Abstract
Rationale: Limited information is available on repeatability of inflammatory parameters in whole induced sputum samples from patients with COPD. Objectives: To study short-term and long-term repeatability in induced sputum samples in 22 patients with moderate to severe, stable COPD (mean age 64 yr, mean FEV1 1.91 L = 65% of predicted). Samples were collected on 71 occasions twice within 1 to 7 days (mean 3.8 days) and on 12 occasions twice with an interval of 3 months while clinically stable. Cell differentials, markers of neutrophilic and eosinophilic inflammation, respiratory membrane permeability and size-selective permeation were assessed. Findings: Parameters of permeability and of size-selective permeation, % eosinophils and % neutrophils showed the best short-term repeatability with intra-class correlation coefficients (Ri) of 0.61 to 0.90, followed by total cell count (TCC) with Ri of 0.52. Repeatability of soluble cell activation markers was less satisfactory (Ri 0.34 to 0.52). Mean short-term within-patient variability for TCC and permeability was approximately 2-fold and for cell activation markers 3-fold; mean between-patients variability was twice as high. Inducing sputum slightly enhanced eosinophil numbers and % neutrophils and decreased % macrophages in successive IS samples. Long-term repeatability was comparable to short-term repeatability but variability increased. Conclusions: Repeatability of parameters assessed in whole sputum is similar as reported previously for sputum plugs. In COPD an induced sputum procedure has a minor pro-inflammatory effect. The current data facilitates power calculations but also indicates that studies using inflammatory markers in sputum may easily be underpowered.
INTRODUCTION
Analysis of induced sputum (IS), which is produced after inhaling nebulized hypertonic saline, has been introduced as a safe and non-invasive method for the assessment of airway inflammation in airway diseases (Citation[1], Citation[2], Citation[3], Citation[4]). In asthma, the number of eosinophils in IS has been used successfully to adjust corticosteroid treatment, leading to a reduction of the number of exacerbations (Citation[5], Citation[6]). IS analysis has also been used to predict the response to corticosteroid treatment in asthma patients (Citation[7]) as well as in patients with chronic obstructive pulmonary disease (COPD)(Citation[8], Citation[9], Citation[10]). Standardization of the methods for induction, collection and analysis of IS samples has consolidated and extended the use of the IS procedure (Citation[11], Citation[12], Citation[13], Citation[14]). The validity of IS analyses strongly depends on the repeatability of the inflammatory parameters derived from it (Citation[15], Citation[16]). Repeatability has predominantly been studied in IS samples from patients with asthma and healthy subjects (Citation[2], Citation[17], Citation[18], Citation[19]).
So far, long-term repeatability has not been studied and 2 studies have investigated short-term repeatability of sputum parameters in COPD, either with a 2-week or a 4-week interval, both during placebo treatment (Citation[20], Citation[21]). Both previous studies used the selected sputum plugs methodology, intended to minimize contamination or dilution with among others saliva. However, selecting plugs excludes the epithelial lining fluid and the soluble phase of the sputum which also contains inflammatory mediators and carries the risk of selection bias. Therefore, analysis of whole sputum samples has become an accepted alternative (Citation[11]), with satisfactory repeatability in IS samples collected from asthmatics (Citation[19]). The present report extends the previous studies by documenting repeatability of inflammatory parameters in whole IS samples from COPD patients collected twice within a 1-week interval and within a 3-month interval.
METHODS AND MATERIALS
Subjects
Patients with clinically stable, smoking-related COPD as described by the Global Initiative for Chronic Obstructive Lung Disease (GOLD)(Citation[22]), were selected from the outpatients' clinic and by advertisements in local newspapers. They were asked to participate in a 16-month study into the effects of systemic and inhaled glucocorticosteroids on parameters of airway inflammation, lung function and exacerbations in COPD (Citation[23]). In line with the most recent version of GOLD (Citation[24]), candidate patients were aged 40–75 years at entry, had middle-age onset of symptoms, a cigarette consumption of at least 15 pack years, a post-bronchodilator FEV1/Vital Capacity (VC) ratio smaller than 0.70 (Citation[22]) and reversibility in Forced Expiratory Volume in the first second (FEV1) < 11% of predicted (25). COPD severity was classified by the post-bronchodilator FEV1, according to the GOLD criteria(Citation[24]). Excluded were patients with a history of asthma, a known allergy and those with a known alpha-1-antitrypsin deficiency.
Study design
Written informed consent was obtained from all patients and the study was conducted according to the declaration of Helsinki and after approval by the local medical ethics committee. Spirometry was measured before and after inhaling 400 μ g of salbutamol or 1000 μ g of terbutaline, hereafter the IS procedure started. For the short-term repeatability, data was used from patients who underwent the IS procedure twice, separated by an interval of 1 to 7 days. A blood sample was drawn for the assessment of albumin (Alb) and alpha-2-macroglobulin (A2M) in serum, usually on the day of the first IS sampling. Treatment was kept unchanged and the patient's condition had to be stable for at least 3 weeks prior to, and in between the 2 assessments. Additional sets of duplicate IS and one blood sampling were obtained with 1 to 7 months interval up to a maximum of 4 duplicate procedures per patient.
Treatment for COPD differed in subsequent duplicate sets of samplings, the visits with duplicate sampling were firstly conducted after a corticosteroid-free wash-out (17 evaluable paired samples), subsequently after 3 weeks oral prednisolone treatment (30 mg daily, 15 samples), and thereafter in a randomized and double-blind manner after 6 months inhaled budesonide treatment (800 μ g daily, 10 samples) and after 6 months inhaled placebo treatment (13 samples). For the short-term repeatability the first set of evaluable paired samples was used. For the long-term repeatability a single IS sample was obtained after 3 months inhaled placebo treatment and compared to the first of the 2 samples obtained after 6 months' placebo treatment (12 sets of samples were evaluable).
Sputum induction and analysis of sputum
After β2-agonist inhalation, sputum was induced by nebulizing increasing concentrations of hypertonic saline (3%, 4% and 5%, respectively) during 3 consecutive periods of 5 minutes (Citation[13], Citation[26]). The procedure was interrupted as soon as an adequate sputum volume (minimally 1 ml) was obtained. After careful mouth-rinsing and nose-blowing, sputum was collected, weighted and liquefied on ice with an equal volume of 10 mM dithiotreitol (DTT). When after 15 min of DTT treatment sputum was still viscous, sputum was liquefied with 150 U/ml of DNAse type I (Sigma). Cytospins were prepared for differential counts after staining with Jenner-Giemsa and Diff Quik (Dade Behring, Leusden, The Netherlands). Differential cell counts were performed in minimally 500 non-squamous cells and cell viability had to be > 90%. The liquid phase was aliquoted and stored at −80°C until analysis. Serum aliquots were stored at −80°C.
Soluble inflammatory markers were measured, investigating neutrophilic inflammation (myeloperoxidase (MPO) and interleukin (IL)-8), eosinophilic inflammation (eosinophil cationic protein (ECP)) and respiratory membrane permeability (the content of A2M and Alb in sputum and the ratio of the A2M and Alb content in sputum over that in serum (Q-A2M and Q-Alb)). The quotient of these two Q-values is the Relative Coefficient of Excretion (RCE), representing the size selective permeation, since A2M is an 11-fold larger molecule than Alb. ECP, MPO and A2M were determined with ELISA, with lower limits of detection of 50 pg/ml, 1 ng/ml and 10 ng/ml respectively, being 2-fold the background absorbance (Citation[27], Citation[28], Citation[29]). For the IL-8 ELISA the antibody pair from R+D Systems was used (MAB 208 and BAF 208, lower limit of detection 1 pg/ml). Alb was determined using an immunoturbidimetric analysis (BN ProSpec, Dade Behring, lower limit of detection 2 μ g/ml). The assays were (after dilution of samples) not affected by DTT. The inter-assay coefficient of variation for all applied assays of soluble parameters was below 10%. Sputum samples with 80% or more squamous cells were considered not evaluable because of contamination with saliva and were excluded (30).
Statistical analysis
Due to obvious non-normal distribution of most sputum data, which disappeared after logarithmic transformation (Skewness < 1.0), these data were base-10 log-transformed before statistical analyses. This transformation was applied for cell counts expressed per gram sputum, soluble markers and % eosinophil count (Citation[20]). Other differential counts showed normal distribution. Values for % eosinophils below 0.2% and zero values were arbitrarily assigned 0.1% to allow logarithmic transformation and an estimation of absolute eosinophil counts (1 of 42 samples in the assessment of short-term repeatability and 1 of 24 samples in the assessment of long-term repeatability). Inflammatory data are shown as median and range. Repeatability was graphically presented as proposed by Bland and Altman (Citation[16]).
Analysis of short-term repeatability was based upon one (the first) duplicate pair of samples per patient. Repeatability is expressed as the intraclass correlation coefficient (Ri), which is the quotient of the between-subjects variance and the total variance (between-subjects + within-subjects) (Citation[15]). This parameter requires a normal distribution of the data and a Ri value of > 0.6 represents a good repeatability (Citation[15]). The within-patient variability of parameters was expressed as the standard deviation of the absolute difference between paired values (log-transformed when applicable) (Citation[20]). The between-patients variability of parameters was expressed as the standard deviation of the values obtained for the first IS sample of each patient (log-transformed when applicable).
A systematic effect of the first sputum induction procedure on the results obtained in the second sampling, obtained within 1 week, was investigated firstly by comparing the data by Student's paired t-test (on log-transformed data), using all available duplicate samples, irrespective of the treatment under which they were obtained. Second, a potential temporal effect of the first induced sputum procedure on the results of the second one was evaluated by studying the relationship between the length of the interval from the first to the second sampling and the change in the parameter in the samples from the first to the second sample with Spearman's test. Third an ANOVA was performed on the difference between the inflammatory parameters on the 2 paired visits and the type of treatment given in between the 2 sampling days. An explorative analysis was performed comparing repeatability (intraclass correlation coefficients) under each of the 4 different conditions (wash-out, prednisolone, inhaled budesonide, inhaled placebo) by ANOVA using all available paired samples. Similarly, an explorative analysis was performed by ANOVA investigating differences in short-term repeatability between the group of patients who also participated in the analysis of long-term repeatability (n = 12) and the group who had no such long-term data (n = 9), mainly due to withdrawal from the study due to exacerbations. Two-sided p-values ≤ 0.05 were considered statistically significant. SPSS for PC was used for the statistical analyses. No power analysis was performed beforehand since the present report describes a post-hoc analysis of data from the long-term parent study for which approximately 20 enrolled patients were required, of whom 16 had to complete the study (Citation[23]).
RESULTS
Patients
Samples were obtained from 22 patients with mild-to-severe COPD (FEV1 34%–98% of predicted) with minimal reversibility (mean 4.7% of predicted). The detailed patient characteristics are shown in . A total of 154 induced sputum samples were analyzed (71 sample pairs and 12 single assessments). The IS procedure was well tolerated. Twenty-one of the 154 samples (14%), affecting 16 of the 71 sample pairs, contained more than 80% squamous epithelial cells or contained a surplus of indiscernible cells. These sample pairs were excluded, leaving 55 paired samples from 21 patients for analyses investigating the possible temporal effects of the first IS procedure. Only the first set of paired samples, obtained from 21 patients (separated by a mean 2.5 days, range 1–7), was used for the analysis of short-term variability and repeatability (9 after corticosteroid wash-out, 7 after inhaled placebo, 4 after inhaled budesonide and 1 after oral prednisolone). In 12 patients, samples were available after both 3 and 6 months of inhaled placebo treatment for the analysis of long-term variability and repeatability. Mean FEV1 at commencing the induced sputum procedure did not differ significantly between study conditions.
Table 1 Demographic and baseline data of patients at enrolment into the study
Short-term variability and repeatability of differential cell counts
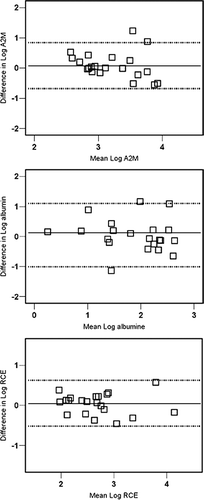
Within-patient variability for Total Cell Count (TCC), non-squamous TCC, absolute neutrophil count and % eosinophil count was approximately 0.3 in the log-transformed data (equivalent with a 2-fold difference, ). Eosinophil counts in 103/g sputum showed the largest mean within-patient variability: 0.5 in log-transformed data, which is equivalent to a 3-fold difference. The between-patients variability of cell counts was approximately twice as high as the within-patients variability and was maximally 1000-fold for the cell counts expressed in 103/g sputum. The repeatability is shown graphically in Bland and Altman plots in . Repeatability, expressed as the intraclass correlation coefficients (Ri) ranged from 0.14 to 0.72, with the lowest values for % lymphocytes and the highest for % eosinophils. Repeatability for differential cell counts was better than that for cell counts per gram sputum. Ri values for TCC and for % neutrophils, eosinophils, macrophages and monocytes were all above the value of 0.6 indicating good repeatability(15) ().
Figure 1 Bland and Altman plots for (a) non-squamous Total Cell Count (106 cells/g sputum), (b) neutrophil count (%) and (c) eosinophil count (%) assessed in 2 induced sputum samples collected with a 1-to 7-day interval. Log-transformed data are shown for Total Cell Count and % eosinophil count and the lines represent the mean difference ± 2 SD.
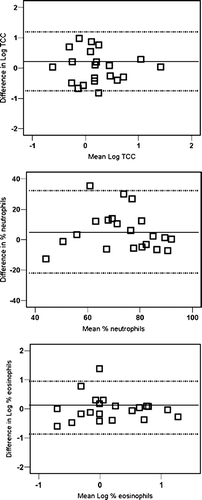
Table 2 Repeatability and variability of inflammatory parameters in two consecutive sputum samples, collected with a 1-to 7-day interval
Short-term variability and repeatability of soluble markers
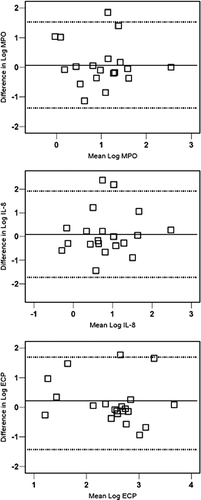
Within-patient variability of permeability parameters was approximately 1.5-to 2-fold (0.2–0.3 in log-transformed data), showing the smallest variability for RCE (). For the cell activation markers ECP and MPO the variability was approximately 3-fold (0.4–0.5 in log-transformed data). The Bland and Altman plots are shown in and . Mean between-patients variability was approximately twice as large as the mean within-patients variability (). Ri-values for soluble parameters ranged from 0.34 to 0.70 with parameters of permeability having the highest Ri values. ().
Short-term repeatability under different study conditions
Repeatability, expressed as the intraclass correlation coefficient, of the cellular and soluble parameters under the 4 different circumstances and treatments: the corticosteroid-free wash-out (n = 17 sets with paired data), oral prednisolone treatment (n = 15), inhaled budesonide treatment (n = 10), and inhaled placebo treatment (n = 13), did not differ significantly (p = 0.84). Short-term repeatability was statistically significantly better in the 12 patients who also provided data for the long-term repeatability than in the 9 patients who were withdrawn from the parent study, mainly due to COPD exacerbations (p = 0.033).
Long-term variability and repeatability
Within-patient variability was somewhat larger with a 3-month interval than with a 1-week interval for cell differentials but similar or smaller for TCC and soluble markers (). Repeatability was quite similar on the long term as on the short term (). Ri-values for TCC, % eosinophil count and for soluble parameters (except ECP) were acceptable (above 0.60) but Ri values were below 0.60 for other differential cell counts.
Table 3 Repeatability and variability of inflammatory parameters in two consecutive sputum samples, collected with a 3-month interval
Effect of the first induced sputum sampling on the parameters in the second sample
Numerically there were small differences in the levels for most parameters in the 2 subsequent IS samples (). Only the slight decrease in % macrophages (from 16.5% in the first to 14.0% in the second sample) reached statistically significance (p < 0.05). There was a tendency for a parallel increase in % neutrophils (from 76.6% to 79.6%, p = 0.067) and in % eosinophils (from 0.8% to 1.2%, p = 0.068).
Table 4 Effect of obtaining induced sputum on inflammatory parameters in a second sputum sample, collected with a 1-to 7-day interval
There was no detectable relation between the duration of the interval between sputum sampling and the difference in levels of cellular or soluble parameters from the first to the second of the duplicate samples. There was also no detectable influence of the type of treatment in between the two paired sampling days and the differences in inflammatory parameters from the first to the second sampling (all p > 0.5).
DISCUSSION
The present study documents the short-term and long-term repeatability of inflammatory parameters in induced whole sputum samples from clinically stable COPD patients with a wide range of disease severity. The intra-class correlation coefficients (Ri, the measure of repeatability) ranged from 0.14 to 0.90 on the short-term, indicative of repeatability ranging from rather poor to very good. Total cell count, % neutrophils, % eosinophils and the soluble parameters for permeability were most repeatable having the lowest measurement errors for consecutive samples collected within a one week's interval. A prerequisite for interpreting these results was that the induced sputum procedure itself had no effect on the results of the second induced sputum test. An important secondary observation from our study was therefore that the IS procedure in patients with COPD induced very limited and clinically irrelevant changes in the cell numbers and levels of inflammatory markers, hereby allowing repeated sampling within one week while monitoring COPD. Additionally, in a subset of patients in an unchanged clinical condition under placebo treatment, the long-term repeatability and variability was assessed with a 3 months interval between sampling. This resulted in similar repeatability and somewhat larger variability compared to the short-term variability.
Short-term repeatability of differential cell counts in the present study was similar to that, determined in two previous studies in COPD using the selected plugs methodology (Citation[20], Citation[21]). Additionally, the treatment which was given in between the duplicate samplings did not significantly affect repeatability. This suggests that the whole sputum methodology can be used as an alternative for the more laborious selected sputum plugs methodology. It must be noted, that Beeh et al. did not use log-transformed cell counts, while in the present study both absolute cell counts and % eosinophils required logarithmic transformation to become normally distributed, as is required for the calculation of repeatability (Citation[15]).
A poor repeatability of % lymphocyte count was observed for both their and our study and may be attributed to the low numbers of these cells and difficulties in accurately identifying lymphocytes. Repeatability of A2M and Alb, as markers of permeability, was high on the short-term, whereas repeatability of soluble cell activation markers like IL-8 and ECP (but not MPO) was less satisfactory. Repeatability of IL-8 and ECP was somewhat lower than that previously reported using the selected plug method. Still, the similar outcomes with the two approaches, whole sputum versus selected plugs, indicate that the method of IS work-up is not critical for the repeatability of most parameters.
A study specifically designed to assess repeatability applying more strictly standardized conditions is likely to have shown improved repeatability. For example, by using a fixed number of days between the subsequent samplings and by continuation of exposure to three periods of 5 minutes of nebulized saline in all patients irrespective of the volume of sputum obtained. The latter aspect in the induced sputum procedure was recommended after the start of the present study and within the present long-term study methodology was kept constant (Citation[11]). The variable duration of the exposure to saline is probably of lesser importance for within-patients comparisons than for between-patients comparisons since individual patients in the present study usually produced a satisfactory quantity of sputum after a similar duration of exposure to saline.
The withdrawal of patients from the parent study (mainly due to COPD exacerbations) may have lead to selective withdrawal of the most instable patients and hereby to exclusion of data with largest variability in the analysis of long-term repeatability. In an explorative analysis it was shown that in the subgroup of 12 patients with more stable disease (defined as those who completed the 6 months' inhaled placebo treatment period) short-term repeatability was better than in the 9 patients who were later withdrawn from the study. This indicates that clinically instable patients have also more fluctuating airway inflammation. This also suggests that selective withdrawal may affect sample size planning of future COPD studies.
Despite these potential methodological shortcomings, our study indicates good repeatability. A 2-fold to 3-fold variation was found for most parameters, which must be attributed to biological intra-individual variation as assay variability was below 10% for all variables. The observed variability data can be used for power calculations of studies evaluating sputum data of COPD patients, using the formula n = 16 × (SD2/Dif2) per group in which SD is the standard deviation of the variability and Dif the difference to be expected between groups or treatments (Citation[31]). The within-patient variability is approximately 2-fold for TCC, neutrophil cell count and permeability and approximately 3-fold for cell activation markers. Therefore, a theoretical cross-over study in COPD patients investigating an intervention capable of inducing a 2-fold reduction or increase of a certain parameter in sputum would necessitate a sample size of 16 to 36 patients.
Obviously, a small surplus of patients must be enrolled to compensate for withdrawal of patients and to compensate for the exclusion of samples with a high % squamous cells. In our study this comprised 14% of the samples. Taking into consideration that the between-patients variability is approximately twice as high as the within-patient variability, parallel and cross-sectional studies will require larger numbers of patients, as do interventions with a smaller effect than 2-fold. Though cell differential showed somewhat larger variability on the long term than on the short term, other parameters showed the same or even smaller variability on the long term.
A secondary objective of the present study was to investigate the potential influence of performing an IS test on the parameters assessed in a second IS test. Obviously, this was a prerequisite for the primary aim of investigating short-term repeatability. The results indicate that there was a small reduction in percentage of macrophages, and a trend for an increase in the percentage of neutrophils and eosinophils in response to a previous IS procedure. Numerically, the overall effects were rather limited and no detectable influence was observed of the length of the interval. Additionally, whether or not a corticosteroid treatment was given in between the two paired sampling days did not affect the outcome. Previous studies into repeated IS procedures in asthmatics or healthy subjects showed larger effects of one or a few days' interval with an increase in % neutrophils and % eosinophils and ECP (Citation[17], Citation[18], Citation[19], Citation[32]).
These effects were larger than those observed in the present study, which is most likely due to the generally longer interval between successive IS procedures in the present study and high % neutrophils in the first IS test, leaving little room for further increase. No difference in neutrophil counts was seen when asthmatics were tested twice with 1 week-interval (Citation[33]), or in COPD patients with a 2-week or a 4-week interval between successive IS tests (Citation[20], Citation[21]). In a recent study in healthy smokers an increase in neutrophils after a sputum induction was observed lasting one day and an increase in eosinophils was observed lasting 2 days (Citation[32]). Taken together, these results suggest that prior induction of sputum has (if any) a small and short-lasting effect on the outcomes of a second IS sample in patients with COPD as included in the present study.
In conclusion, repeatability of the measurement of cell counts and soluble inflammatory parameters in IS of patients with COPD is generally acceptable to good when using the whole sputum sample methodology. Prior sputum induction does not, or only minimally influences inflammatory parameters, hereby allowing repeated sputum sampling within 1 week. The current findings provide values to size future studies with induced sputum in COPD to investigate airway inflammation and to quantify the effects of therapeutic interventions.
We gratefully acknowledge P. Teiwes, who performed most of the lung function tests and the collection of induced sputum. The statistical advice of B.A. Hutten and P.P.H.M. Gobbens is greatly appreciated. The study was supported by an unrestricted research grant from AstraZeneca, The Netherlands under study number BN-00P-0087. Part of the present data was presented at the ERS congress in Glasgow, September 2004.
REFERENCES
- Pin I, Gibson P G, Kolendowicz R, Girgis-Gabardo A, Denburg J A, Hargreave F E, Dolovich J. Use of induced sputum cell counts to investigate airway inflammation in asthma. Thorax 1992; 47: 25–29
- Pizzichini E, Pizzichini M MM, Efthimiadis A, Evans S, Morris M M, Squillace D, Gleich G J, Dolovich J, Hargreave F E. Indices of airway inflammation in induced sputum: reproducibility and validity of cell and fluid-phase measurements. Am J Respir Crit Care Med 1996; 154: 308–317
- Fahy J V, Wong H, Liu J, Boushey H A. Comparison of samples collected by sputum induction and by bronchoscopy from asthmatics and healthy subjects. Am J Respir Crit Care Med 1995; 152: 53–58
- Wilson A, Leigh R, Hargreave F, Pizzichini M, Pizzichini E. Safety of sputum induction in moderate-to-severe smoking-related Chronic Obstructive Pulmonary Disease. COPD: Journal of Chronic Obstructive Pulmonary Disease 2006; 3: 89–93
- Green R H, Brightling C E, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw A J, Pavord I D. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 2002; 360: 1715–1721
- Jayaram L, Pizzichini M M, Cook R J, Boulet L P, Lemiere C, Pizzichini E, Cartier A, Hussack P, Goldsmith C H, Laviolette M, Parameswaran K, Hargreave F E. Determining asthma treatment by monitoring sputum cell counts: effect on exacerbations. Eur Respir J 2006; 27: 483–494
- Bacci E, Cianchetti S, Bartoli M L, Dente F L. Di Franco A Vagaggini B Paggiaro P. Low sputum eosinophils predict the lack of response to beclomethasone in symptomatic asthmatic patients. Chest 2006; 129: 565–572
- Brightling C E, Monteiro W, Ward R, Parker D, Morgan M DL, Wardlaw A J, Pavord I D. Sputum eosinophilia and short term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2000; 356: 1480–1485
- Brightling C E, McKenna S, Hargadon B, Birring S, Green R, Siva R, Berry M, Parker D, Monteiro W, Pavord I D, Bradding P. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 2005; 60: 193–198
- Leigh R, Pizzichini M MM, Morris M M, Maltais F, Hargreave F E, Pizzichini E. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J 2006; 27: 964–971
- Paggiaro P L. Sputum induction. Eur Respir J 2002; 20: 3–8
- Holz O, Kips J C, Magnussen H. Update on sputum methodology. Eur Respir J 2000; 16: 355–359
- Efthimiadis A, Spanevello A. Methods of sputum processing for cell counts, immunocytochemistry and in situ hybridisation. Eur Respir J 2002; 20: 19s–23s
- Kips J C. The use of induced sputum in clinical trials. Eur Respir J 2002; 20: 47s–50s
- Chinn S. Repeatability and method comparison. Thorax 1991; 46: 454–456
- Bland J M, Altman D G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; i: 307–310
- Holz O, Richter K, Jorres R A, Speckin P, Mucke M, Magnussen H. Changes in sputum composition between two inductions performed on consecutive days. Thorax 1998; 53: 83–86
- Nightingale J A, Rogers D F, Barnes P J. Effect of repeated sputum induction on cell counts in normal volunteers. Thorax 1998; 53: 87–90
- In't Veen J CCM, de Gouw H WFM, Smits H H, Sont J K, Hiemstra P S, Sterk P J, Bel E H. Repeatability of cellular and soluble markers of inflammation in induced sputum from patients with asthma. Eur Respir J 1996; 9: 2441–2447
- Brightling C E, Monterio W, Green R H, Parker D, Morgan M DL, Wardlaw A J, Pavord I D. Induced sputum and other outcome measures in chronic obstructive pulmonary disease: safety and repeatability. Respir Med 2001; 95: 999–1002
- Beeh K M, Beier J, Kornmann O, Mander A, Buhl R. Long-term repeatability of induced sputum cells and inflammatory markers in stable, moderately severe COPD. Chest 2003; 123: 778–783
- Pauwels R A, Buist A S, Calverley P MA, Jenkins C R, Hurd S S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163: 1256–1276
- Boorsma M, Lutter R, van de Pol M A, Jansen H M, Jonkers R E. Inhaled budesonide suppresses airway and systemic eosinophilic inflammation in COPD. No predictive value of a prednisolone test treatment[Abstract]. Eur Respir J 2005; 26: 290s
- Fabbri L M, Hurd S S. Global strategy for the diagnosis, management and prevention of COPD: 2003 update. Eur Respir J 2003; 22: 1–2
- Quanjer P hH, Tammeling G J, Cotes J E, Pedersen O F, Yernault J C. Lung volumes and forced ventilatory flows. Report working party: Standardisation of lung function tests European Community for steel and coal. Official statement of the European Respiratory Society. Eur Respir J 1993; 6: 5–40
- Nocker R ET, Out T A, Weller F R, de Riemer M J, Jansen H M, van der Zee J S. Induced sputum and bronchoalveolar lavage as tools for evaluating the effects of inhaled corticosteroids in patients with asthma. J Lab Clin Med 2000; 136: 39–49
- Reimert C M, Venge P, Kharazmi A, Bendtzen K. Detection of eosinophilic cationic protein (ECP) by an enzyme-linked immunosorbent assay. J Immunol Meth 1991; 138: 285–290
- Bresser P, Out T A, van Alphen L, Jansen H M, Lutter R. Airway inflammation in nonobstructive and obstructive chronic bronchitis with chronic Haemophilus influenzae airway infection. Am J Respir Crit Care Med 2000; 162: 947–952
- Out T A, Jansen H M, van Steenwijk R P, de Nooijer M J, van de Graaf E A, Zuijderhoudt F MJ. ELISA of ceruloplasmin and alpha-2-macroglobulin in paired bronchoalveolar lavage fluid and serum samples. Clin Chim Acta 1987; 165: 277–288
- Fahy J V, Boushey H A, Lazarus S C, Mauger E A, Cherniack R M, Chinchilli V M, Craig T J, Drazen J M, Ford J G, Fish J E, Israel E, Kraft M, Lemanske R F, Martin R J, McLean D, Peters S P, Sorkness C, Szefler S J. Safety and reproducibilty of sputum induction in asthmatic subjects in a multicentre study. Am J Respir Crit Care Med 2001; 163: 1470–1475
- Swinscow T DV. Statistics at Square One (Ed 9). BMJ publishing group 1997
- van der Vaart H, Postma D S, Timens W, Kauffman H F, Hylkema M N, ten Hacken N HT. Repeated sputum inductions induce a transient neutrophilic and eosinophilic response. Chest 2006; 130: 1157–1164
- Bacci E, Cianchetti S, Carnevalli S, Bartoli M L, Dente F L, Di Franco A, Giannini D, Vagaggini B, Paggiaro P L. Induced sputum is a reproducible method to assess airway inflammation in asthma. Mediators Inflamm 2002; 11: 293–298