Abstract
Background: Responsiveness to pharmacologic agents may differ among subpopulations compared with the general population. In patients of African descent, possible differences have been observed for inhaled beta-agonists. However, pharmacologic responsiveness to a long-acting anticholinergic has not been prospectively evaluated. Methods: An 8-week, randomized, placebo-controlled clinical trial was conducted to assess the efficacy of the once-daily, inhaled anticholinergic tiotropium in COPD patients of African descent. African-American COPD patients ≥40 years, FEV1 ≤ 65% predicted, FEV1/FVC ≤70% were included. Spirometry (pre-study drug, and 0.5, 1, 2 and 3 hours post-dose) and the University of California, San Diego Shortness of Breath Questionnaire (SOBQ) were performed at baseline and at 4 and 8 weeks. Data on use of rescue medication and on adverse events (including COPD exacerbations) were also collected. Results: Randomized patients (n = 166) were (mean ± SD) 62.5 ± 9.3 years; baseline mean FEV1 1.02 ± 0.37 L (41 ± 13% predicted); 67.5% were male. A total of 160 patients were eligible for efficacy evaluation. At 8 weeks, mean FEV1 AUC0 − 3 response was 180 mL greater with tiotropium (n = 78) than with placebo (n = 82), (p < 0.0001). Difference (tiotropium–placebo) for mean peak FEV1 response was 182 mL (p < 0.0001) and 122 mL (p = 0.002) for mean trough FEV1 response. There were no significant differences in SOBQ or use of rescue medication between the groups. No patients in the tiotropium group experienced a COPD exacerbation compared with 12 in patients receiving placebo. Conclusion: Tiotropium significantly improved pulmonary function in African-American COPD patients.
Key words: :
| ABBREVIATIONS | ||
| AUC0 − 3 | = | area under the curve from 0 to 3 hours |
| COPD | = | chronic obstructive pulmonary disease |
| FEV1 | = | forced expiratory volume in one second |
| FVC | = | forced vitalcapacity |
| L | = | liter |
| mL | = | milliliter |
| MRC | = | Medical Research Council |
| n | = | number |
| SD | = | standard deviation |
| SOBQ | = | Shortness of Breath Questionnaire |
| UCSD | = | University of California at San Diego |
INTRODUCTION
Epidemiological data suggest that patients of African descent have increased susceptibility to certain illnesses, especially those of the respiratory system (Citation[1]). In addition, potential differences in disease state and drug responsiveness have raised questions about whether outcomes of clinical trials performed in predominantly Caucasian populations can be generalized to subpopulations, particularly African Americans (Citation[2], Citation[3], Citation[4], Citation[5], Citation[6], Citation[7], Citation[8], Citation[9], Citation[10]).
Tiotropium (Spiriva, Boehringer Ingelheim GmbH & Co. KG, Ingelheim, Germany) is a once-daily, inhaled anticholinergic that provides sustained bronchodilation through prolonged M3-receptor blockade (Citation[11]). In previous randomized, controlled clinical trials, tiotropium 18 mcg daily was shown to improve lung function, dyspnea, and health-related quality-of-life, and to reduce the frequency of COPD exacerbations in COPD patients (Citation[12], Citation[13], Citation[14]).
The majority of participants in prior tiotropium investigations were Caucasian (Citation[12], Citation[13], Citation[14]). However, a subgroup analysis of a 6-month, multi-center clinical trial in COPD patients in the Veterans Affairs Medical System demonstrated improvements in lung function in the African American subgroup (n = 149) comparable to those seen in the total study population (n = 1,829) (Citation[15]). The purpose of the present study was to prospectively test the hypothesis that maintenance treatment with tiotropium results in significant improvements in lung function in African American COPD patients.
METHODS
Study design
The study was an 8-week, randomized, double-blind, placebo-controlled, parallel-group clinical trial prospectively designed to evaluate the efficacy of tiotropium in COPD patients of African descent in the United States. The primary efficacy outcome was average improvement in forced expiratory volume in 1 second over the first 3 hours (FEV1 AUC0 − 3) after drug administration after 8 weeks of treatment. Secondary efficacy outcomes included spirometric endpoints of trough and peak FEV1 and trough, peak, and average (over 3 hours) forced vital capacity (FVC AUC0 − 3). Use of rescue albuterol and dyspnea were also assessed as secondary outcomes.
Participants
Patients of African descent ≥40 years of age with a smoking history of ≥10 pack-years and a diagnosis of COPD were included in the study. African descent was defined as having origins in one of the black racial groups of Africa (Citation[16]). Identification as being of African descent was based on patients reporting themselves as such. Strategies to facilitate recruitment of this subpopulation included involving research centers with strong ties to local African-American communities and reaching out to organizations representing these patients. Patients were included if they had an FEV1 ≤ 65% of predicted (Morris) adjusted for race, (Citation[17], Citation[18]) an FEV1/FVC of ≤70%, and a Medical Research Council (MRC) dyspnea questionnaire score of ≥2 (Citation[19]).
Patients were excluded if they had significant disease other than COPD which, in the opinion of the investigator, may have put the patient at risk, confounded the results, or otherwise affected the ability of the patient to participate. Patients were also excluded if they had a history of asthma, allergic rhinitis, atopy, clinically evident bronchiectasis, life-threatening pulmonary obstruction, a respiratory tract infection or COPD exacerbation within 4 weeks of the first study visit; or if they recently participated in pulmonary rehabilitation. In addition, patients were excluded if they were taking systemic corticosteroids at unstable doses or in daily doses of ≥10 mg prednisone (or its equivalent), if they previously or currently used commercially available tiotropium (SPIRIVA HandiHaler, Boehringer Ingelheim GmbH & Co. KG, Ingelheim, Germany), if they used oral beta-agonists or if they used oxygen for > 1 hour per day.
The study was conducted in 20 investigational sites in the United States. The protocol was approved by institutional review boards. Written informed consent was obtained from all patients.
Intervention
Following a 2-week screening period, patients were randomized (1:1) to receive either tiotropium (18 mcg) or placebo once daily via the HandiHaler device (Boehringer Ingelheim GmbH & Co. KG, Ingelheim, Germany). Patients took study medication at approximately the same time each morning. On days with clinic visits, study medication was administered after pre dose spirometry. The goal of ≥80% compliance was assessed by requiring patients to bring all used and unused capsules to clinic visits at weeks 4 and 8 for capsule counts.
During the treatment period, patients were permitted to continue using previously prescribed respiratory medications including long-acting β2-agonists, theophylline, inhaled corticosteroids, and modest doses of oral corticosteroids. Open-label anticholinergics were withheld 8 hours prior to randomization and for the duration of the treatment period. Patients were supplied with albuterol for use as rescue medication. Use of antibiotics was not restricted and theophylline preparations and corticosteroids were permitted for COPD exacerbations.
Medication washout procedures were followed prior to each study visit. Long-acting β2-agonists (alone or in combination with inhaled corticosteroids) and short-acting theophylline preparations were withheld for 24 hours; long-acting theophylline preparations were withheld for 48 hours; and short-acting β2-agonists were withheld for 8 hours prior to study visits. Inhaled corticosteroids administered as monoproduct were withheld on the morning of each visit.
PROCEDURES
Spirometry
Following medication washout procedures, spirometry was performed prior to the first dose of study medication (baseline) and at week 4 and week 8. At each visit, spirometry was performed 10 minutes prior to, and 30 minutes, 1 hour, 2 hours, and 3 hours after administration of study drug. Testing was performed in triplicate with the patient in a seated position and results were reported in accordance with the American Thoracic Society recommendations (Citation[20]). If antibiotics or systemic steroids were prescribed for a respiratory infection or COPD exacerbation, the testing was postponed for up to 7 days after the last dose.
Use of rescue medication
Patients recorded the number of times rescue medication (albuterol) was used (daytime and nighttime) on a patient record. The baseline value was determined from rescue medication use during the 2 week run-in period.
Dyspnea
The University of California at San Diego Shortness of Breath Questionnaire (SOBQ) was selected to measure dyspnea because it is a unique 24-item questionnaire that allows patients to self-report the degree of shortness of breath in relation to 21 different activities of daily living (Citation[21]). The questionnaire was completed by patients prior to spirometric evaluations at baseline and at weeks 4 and 8. Patients rated the severity of their shortness of breath using a 6-point scale (0 = not at all to 5 = maximal or unable to perform activity because of breathlessness). The SOBQ total score has a range of 0 to 120. A decrease in score indicates an improvement in breathlessness.
Safety
Safety data was collected through adverse event reporting from the time each patient signed informed consent to 30 days after the last dose of study medication. Exacerbations of COPD were reported as adverse events. The investigator decided whether worsening of symptoms was severe enough to be considered an exacerbation of COPD as there was no a priori definition. Serious adverse events were defined as events which resulted in prolonged hospitalization, were immediately life-threatening, were deemed serious by the investigator, or resulted in death.
Statistical analysis
Assuming a standard deviation of 215 mL for FEV1 AUC0 − 3 (based on the collective data from tiotropium clinical trials), we estimated that 30 patients per treatment group would be required to detect a 190 mL difference between tiotropium and placebo in the mean FEV1 AUC0 − 3, and a sample size of 69 patients per treatment group would be required to detect a 120 mL difference between tiotropium and placebo in mean trough FEV1 at 5% level of significance and 90% power. The study was planned for 75 patients to complete the study per treatment group to allow for detecting treatment differences in both FEV1 AUC0 − 3 and trough FEV1 (nQuery Advisor, version 4.0, Statistical Solutions, Saugus, MA, USA).
For the primary spirometric endpoint, area under the curve was calculated using the trapezoid rule. The area was normalized by dividing by 3 to express units in liters and estimate average improvement in FEV1 over the 3-hour post dose observation period. The statistical model was analysis of covariance with terms for treatment and center and baseline FEV1 as covariate. Baseline FEV1 was defined as FEV1 measured prior to first dose of study medication at randomization visit (Visit 2). Missing values due to worsening of disease were imputed by the patient's least favorable observation carried forward while missing values due to other reasons were imputed by last observation carried forward method. An analysis was performed to examine the possibility that differences between treatments depended on the center by including treatment-by-center into a preliminary model. The interaction was dropped from the final model. Statistical significance was considered at p < 0.05.
Analysis of covariance was also used for secondary spirometric endpoints using the same statistical model as the primary analysis. Mean daily numbers of occasions of rescue medication use for each week and SOBQ total score were compared using analysis of covariance with treatment and center in the model and baseline as a covariate.
RESULTS
Study population
A total of 240 patients signed informed consent and were screened for participation in the trial. Of these, 166 patients met entry criteria and were randomized to receive either tiotropium (n = 80) or placebo (n = 86). A total of 4 patients in the tiotropium group and 7 in the placebo group prematurely discontinued after randomization (none in the tiotropium group and 6 in the placebo group due to adverse events). The percent of patients with ≥80% compliance to study medication was similar between groups at week 4 (tiotropium = 91.1, placebo = 88.9) and slightly higher in the tiotropium group at week 8 (tiotropium = 92.1, placebo = 85.7).
A total of 160 patients were eligible for efficacy evaluation. All patients were of African descent. The overall COPD disease characteristics profile was similar across treatment groups (). The tiotropium group had a lower percentage of females and a slightly higher percentage of current smokers compared with placebo ().
Table 1 Baseline characteristics of patients in the tiotropium and placebo groups
Prior to enrollment in the study, 84.3% of patients were taking pulmonary medications. The use of pulmonary medications at baseline is reported in .
Table 2 Baseline use of respiratory medications in the tiotropium and placebo groups (% of patients)
Spirometric outcomes
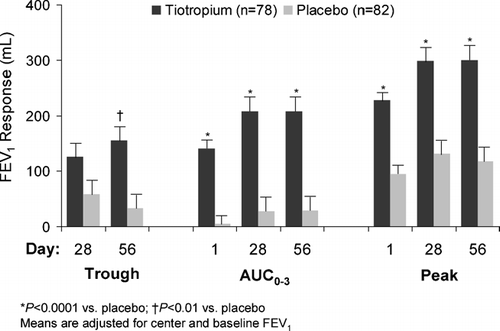
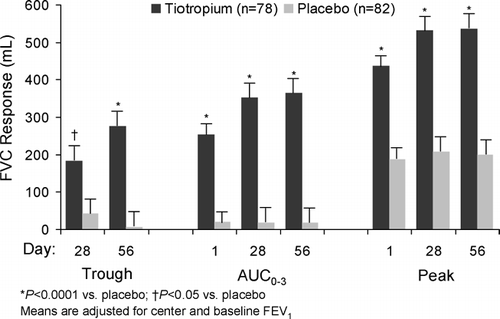
Treatment with tiotropium resulted in significant improvements in airflow as measured by the primary endpoint of FEV1 AUC0 − 3 at week 8 (). Secondary spirometric endpoints of mean trough, peak and AUC0 − 3 FEV1 responses were significantly greater with tiotropium than with placebo except for trough FEV1 response at week 4 (). Mean trough, peak and AUC0 − 3 FVC responses were significantly higher with tiotropium on all test days ().
Use of rescue medication
At baseline, the number of occasions of rescue medication use per day was similar in both groups (tiotropium = 2.8, placebo = 2.9). The weekly means for total daily rescue medication use were not significantly different for patients receiving tiotropium or placebo and ranged from approximately 2.6 to 3.0 uses per day. There were also no significant differences between the tiotropium and placebo groups in weekly means for daytime (ranged from 1.8 to 2.1 uses per day) or nighttime (ranged from 0.8 to 1.0 uses per day) rescue medication use.
Dyspnea
The overall total score for shortness of breath as measured by the SOBQ was 59.8 at baseline indicating moderate shortness of breath. Mean total SOBQ scores were not significantly different between tiotropium and placebo groups after 4 weeks (tiotropium = 58.1, placebo = 54.1) or 8 weeks (tiotropium = 59.9, placebo = 57.3).
Safety
A total of 42 patients (25.3%) experienced adverse events during the treatment period. Fewer patients with tiotropium (n = 11 [13.8%]) experienced adverse events than with placebo (n = 31 [36%]). Serious adverse events were similar in the tiotropium and placebo groups (n = 5 [6.3%] vs. n = 7 [8.1%], respectively). One death was reported during treatment period due to a newly-diagnosed small cell carcinoma in a patient in the placebo group.
Seventeen adverse events were attributed to the respiratory system organ class, the most common of which were reported as exacerbations of COPD by the study investigators. None of the patients in the tiotropium group experienced a COPD exacerbation whereas 12 patients in the placebo group experienced ≥1 COPD exacerbation. Although there was no pre-specified definition of COPD exacerbation, 11 of the 12 COPD exacerbations required treatment with antibiotics or steroids or both and 3 required hospitalization.
DISCUSSION
Under representation of minority populations in clinical trials is well recognized (Citation[22]). Evidence also suggests that members of certain subgroups have increased susceptibility to some disease states, especially respiratory diseases (Citation[1], Citation[2], Citation[3], Citation[4], Citation[5], Citation[6]) and responses to therapeutic interventions that differ from the general population (Citation[7], Citation[8], Citation[9], Citation[10]). The present study is one of the first studies designed to prospectively evaluate the efficacy of a pharmacologic intervention in African American patients with COPD. Results of this study confirm that treatment with tiotropium results in significant improvements in lung function in African Americans with COPD. These findings are similar to a post hoc analysis of 149 African American patients who participated in a previous study (n = 1,829) (Citation[14], Citation[15]). Observed improvements in spirometric indices in the present study are also consistent with findings in the general population (Citation[12], Citation[13]).
Overall use of rescue medication was relatively low at baseline and remained low during the treatment period; however, in contrast to previous studies, there was no significant difference between treatment groups. In the previous 1-year, placebo-controlled trials, baseline albuterol use ranged from 3.4 to 3.7 occasions per day and was significantly reduced with tiotropium compared with placebo (3.2 vs. 4.1 doses day, respectively, p < 0.01) (Citation[12]). The difference from previous studies could be due to a higher percentage of patients in the tiotropium group taking short-acting or long-acting β2-agonists at baseline in the present study. Underlying genetic differences may also have contributed to differences in β2-agonist responsiveness in African Americans (Citation[23], Citation[24], Citation[25]).
In contrast to previous clinical trials using the Transition Dyspnea Index to measure dyspnea (Citation[12], Citation[26]), the present study showed no significant difference in dyspnea (measured by the SOBQ) between tiotropium and placebo. These findings may reflect differences in the perception of dyspnea between the African American subgroup and the general population, or they may reflect methodological issues related specifically to the SOBQ. The SOBQ was developed for use in the setting of pulmonary rehabilitation, and has been validated in a rehabilitation population and used in the National Emphysema Treatment Trial (Citation[21], Citation[27]); however, it has not been validated in the setting of a pharmacologic intervention or in the subpopulation under study. Use of the SOBQ in this study provided the opportunity to explore use of this instrument in a setting of pharmacologic intervention. To fully assess the impact of potential cultural bias, further exploration using instruments validated in this specific subpopulation would be needed.
In the present study, a substantial difference between treatment groups in incidence of exacerbations was observed (no exacerbations with tiotropium vs. 12 with placebo). The magnitude of this difference is greater than that observed in previous studies (Citation[12], Citation[14], Citation[28]). Neiwoehner and colleagues reported that tiotropium significantly reduced the percentage of patients experiencing 1 or more exacerbations over a 6-month period compared with placebo (28% vs. 32%, respectively, p < 0.05) (Citation[14]). In the combined 1-year, placebo-controlled studies, Casaburi and colleagues reported a similar significant reduction in the percentage of patients experiencing exacerbations with tiotropium compared with placebo (36% vs. 42%, respectively, p < 0.05) (Citation[12]).
Since the duration of the present study was only 8 weeks, one might question whether withdrawal of previously prescribed anticholinergic in patients in the placebo group may have been a factor leading to increased exacerbations. However, of 12 patients with exacerbations, 7 were not receiving anticholinergics prior to study entry. It is unclear whether the large reduction in exacerbations with tiotropium may be a signal of an intrinsic response of African American patients to tiotropium. Prospective studies would be required to more fully explore this possibility.
This study had a number of limitations. Although this study showed improvements in lung function with tiotropium in African American COPD patients comparable to improvements demonstrated in largely Caucasian study populations, the relatively short duration of the study (8 weeks) may be a limitation. Although pharmacodynamic steady state of tiotropium is achieved within approximately 8 days, peak effects in other patient-reported outcomes may not be realized in this time. Additionally, because activity levels were not monitored, it is not possible to determine whether lack of improvements in dyspnea and use of rescue medication occurred with stable activity levels or whether patients in the tiotropium group may have increased their activities leading to continued need for rescue albuterol and a lack of an observed change in dyspnea.
The importance of studying therapeutic interventions in specific subpopulations is illustrated by experience with other pharmacologic agents. A large body of evidence suggests that race may be a determinant in responsiveness to different classes of antihypertensive therapy (Citation[8], Citation[9], Citation[10]). Racial differences in responsiveness to agents targeting the beta adrenergic receptor have also been suggested (Citation[29], Citation[30]). A large-scale study involving 26,355 subjects with asthma revealed an association between treatment with salmeterol and a small but statistically significant increase in respiratory-related deaths and life-threatening experiences that was more pronounced in the African American subgroup (Citation[7]). β2-agonist receptor polymorphisms have been linked to diminished responsiveness to β -agonist medication, and may be more prevalent in certain racial minorities (Citation[23], Citation[24], Citation[25]). Further prospective evaluations may determine whether clinical findings are related to genetic differences or race is a surrogate for environmental or social differences between groups.
In conclusion, the present study provides evidence that treatment with tiotropium results in significant improvements in lung function in African Americans with COPD and supports the known favorable safety profile of tiotropium. Additional studies are needed to further explore the potential impact of tiotropium on exacerbations of COPD and other patient-centered outcomes in this population.
ACKNOWLEDGMENTS
We wish to acknowledge the contributions of our co-investigators and study staff at their sites: Dr. Peter Arcuri, Dr. Dick D. Briggs Jr., Dr. Cynthia Brown, Dr. Geoffrey L. Chupp, Dr. Christopher B. Cooper, Dr. J. Allen D. Cooper, Dr. Claudia Cote, Dr. Charles Fogarty, Dr. Nichola Hanania, Dr. David G. Hill, Dr. Thomas Kaelin Jr., Dr. Marvin Lesser, Dr. Michael Littner, Dr. Timothy Moriarity, Dr. Gerry San Pedro, Dr. Gregory Schilero, and Dr. Warren Summer. We also wish to acknowledge Kenichi Inoue and Rosemary Steinberg of Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT for their assistance in this trial.
Trial number: 205.294; ClinicalTrials.gov identifier NCT00106821.
Dr. Gerard J. Criner is a consultant for Boehringer Ingelheim, Otsuka Pharmaceuticals, Schering-Plough, Shire, Actelion and Ortho-Biotech; is on the advisory board of Schering-Plough, Ortho-Biotech and Otsuka Pharmaceuticals; and has received industry-sponsored grants and honoraria for speaking from Boehringer Ingelheim. Dr. Amir Sharafkhaneh is on the advisory board of Pfizer and DEY and has received industry-sponsored grants and honoraria for speaking for Boehringer Ingelheim, Pfizer, GlaxoSmithKline, and DEY. Dr. Rick Player has received honoraria for speaking from Boehringer Ingelheim and Schering-Plough. Dr. Cara Cassino, Dr. Craig S. Conoscenti, Ms. Mary Theresa Keyser, and Mr. Philip Johnson are employees of Boehringer Ingelheim. The study was funded by Boehringer Ingelheim and Pfizer.
REFERENCES
- NHLBI Working Group. Respiratory diseases disproportionately affecting minorities. Chest 1995; 108: 1380–1392, [published erratum appears in Chest 1996; 109:295]
- Mannino D M, Reichert M M, Davis K J. Lung function decline and outcomes in an adult population. AmJ Respir Crit Care Med 2006; 173: 985–990
- Chatila W M, Hoffman E A, Gaughan J, Robinswood G B, Criner G J, for the National Emphysema Treatment Trial Research Group. Advanced emphysema in African American and white patients: Do differences exist?. Chest 2006; 130: 108–118
- Chatila W M, Wynkoop W A, Vance G, Criner G J. Smoking patterns in African Americans and whites with advanced COPD. Chest 2004; 125: 15–21
- Dransfield M T, Davis J J, Gerald L B, Bailey W C. Racial and gender differences in susceptibility to tobacco smoke among patients with chronic obstructive pulmonary disease. Resp Med 2006; 100: 1110–1116
- National Heart, Lung, and Blood Institute. Chronic Obstructive Pulmonary Disease Data Fact Sheet. U.S. Department of Health and Human Services, http://www.nhlbi.nih.gov/health/public/lung/other/copd_fact.htm March 2003. Available from. Accessed August 14, 2006
- Nelson H S, Weiss S T, Bleeker E R, Yancey S W, Dorinsky P M, the SMART Study Group. The salmeterol multicenter asthma research trial, a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006; 126: 15–26
- Kalus J S, Nappi J M. Role of race in the pharmacotherapy of heart failure. Ann Pharmacother 2002; 36: 471–478
- Exner D V, Dries D L, Domanski M J, Cohn J N. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. N EnglJ Med 2001; 344: 1351–1357
- Carson P, Ziesche S, Johnson G, Cohn J N, for the Vasodilator-Heart Failure Trial Study Group. Racial differences in response to therapy for heart failure: analysis of the vaso-dilator-heart failure trials. J Card Fail 1999; 5: 178–187
- Disse B, Speck G A, Rominger K L, Witek T J, Hammer R. Tiotropium (Spiriva): mechanistical considerations and clinical profile in obstructive lung disease. Life Sci 1999; 64: 457–464
- Casaburi R, Mahler D A, Jones P W, Wanner A, San Pedro G, ZuWallack R L, Menjoge S S, Serby C W, Witek T J. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur RespirJ 2002; 19: 217–224
- Vincken W, van Noord J A, Greefhorst A P, Bantje T A, Kesten S, Koducki L, Cornelissen P JG. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur RespirJ 2002; 19: 209–216
- Celli B, Zu Wallack R, Wang S, Kesten S. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator. Ann Intern Med 2005; 143: 317–326
- Niewoehner D, Rice K, Cote C, Korducki L, Cassino C, Kesten S. Characteristics of COPD and response to once-daily tiotropium in African Americans in the VA medical system. Proc American Thorac Soc 2005; 2: A543
- Agency-wide Race and Ethnicity Working Group from the U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health, Office of the Commissioner. Guidance for industry: collection of race and ethnicity data in clinical trials (Draft guidance, January 2003). U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research, Center for Biologics Evaluation and Research, Center for Devices and Radiological Health, Office of the Commissioner. Rockville, MD, 2003
- Morris J F, Koski A, Temple W P, Claremont A, Thomas D R. Fifteen year interval spirometric evaluation of the Oregon predictive equations. Chest 1988; 93: 123–127
- Hankinson J L, Odencrantz J R, Fedan K B. Spirometric reference values from a sample of the general US population. AmJ Respir Crit Care Med 1999; 159: 179–187
- Bestall J C, Paul E A, Garrod R, Garnham R, Jones P W, Wedzicha J A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54: 581–586
- American Thoracic Society. Standardization of spirometry – 1994 update: statement of the American Thoracic Society. Am J Respir Crit Care Med 1995; 152: S77–S120
- Eakin E G, Resnikoff P M, Prewitt L M, Ries A L, Kaplan R M. Validation of a new dyspnea measure: The UCSD Shortness of Breath Questionnaire, University of California, San Diego. Chest 1998; 113: 619–624
- Evelyn B, Toigo T, Banks D, Pohl D, Gray K, Robins B, Ernat J. Participation of racial/ethnic groups in clinical trials and race-related labeling: A review of new molecular entities approved 1995–1999. US Food and Drug Administration, Rockville, MD 2001, http://www.fda.gov/cder/reports/race_ethnicity/race_ethnicity_report.htm Available at. Accessed August 15, 2006
- Israel E, Drazen J M, Liggett S B, Boushey H A, Cherniack R M, Chinchilli V M, Cooper D M, Fahy J V, Fish J E, Ford J G, Kraft M, Kunselman S, Lazarus S C, Lemanske R F, Jr, Martin R J, McLean D, Peters S P, Silverman E K, Sorkness C A, Szefler S J, Weiss S T, Yandava C N, for the National Heart, Lung, and Blood Institute's Asthma Clinical Research Network. The effect of polymorphisms of the β2-adrenergic receptor on the response to regular use of albuterol in asthma. AmJ Respir Crit Care Med 2000; 162: 75–80
- Israel E, Chinchilli V M, Ford J G, Boushey H A, Cherniack R, Craig T J, Deykin A, Fagan J K, Fahy J V, Fish J, Kraft M, Kunselman S J, Lazarus S C, Lemanske R F, Jr, Liggett S B, Martin R J, Mitra N, Peters S P, Silverman E, Sorkness C A, Szefler S J, Wechsler M E, Weiss S T, Drazen J M, for the National Heart, Lung, and Blood Institute's Asthma Clinical Research Network. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomized, placebo-controlled, cross-over trial. Lancet 2004; 364: 1505–1512
- Lima J J, Thomason D B, Mohamed M HN, Eberle L V, Self T H, Johnson J A. Impact of genetic polymorphisms of the β2-adrenergic receptor on albuterol bronchodilator pharmacodynamics. Clin Pharmacol Ther 1999; 65: 519–525
- Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax 2003; 58: 399–404
- National Emphysema Treatment Trial Research Group. A randomized trial comparing lung-volume–reduction surgery with medical therapy for severe emphysema. N EnglJ Med 2003; 348: 2059–2073
- Dusser D, Bravo M L, Iacono P, on behalf of the Mistral study group. The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur RespirJ 2006; 27: 547–555
- Johnson J A, Burlew B S, Siles R N. Racial differences in β -adrenoreceptor-mediated responsiveness. J Cardiovasc Pharmacol 1995; 25: 90–96
- Rutledge D R, Wallace A, Steinberg J D, Cardozo L, Lavine S J. Racial differences in drug response: isoproterenol effects before and after propranolol. Pharm Res 1991; 8: 754–757