Abstract
Subjects with severe chronic obstructive pulmonary disease (COPD) may have marked differences in emphysema severity on chest computed tomography (CT) scans. Although many patients with severe COPD will have chest CTs performed during their clinical care, chest CTs have not been widely included in epidemiologic and genetic studies of COPD. We sought to determine whether chest CT scans performed for clinical indications can provide useful data in an epidemiologic study of COPD and to determine whether chest CT scans can be used to define subtypes of severe, early-onset COPD. Clinical chest CT scans on 91 probands in the Boston Early-Onset COPD Study were retrospectively reviewed by 2 pulmonologists and 1 to 2 chest radiologists, using a semi-quantitative emphysema severity score, ranging from 0–24. 88 of 91 chest CT scans were suitable for emphysema analysis. There was a wide range of emphysema severity, from mild to severe (1.3–23.7). Emphysema-predominant subjects (upper 3 quartiles of emphysema scores) had more severe airflow obstruction than airway-predominant subjects (lowest quartile of emphysema scores): FEV1 17.4% vs. 22.4% predicted, p = 0.009. A higher percentage of airway-predominant subjects had a positive bronchodilator response (28.6% vs. 6.7%, p = 0.009). Airway-predominant subjects also had a higher frequency of physician-diagnosed asthma (p = 0.04) and a trend towards higher serum immunoglobulin E levels (p = 0.09). Analysis of siblings of early-onset COPD probands suggested a genetic contribution to the subgroups. Using clinical chest CT scans, we were able to identify an airway-predominant subgroup with asthma-like features among subjects with severe, early-onset COPD.
Key words: :
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a heterogeneous syndrome, with variable contributions from emphysema, chronic bronchitis, and small airway disease occurring in each patient. Spirometric measures, such as the forced expiratory volume in 1 second (FEV1), have traditionally been used to categorize COPD severity (1). Spirometry captures airflow obstruction due to the combination of processes listed above, but fails to reflect the disease heterogeneity. Chest computed tomography (CT) scans can help to delineate the anatomic processes that lead to airflow obstruction (). Despite the importance of accurate phenotypic characterization for COPD research, epidemiologic and genetic studies of COPD have not routinely included chest CT scans. Several factors have likely hindered the widespread acquisition of chest CT scans in large observational studies of COPD, including cost, availability, and difficulty with standardization of imaging across multiple centers (Citation[2], Citation[3]). However, many patients with severe COPD will have chest CT scans ordered for a variety of indications as part of their clinical care, such as evaluation for lung volume reduction surgery (LVRS) or lung transplantation, work-up of lung nodules, or assessment for bronchiectasis (Citation[4]).
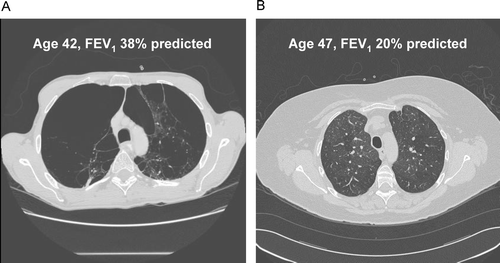
Figure 1 Computed tomography (CT) scans from two participants in the Boston Early-Onset COPD Study, demonstrating the disconnect between emphysema severity on CT scans and lung function testing in patients with COPD. Subject A has severe emphysema yet higher lung function (emphysema severity score = 21.7; FEV1 = 38% predicted) than Subject B, who has minimal emphysema and lower lung function (emphysema severity score = 4.0; FEV1 = 20% predicted). The mosaic attenuation in Subject B is consistent with airways disease as a cause for the severe airflow obstruction.
Since 1994, the Boston Early-Onset COPD Study has been recruiting probands with severe chronic airflow obstruction at a young age and their relatives in order to study the genetic epidemiology of COPD (5). Many of these patients have been ascertained through the LVRS and lung transplant programs at Brigham and Women's Hospital (BWH) and Massachusetts General Hospital (MGH), and they have had chest CT scans performed during their evaluations; other probands have had chest CT scans ordered at a variety of other institutions for clinical indications. This cohort of severe COPD patients is an ideal population in which to test the following two hypotheses: (Citation[1]) Chest CT scans performed for clinical indications can provide useful, semi-quantitative emphysema data in an epidemiologic study of COPD, and (Citation[2]) Standardized grading of emphysema on clinical chest CT scans can be used to define subtypes of severe, early-onset COPD.
METHODS
Study subjects
Patients with severe, early-onset COPD were recruited primarily through the lung transplant and LVRS programs at BWH and MGH and through outpatient pulmonary practices at these hospitals and elsewhere (Citation[5]). Inclusion criteria included physician-diagnosed COPD with an FEV1 less than 40% predicted at an age less than 53 years. Exclusion criteria included severe alpha1-antitrypsin deficiency (e.g., PiZ, PiSZ) or a diagnosis of other significant lung diseases (e.g., interstitial lung disease, lung cancer other than a solitary pulmonary nodule) or other disease processes that may affect pulmonary function tests (e.g., neuromuscular weakness). All available first-degree relatives and older second-degree relatives were invited to participate. After written informed consent, subjects completed a study questionnaire (Citation[6]), spirometry before and after inhaled bronchodilator (performed according to American Thoracic Society (ATS) recommendations (Citation[7])), and a blood draw for DNA extraction and measurement of serum Immunoglobulin E (IgE) levels, in a subset of families (Citation[8]). The study was approved by the Institutional Review Board of Partners Healthcare.
Radiographic analysis
Chest CT scans were not required for inclusion into the Boston Early-Onset COPD Study, but 91 probands had available chest CTs which had been ordered for clinical indications at the discretion of their local physicians. Copies of chest CT scans were retrieved from hospital film libraries. For subjects with multiple chest CTs, the scan performed most proximal to study enrollment was used. Pre-operative studies were collected for those subjects who had undergone LVRS or lung transplantation.
All chest CT scans were read independently by one chest radiologist and two pulmonary physicians. Inspiratory images were read on hardcopy film, using available lung windows (e.g., width -1500 to -2000 HU, level 500 to 600 HU). Readers were aware that all patients had severe COPD, but were blinded to other clinical data. Emphysema severity scores were determined using a modification of the scoring system from the National Emphysema Treatment Trial (9–11), assigning a score from 0–4 for the upper (apex to aortic arch), mid (aortic arch to right inferior pulmonary vein), and lower (right inferior pulmonary vein to diaphragm) portions of each lung (). We used a score of 0.5 to denote trivial emphysema (< 5% lung affected). Scores across the 6 regions were totaled for a maximum possible score of 24. All readers had been uniformly trained to use this scoring system.
Table 1 Emphysema severity scale
Total scores were averaged across 3 readers for analysis. Substantial discrepancies were defined by more than 6 points separating the highest and lowest total score among the 3 readers. Discrepancies were resolved in one of two ways. (Citation[1]) If one reader's score was a clear outlier (≥ 6 points from the other 2 scores), then that score was dropped, and the other two readers' scores were averaged together. (Citation[2]) If there was no clear outlier, then the highest and lowest scores were dropped; the CT scan was read by an additional chest radiologist and this score was averaged with the remaining initial score (the middle score).
Statistical analysis
Differences between groups of probands were compared using t-tests and linear regression for continuous outcomes and contingency table analysis and logistic regression for binary outcomes. Regression models were adjusted for age, sex, and pack-years of smoking. Survival analysis was performed with the Kaplan–Meier method and Cox proportional hazards regression (Citation[12]). Differences between siblings of probands were compared using mixed models for continuous outcomes and generalized estimating equations for binary outcomes, in order to account for correlation among multiple siblings in a family (Citation[13]). Statistical analyses were performed with SAS 9.1 (Cary, NC).
RESULTS
CT scan quality
Of the 160 severe, early-onset COPD probands enrolled as of August 1, 2004, 91 had a chest CT scan available for interpretation. CT scans were performed between May 1993 and May 2004 at a total of 11 different institutions. The majority of the studies were performed at BWH (N = 66) or MGH (N = 16). Three of the 91 CTs were unusable for analysis; 2 were missing images and 1 had poor image quality. CT scans were performed a median of 20 days prior to spirometry, but there was substantial variability in the timing (interquartile range 13 months). Of the 88 CTs used in the analysis, 3 had only thick section (10 mm) cuts. The remaining CTs included at least 3 high resolution (1–1.25 mm) images. Of the 88 CT scans, 40 had substantial discrepancies between the 3 readers. For 26 scans, there was one outlier reading that could be removed. For 14 scans, review by the second chest radiologist was required.
CT phenotypes in severe, early-onset COPD
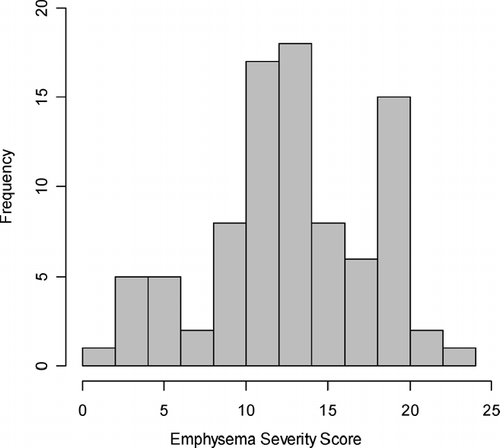
The average emphysema severity score across all 88 probands was 13.0. Scores ranged from 1.3, representing mild emphysema, to 23.7, representing severe emphysema (). For subsequent statistical analyses, subjects were divided into two groups. Subjects within the upper three quartiles of emphysema severity score (average scores > 10.25) were considered to have emphysema-predominant disease. Subjects in the lowest quartile were considered airway-predominant. Since most subjects had at least moderate emphysema, we chose to include the top three quartiles in the emphysema-predominant group, as opposed to dividing subjects in half along the median of emphysema severity scores.
Figure 2 Distribution of computed tomography emphysema severity scores in the Boston Early-Onset COPD Study.
shows the comparison between the emphysema-predominant and airway-predominant probands. Emphysema-predominant subjects had significantly lower values of FEV1, both pre- and post-bronchodilator, compared to airway-predominant subjects; there was no difference in FEV1/FVC ratio between the two groups. All subjects were classified in GOLD stages 3 to 4 based on their FEV1 values, though post-bronchodilator spirometry was not available in all subjects. Emphysema-predominant subjects tended to have a greater smoking history. The two groups were similar in terms of age, sex, and symptoms of chronic bronchitis and COPD exacerbations.
Table 2 Differences between emphysema-predominant (N = 66) and airway-predominant (N = 22) probands in the Boston Early-Onset COPD Study
There were significant differences in asthma-related phenotypes between the airway-predominant and emphysema-predominant groups (). The airway-predominant probands had significantly greater bronchodilator responsiveness, whether defined in absolute volume, as a percent of predicted FEV1, or as a binary outcome according to the ATS criteria of a change in FEV1 of at least 200 ml and at least 12% of baseline (Citation[14]). Serum IgE levels were measured in a subset of patients, and the airway-predominant subjects had a trend towards higher levels. Significantly more probands in the airway-predominant group had a history of physician-diagnosed asthma; a slightly greater percentage had a history of attacks of wheezing, though the difference was not statistically significant.
Table 3 Asthma-related phenotypes in emphysema-predominant (N = 66) and airway-predominant (N = 22) probands in the Boston Early-Onset COPD Study.
In a previous publication, we had ascertained the vital status as of November 1, 2002 for probands enrolled prior to that date (Citation[12]). Survival data was available on 82 of the 88 subjects in the current report. There were 21 deaths in the 82 probands, with a median survival of 7.0 years, similar to the results for all 139 probands in the previous paper. There was no difference in survival between the emphysema-predominant and the airway-predominant subjects, using Cox proportional hazards models adjusted for age, sex, and pack-years of smoking.
Siblings of early-onset COPD probands
The Boston Early-Onset COPD Study was designed to study the genetic epidemiology of COPD. In order to assess for potential genetic contributions to the CT scan phenotypes, we analyzed characteristics of the 144 siblings of the early-onset COPD patients; clinical chest CT scans were not available for the siblings. Compared to siblings of the emphysema-predominant probands, siblings of the airway-predominant probands showed trends towards lower risk of airflow obstruction (Odds Ratio [OR] 0.33, 95% Confidence Interval [CI] 0.09, 1.16) and higher risks of bronchodilator response as a binary trait based on the ATS definition (OR 3.47, 95% CI 0.90, 13.38) and physician diagnosed asthma (OR 2.84, 95% CI 0.92, 8.76). Siblings of the airway-predominant probands also had higher (log10) IgE levels than siblings of the emphysema-predominant probands (1.67 vs. 1.38, p = 0.048). There was no difference in spirometry or bronchodilator response measured a quantitative trait. Effect estimates were similar in an analysis restricted to siblings who were current or former smokers.
DISCUSSION
In a retrospective analysis of chest CT scans on probands in the Boston Early-Onset COPD Study, we demonstrated that almost all of the clinically ordered CT scans could provide suitable semi-quantitative emphysema data for use in a genetic epidemiology study of COPD. We found a wide distribution in emphysema severity among this cohort of patients who all had severe chronic airflow obstruction. Based on CT emphysema scores, we were able to define an emphysema-predominant subtype and an airway-predominant subtype. The emphysema-predominant subjects had more severe airflow obstruction, while the airway-predominant subjects had more asthma-like characteristics, such as increased bronchodilator responsiveness and higher serum IgE levels. Analysis of siblings suggested a genetic contribution to these subgroups, based on similarities in asthma-like features.
Previous authors have demonstrated correlations between semi-quantitative emphysema severity scores and measures of airflow obstruction (Citation[10], Citation[15], Citation[16]). In the present study we found a similar association; the group of subjects with higher emphysema severity scores, the emphysema-predominant group, had a lower average FEV1 than the subjects with less emphysema. Similar to our study, Boschetto et al. found lower FEV1 values in 12 patients with emphysema on HRCT compared to 12 patients without emphysema, among patients with airflow obstruction (FEV1 < 70% of predicted, FEV1/FVC < 0.7) (Citation[17]). However, the CT scans used in several previous studies, including the study by Boschetto, were prospectively obtained, at a single institution on the same CT scanner for all subjects in each study (Citation[10], Citation[16], Citation[17]). In one study, CTs were retrospectively reviewed; Sanders and colleagues reviewed CT scans performed on a single scanner at a single institution over 15 months, screening 568 patient records to find 60 eligible study subjects (Citation[15]). We were able to obtain similar information from a retrospective review of CT scans performed over 11 years at multiple institutions. CT scans were available for 91 of 160 probands, and data were useable in 88 of the 91 CT scans available.
Although chest CT scans are not routinely recommended in the evaluation and management of COPD (Citation[1]), many patients with severe COPD will have a chest CT performed as part of their clinical care, including evaluations for LVRS and lung transplantation (Citation[18], Citation[19]). CT screening for lung cancer in smokers, regardless of airflow obstruction, is also a potential indication for CT scans in patients with varying severity of COPD (Citation[20]). Prospective collection of CT data in epidemiologic studies and clinical trials of COPD will allow for computerized image analysis and longitudinal follow-up (Citation[21]). For studies in which prospective CT scanning is not possible, we have demonstrated that retrospective review of clinical CT scans can still provide some useful information.
In the Boston Early-Onset COPD Study probands, we were able to identify a wide range of emphysema severity, from minimal to severe. Based on the quartile distribution, we defined an emphysema-predominant group and a group with less emphysema, which we termed the airway-predominant group. We were not able to quantitatively assess the small airways in the retrospective review of CT scans, though we assume that the probands without significant emphysema must have substantial airway disease as the cause of their severe chronic airflow obstruction, since emphysema and airway disease are the two major processes that lead to chronic airflow obstruction. Airway disease has been more challenging to assess than emphysema, but airway wall thickness can be measured as a continuous variable using computerized image analysis (Citation[22]). However, we could not perform a quantitative airway analysis on our hardcopy films. Therefore, our arbitrary distinction will miss the potential overlap of emphysema and airway disease within COPD subjects.
Nonetheless, we were able to distinguish significant differences between the emphysema-predominant and airway-predominant subjects. The airway-predominant probands tended to have an asthma-like phenotype, with greater bronchodilator responsiveness, higher IgE levels, and more frequent history of physician-diagnosed asthma, with the latter trait being more subjective than the former two. This is consistent with the Dutch hypothesis, which proposes a common origin for asthma and COPD; host factors of atopy and airway hyperresponsiveness are postulated to predispose an individual to chronic airflow obstruction (Citation[23]). However, one should not conclude that the airway-predominant subjects have asthma, as it is usually defined in clinical practice. The airway-predominant patients still have very severe chronic airflow obstruction, even post-bronchodilator, and almost all have a history of cigarette smoking. In addition, their mortality rate is similar to the emphysema-predominant patients. The median survival of 7 years in our study, in both subgroups, was substantially shorter than the median survival of 17.7 years in 279 asthma patients of similar ages in a Danish study, but more similar to the median survival of 8.5 years in 869 patients with COPD (Citation[24]).
There was a suggestion of a genetic contribution to the radiographic phenotypes in severe, early-onset COPD. Our group has previously demonstrated a genetic effect on bronchodilator responsiveness in the Boston Early-Onset COPD Study (8); current and former smoking first-degree relatives of the early-onset COPD probands had greater bronchodilator responsiveness than control subjects. However, serum IgE levels did not show a similar genetic effect when the entire cohort was considered, compared to the present analysis of CT subgroups. In a sibling-pair study of COPD, Coxson and colleagues found significant genetic contributions to several quantitative measures of emphysema based on high-resolution CT scans (Citation[25]). Without CT scans on the siblings, we were not able to perform a similar heritability analysis for the semi-quantitative emphysema severity score.
Besides the availability of clinical CT scans only on the early-onset COPD probands, our study has additional limitations. Only 91 of the 160 probands in the Boston Early-Onset COPD Study had CT scans available for analysis, leading to limited power to detect differences between the CT scan subgroups. The high number of discordant readings in our study may be partially due to the substantial variability in CT scans that were available. CTs were performed on different scanners in different institutions using different protocols, and the films were presented in various formats, with varying numbers of high-resolution images. However, the use of multiple readers and a formula for resolving discordances allowed us to extract semi-quantitative emphysema data for epidemiologic analysis. Measurement of carbon monoxide diffusing capacity (DLCO) might also be expected to correlate with emphysema severity. Since the majority of subjects in the Boston Early-Onset COPD Study were enrolled during a home visit, DLCO measurement was not available.
Clearly, prospectively obtained, standardized high-resolution CT scans would be ideal to delineate and quantify COPD-related phenotypes. However, the retrospective review of clinically obtained CT scans allowed for the identification of subgroups within a cohort of patients with severe, early-onset COPD; these subgroups may have a genetic contribution. Future epidemiologic, genetic, and clinical studies of COPD might benefit from the use of more precise phenotypes, based on chest CT scans, in order to disentangle the heterogeneous syndrome of COPD.
Supported by U.S. National Institutes of Health grants HL080242 (CPH), HL71393 (EKS), HL68926 (EKS), and HL075478 (EKS), a grant from the Alpha-1 Foundation (CPH), an American Lung Association Career Investigator Award (EKS), and a Clinical Innovator Award from the Flight Attendant Medical Research Institute (FLJ).
The authors thank Laura Kaufman for her assistance in cataloging the CT films and Scott Weiss, Frank Speizer, John Reilly, Jeffrey Drazen, Hal Chapman, Leo Ginns and Steve Mentzer for their roles in developing the Boston Early-Onset COPD Study.
REFERENCES
- Pauwels R A, Buist A S, Calverley P M, Jenkins C R, Hurd S S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001; 163: 1256–1276
- Cosio M G, Snider G L. Chest computed tomography: is it ready for major studies of chronic obstructive pulmonary disease?. Eur Respir J 2001; 17: 1062–1064
- Stoel B C, Stolk J. Optimization and standardization of lung densitometry in the assessment of pulmonary emphysema. Invest Radiol 2004; 39: 681–688
- Lynch D A, Menon P. Imaging of lung disease. Baum's Textbook of Pulmonary Diseases, 7th Ed., J D Crapo, J Glassroth, J B Karlinsky, T E King. Lippincott Williams and Wilkins, Philadelphia 2004; 1–33
- Silverman E K, Chapman H A, Drazen J M, Weiss S T, Rosner B, Campbell E J, O'Donnell W J, Reilly J J, Ginns L, Mentzer S, Wain J, Speizer F E. Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis. Am J Respir Crit Care Med 1998; 157: 1770–1778
- Ferris B G. Epidemiology Standardization Project (American Thoracic Society). Am Rev Respir Dis 1978; 118: 1–120
- American Thoracic Society. Standardization of spirometry, 1994 Update. Am J Respir Crit Care Med 1995; 152: 1107–1136
- Celedon J C, Speizer F E, Drazen J M, Weiss S T, Campbell E J, Carey V J, Reilly J J, Ginns L, Silverman E K. Bronchodilator responsiveness and serum total IgE levels in families of probands with severe early-onset COPD. Eur Respir J 1999; 14: 1009–1014
- National Emphysema Treatment Trial Research Group. Patients at high risk of death after lung-volume-reduction surgery. N Engl J Med 2001; 345: 1075–1083
- Bergin C, Muller N, Nichols D M, Lillington G, Hogg J C, Mullen B, Grymaloski M R, Osborne S, Pare P D. The diagnosis of emphysema. A computed tomographic-pathologic correlation. Am Rev Respir Dis 1986; 133: 541–546
- Hersh C P, Washko G R, Jacobson F L, Gill R, San Jose Estepar R, Reilly J J, Silverman E K. Inter-observer variability in the determination of upper lobe predominant emphysema. Chest 2007; 131: 424–431
- Hersh C P, DeMeo D L, Al-Ansari E, Carey V J, Reilly J J, Ginns L C, Silverman E K. Predictors of survival in severe, early onset COPD. Chest 2004; 126: 1443–1451
- Fitzmaurice G M, Laird N M, Ware J H. Applied Longitudinal Analysis. John Wiley & Sons, Hoboken 2004
- American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis 1991; 144: 1202–1218
- Sanders C, Nath P H, Bailey W C. Detection of emphysema with computed tomography. Correlation with pulmonary function tests and chest radiography. Invest Radiol 1988; 23: 262–266
- Eda S, Kubo K, Fujimoto K, Matsuzawa Y, Sekiguchi M, Sakai F. The relations between expiratory chest CT using helical CT and pulmonary function tests in emphysema. Am J Respir Crit Care Med 1997; 155: 1290–1294
- Boschetto P, Miniati M, Miotto D, Braccioni F, De Rosa E, Bononi I, Papi A, Saetta M, Fabbri L M, Mapp C E. Predominant emphysema phenotype in chronic obstructive pulmonary disease patients. Eur Respir J 2003; 21: 450–454
- Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood D E. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348: 2059–2073
- Kazerooni E A, Chow L C, Whyte R I, Martinez F J, Lynch J P. Preoperative examination of lung transplant candidates: value of chest CT compared with chest radiography. AJR Am J Roentgenol 1995; 165: 1343–1348
- Henschke C I, Yankelevitz D F, Libby D M, Pasmantier M W, Smith J P, Miettinen O S. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med 2006; 355: 1763–1771
- Newell J D, Jr, Hogg J C, Snider G L. Report of a workshop: quantitative computed tomography scanning in longitudinal studies of emphysema. Eur Respir J 2004; 23: 769–775
- Nakano Y, Wong J C, de Jong P A, Buzatu L, Nagao T, Coxson H O, Elliott W M, Hogg J C, Pare P D. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med 2005; 171: 142–146
- Postma D S, Boezen H M. Rationale for the Dutch hypothesis. Allergy and airway hyperresponsiveness as genetic factors and their interaction with environment in the development of asthma and COPD. Chest 2004; 126: 96S–104S
- Ringbaek T, Seersholm N, Viskum K. Standardised mortality rates in females and males with COPD and asthma. Eur Respir J 2005; 25: 891–5
- Coxson H O, Lake S, Muller N L, Pare P D, Pillai S G, Anderson W H, Lomas D A, Silverman E K. Heritability of quantitative emphysema phenotypes in Chronic Obstructive Pulmonary Disease [abstract]. Proc Am Thorac Soc 2005; 2: A138
