoxaemia. Thorax 1996; 51(1)44–47
- Janssens J P, Rochat T, Frey J G, Dousse N, Pichard C, Tschopp J M. Health-related quality of life in patients under long-term oxygen therapy: a home-based descriptive study. Respir Med 1997; 91(10)592–602
- van Manen J G, Bindels P J, Dekker F WI, Jzermans C J, van der Zee J S, Schade E. Risk of depression in patients with chronic obstructive pulmonary disease and its determinants. Thorax 2002; 57(5)412–416