Abstract
Rationale: Arformoterol, a single isomer long-acting β2-agonist, was developed as an inhalation solution for the maintenance treatment of bronchoconstriction in COPD. Methods: The pulmonary function efficacy of nebulized arformoterol (15 μ g BID, 25 μ g BID, 50 μ g QD) and salmeterol MDI (42 μ g BID) versus placebo was assessed in 1456 subjects (mean FEV1 1.2L, mean predicted 41%). Data were pooled from 2 identical, 12-week, double-blind, randomized trials. The percent change in trough FEV1, percent change in FEV1 average AUC(0 - 12 hrs) and peak percent change FEV1 from predose were analyzed. Results: Improvement in trough FEV1 averaged over 12 weeks was greater for arformoterol and salmeterol versus placebo (mean differences from placebo [95% CI] arformoterol–15 μ g BID: 11.4% [8.4, 14.3]; 25 μ g BID: 15.4% [12.2, 18.6]; 50 μ g QD: 10.9% [7.9, 13.9]); salmeterol: (11.6% [8.8, 14.4]). Greater improvements versus placebo occurred after the first dose (mean differences between arformoterol and placebo for trough FEV1: 13–19%; FEV1 AUC(0 - 12 hrs): 19–24%; peak percent change: 20–25%) and at week 12 (trough FEV1: 10–13%; FEV1 AUC(0 - 12 hrs): 6–13%; peak percent change: 7–14%); all 95% CIs excluded zero. Increases in FEV1 AUC(0 - 12 hrs) and peak percent change were greater for arformoterol than for salmeterol (95% CIs excluded zero). After 12 weeks, 78–87% of arformoterol subjects had ≥ 10% increases in FEV1 from pre-dose (56% salmeterol, 44% placebo); the median time to response was 3–13 minutes (142 minutes salmeterol). Conclusions: In these trials, COPD subjects administered nebulized arformoterol demonstrated significant and sustained improvement in lung function over 12 weeks.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) affects approximately 24 million people in the United States (Citation[1]). The estimated number of COPD patients has increased by 41.5% since 1982 and currently approximately 11% of the U.S. population is affected by this condition (Citation[2]). COPD is characterized by the presence of progressive airflow obstruction. While the airway obstruction may be only partially reversible, bronchodilator therapy is a key component of its management. Among available bronchodilators, β 2-agonists play a major role in maintenance therapy of COPD (Citation[2], Citation[3]). Most currently available inhaled bronchodilators are administered using metered dose inhalers (MDI) or dry powder inhalers (DPI). For some patients, nebulization may be the preferred route of administration (Citation[4], Citation[5]). Until recently, there were no long-acting β2-agonists (LABA) formulated as an inhalation solution suitable for use in a nebulizer.
Arformoterol is the (R,R)-isomer of formoterol (Citation[6], Citation[7]). In preclinical studies the potency of arformoterol is 2-fold greater than racemic formoterol (Citation[7]). By contrast, the (S,S)-isomer of formoterol is 1000-fold less potent as a β -agonist than the (R,R)-isomer, based on receptor stimulation, smooth muscle relaxation, and inhibition of spasmogen responses (Citation[8], Citation[9]).
The purpose of these identically designed, but independently conducted, Phase III clinical trials was to evaluate the effects of 3 doses of nebulized arformoterol on lung function in subjects with COPD. The results in each of the individual trials were consistent with each other. Therefore, the pooled findings presented in this report provide an accurate and stable estimate of the treatment effects.
MATERIALS AND METHODS
Both trials were Phase III, multicenter, randomized, double-blind, double-dummy, parallel group designs. Studies were conducted according to FDA regulations and guidelines, which encompass all principles established by the Declaration of Helsinki (Citation[10]). The institutional review boards at each of the 124 study sites approved the protocol, and a written informed consent was obtained from each subject.
Subjects
Subjects had non-asthmatic COPD (including chronic bronchitis and/or emphysema). Subjects were 35 years of age and older, had a baseline FEV1 ≤65% of predicted (Citation[11]) but at least 0.70 L, a FEV1/forced vital capacity (FVC) ratio ≤70%, ≥15 pack-year smoking history, and breathlessness severity from the Medical Research Council dyspnea scale of ≥2 (i.e., shortness of breath when hurrying on the level or walking up a slight hill) (Citation[12]). Subjects with life-threatening/unstable respiratory status within 30 days before screening; asthma or any chronic respiratory disease (including sleep apnea) other than COPD; lung resection of more than 1 full lobe; and/or continuous supplemental oxygen were excluded.
Study design
After screening, subjects entered a 2-week, single-blind placebo run-in period. Following the run-in, eligible subjects were randomized to one of the following 5 regimens: arformoterol 15 μ g BID, arformoterol 25 μ g BID, arformoterol 50 μ g QD, salmeterol MDI 42 μ g BID, or placebo. Salmeterol MDI 42 μ g BID was chosen as the active control as it was the only LABA approved for the treatment of COPD in the US at the time the trials were initiated. Serial spirometry was performed in all subjects in duplicate before and after dosing (immediately post-first dose, and at 15 and 40 minutes, 1, 2, 3, 4, 5, 6, 8, 10, 12, 23, and 24 hours post-first dose) at Weeks 0, 6, and 12. Albuterol MDI (VENTOLIN®, GlaxoSmithKline, Research Triangle Park, NC) and ipratropium MDI (ATROVENT®, Boehringer Ingelheim, Ridgefield, CT) were provided as rescue and supplemental medications, respectively, for use throughout the trial. Subjects were instructed in the use of these medications and told to withhold them for 6 hours prior to each clinic visit.
Study endpoints
The primary endpoint was the percent change in trough FEV1 from baseline (prior to the first dose at Week 0) analyzed over the double-blind period. Trough FEV1 was the morning value obtained at the end of the dosing interval, 12 hours after the evening dose for the BID treatment arms and 24 hours after the morning dose for the QD treatment arm. The percent change in trough FEV1 was also analyzed separately at Weeks 0, 6, and 12.
The key secondary endpoint, the percent change in FEV1 average area under the curve (FEV1 AUC(0 - 12 hrs)) measured from the predose value was analyzed over the 12-week double-blind period and separately at Weeks 0, 6, and 12. Additional spirometry endpoints included: peak percent change in FEV1 from visit predose, peak percent predicted FEV1, and time to onset of response (10% increase in FEV1 from visit predose) performed over 12 hours.
Subgroup analyses of the primary and key secondary endpoints were conducted post hoc. These subgroups included: age (< 65, 65–74, and ≥75 years), race (Caucasian, Black, and other), gender, severity of airway obstruction similar to that described by the Global Initiative for Obstructive Lung Disease (GOLD; < 30%; ≥ 30 to < 50%; and ≥ 50% baseline percent predicted FEV1), FEV1 reversibility at study entry (< 10% reversibility, ≥ 10 to < 18% reversibility, and ≥ 18% reversibility), and regular inhaled or systemic corticosteroid use at study entry.
Statistical methods
Data were compiled, and statistical analyses for this study were performed by Sepracor Inc. and were fully available to all authors. All authors were involved in the preparation of the manuscript. As the results of the 2 individual trials were similar, the data from the trials were pooled to provide a more precise estimate of the treatment effects. All analyses were based on pooled data and performed on the intent-to-treat (ITT) population (randomized subjects who received at least one dose of blinded study medication).
Descriptive statistics were calculated by treatment group for baseline variables and each efficacy endpoint. The difference in treatment means (and 95% confidence intervals) were estimated post hoc for outcomes using the pooled data. If the 95% CI for the treatment difference excluded zero, it was considered statistically significant in testing the hypothesis of no treatment difference at the 5% level. Spirometry measurements collected within 6 hours following rescue/supplemental medication use were excluded.
The median time to 10% response was estimated using the Kaplan-Meier product-limit method; 95% confidence intervals were estimated (Citation[13]). Linear interpolation was used to estimate the time of 10% response. Subjects with no response within 12 hours after first dosing were censored at the time of their 12-hour assessment. For this endpoint, spirometry measurements collected after rescue/supplemental medication use were excluded. Nonresponders with missing or excluded data prior to 12 hours were censored at the time of their last valid FEV1 measurement.
RESULTS
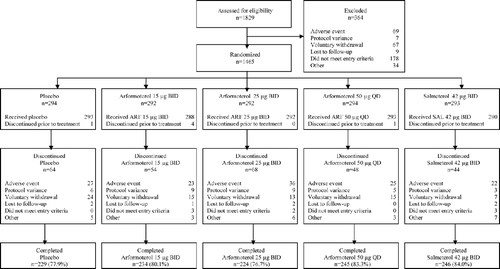
There were 1829 subjects enrolled in the combined trials. Of these subjects, 1465 were randomized and 1456 took study medication (intent-to-treat: 293 placebo, 288 arformoterol 15 μ g BID, 292 arformoterol 25 μ g BID, 293 arformoterol 50 μ g QD, 290 salmeterol). The overall rate of completion was 81% and was similar for all treatment groups (). The most common reason for discontinuation of double-blind treatment was an adverse event (133 subjects, 9.1%; ). COPD adverse events were the most frequently reported adverse event leading to discontinuation and occurred with a similar frequency in all groups: 3.1% in the placebo group, 1.4% in the arformoterol 15 μ g BID group, 2.4% in the arformoterol 25 μ g BID, 2.4% in the arformoterol 50 μ g QD group, and 2.8% in the salmeterol group. The groups were well balanced for age, race, gender, and baseline disease. Subjects had moderate to severe COPD by GOLD PFT criteria ().
Table 1 Demographics and baseline disease.
The active treatments provided substantial bronchodilation over the 24-hour interval and at the 24-hour trough after the first dose (at Week 0) and after 12 weeks of dosing (). There was some decline in the degree of bronchodilation at the end of the dosing interval in all groups between Week 0 and Week 6, although little additional decline was observed between Weeks 6 and 12. Despite these decreases in response, significant improvement in lung function relative to placebo was observed throughout the 12 weeks of treatment (). There were no statistically significant differences between arformoterol and salmeterol at most time points for trough FEV1.
Figure 2 Mean change in FEV1 from baseline over the 24-hour dosing interval after the first dose at Week 0 (A) and at Week 12 (B). There was substantial bronchodilation relative to placebo for the active treatments over the 24-hour interval and at the 24-hour trough at both time points.
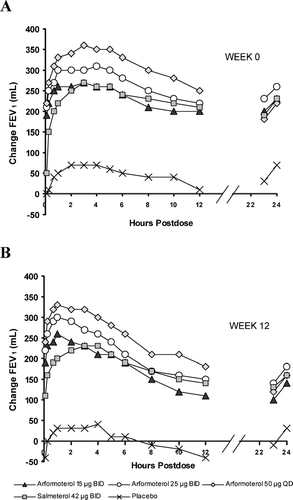
Table 2 Percent change in trough FEV1.
FEV1 AUC(0 - 12 hrs), a measure of bronchodilation over the 12 hours after dosing measured from the predose value, was improved at all time points for the active treatments compared to placebo. Significantly greater improvement was observed for all arformoterol doses than for salmeterol for this endpoint (). The results were similar for mean peak percent change in FEV1 (). Peak percent predicted FEV1 values were also greater in the active treatment groups compared with placebo at all weeks (data not shown).
Table 3 Percent change in FEV1 area under the curve over 12 hours (FEV1 AUC(0 - 12 hrs).
Table 4 Peak percent change in FEV1.
In a responder analysis, a large proportion of subjects in the active treatment groups achieved a greater than 10% increase in FEV1 compared with values before dosing at all visits. At Week 12, 78–87% of subjects in the arformoterol groups had a ≥ 10% response, compared with 56% and 44% for the salmeterol and placebo groups, respectively. The median time to achieve this response at Week 12 was 3–13 minutes in the arformoterol groups, 142 minutes in the salmeterol group, and 353 minutes in the placebo group ().
Table 5 Percentage of responders and median time to onset of response
Morning and evening daily mean PEFR values increased in the first 3 weeks of the double-blind period in all active treatment groups. These increases were stable and maintained through 12 weeks of dosing (data not shown). All active treatment groups showed improvement in the primary and key secondary efficacy endpoints for all age and gender categories. Subjects in racial categories other than Caucasian, however, were too few in number for meaningful analysis (data not shown).
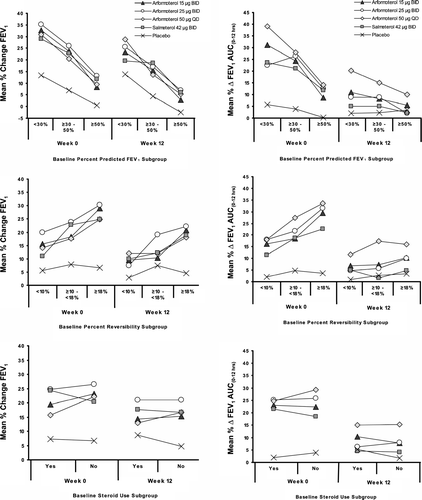
The analyses of the change in trough FEV1 stratified by the degree of pulmonary function impairment at study entry demonstrated that subjects in all treatment groups with more severe FEV1 compromise had greater improvements in lung function than those with less severe disease (). The results were similar for changes in FEV1 AUC(0 - 12 hrs), with subjects in the < 30% and ≥ 30 to < 50% predicted FEV1 subgroups having greater improvement than those in the ≥ 50% predicted group. Similarly, subjects with greater baseline FEV1 reversibility were also observed to have greater improvements in lung function than less reversible subjects. The active treatments produced greater improvements in trough FEV1 and FEV1 AUC(0-12 hrs) than placebo regardless of baseline corticosteroid use ().
Figure 3 Subgroup analysis of the percent change in morning trough FEV1 (left column) and the percent change in FEV1 AUC(0 - 12 hrs) (right column) by baseline percent predicted FEV1, percent reversibility, steroid-use. Subjects with more severe FEV1 compromise or greater FEV1 reversibility at baseline had greater percent improvement in lung function than those with less severe or less reversible disease. The active treatments improved trough FEV1 and FEV1 AUC(0 - 12 hrs) to a greater extent than placebo regardless of baseline corticosteroid use.
The proportion of subjects with protocol-defined COPD exacerbations was similar for the active treatment groups. The overall adverse event profiles for the 3 doses of arformoterol were similar to placebo and the active control salmeterol, including COPD adverse events. The arformoterol and placebo groups had a similar occurrence of the following cardiovascular adverse events: chest pain (arformoterol 3.4–6.6%; placebo: 6.6%), palpitation (arformoterol: 0–1.4%; placebo: 1.0%), and tachycardia (arformoterol: 0.3–1.0%; placebo: 1.4%). Extensive ECG and Holter monitoring data did not suggest any other cardiac consequences with the active treatments (data not shown). Dose-related increases in the β -mediated events of tremor, nervousness, and insomnia occurred with increasing doses of arformoterol ().
Table 6 Safety summary.
There were 5 deaths: hepatic lacerations secondary to a motor vehicle accident (arformoterol 15 μ g BID); complications from surgery for diverticulosis-induced rectal bleeding (arformoterol 15 μ g BID); complications following surgery for elective abdominal aortic aneurysm (arformoterol 15 μ g BID); disseminated malignancy (arformoterol 25 μ g BID); and aortic dissection (arformoterol 50μ g QD).
DISCUSSION
Recent COPD therapy guidelines state that long-acting bronchodilator treatment is indicated in patients whose lung function response is of moderate or worse severity (Citation[4], Citation[5]). Consensus opinion suggests that nebulized inhalation treatment may be most beneficial for patients (a) who are unable to perform single-breath inhalation with DPI/MDI devices; (b) who have repeated episodes of airflow obstruction despite DPI/MDI use; or (c) with severe lung function compromise (Citation[4]). These trials demonstrated that all doses of arformoterol provided effective long-acting bronchodilation, and the results suggest that arformoterol may be a treatment option for such patients.
A recently published review of minimal clinically important differences in COPD outcomes concluded that increases in trough FEV1 (i.e., at the end of dosing interval) of 100 mL or greater were clinically meaningful (Citation[14]). Improvements of this magnitude or greater were associated with subjects' perceptions of improvement and decreases in the frequency of COPD exacerbations, and are of particular benefit in subjects with baseline FEV1 < 50% predicted. The majority (> 75%) of subjects in these studies had baseline lung function of this or greater severity. After 12 weeks, arformoterol-treated subjects with baseline FEV1 < 30% predicted had mean improvements in morning trough FEV1 ranging from approximately 170 to 230 mL. Subjects with baseline FEV1 between 30% and < 50% predicted had mean improvements ranging from approximately 150 to 180 mL (). These findings suggest that subjects with moderate and severe COPD achieve clinically meaningful and sustained lung function improvement with nebulized arformoterol that persists with long-term (12 weeks) daily treatment.
In addition to significant improvement in airway function at the end of the dosing interval, substantial daily increases in lung function from predose (i.e., trough) levels were also observed at Weeks 6 and 12 (FEV1 AUC0 - 12 hrs) and peak percent change in FEV1). These results indicate that nebulized arformoterol provides meaningful increases in FEV1 in addition to trough improvement that is maintained with long-term treatment. Even after 12 weeks of daily treatment, 78–87% of arformoterol-treated subjects achieved an additional FEV1 improvement of at least 10% from predose (trough) levels, with a median time to achieve this response of 10–13 minutes. By contrast, only 56% of salmeterol-treated subjects had a 10% improvement at Week 12. As study drug was administered in the morning, the response seen in the placebo group (44% at Week 6; 55% at Week 12) may have been due, in part, to the known circadian improvement in lung function observed during daylight hours. The extent to which this greater and faster airway function improvement observed with daily arformoterol treatment may benefit patients, (e.g., improved patient compliance), is not known.
Tolerance, as indicated by a diminution in bronchodilator efficacy with prolonged regular treatment, was observed to a limited extent in all treatment groups. The mean improvement in trough FEV1 at Week 12 was about one-third less in all active treatment groups than that observed after the first dose (). Despite the declines, statistically significant and clinically meaningful improvement in comparison with placebo was seen for this and other pulmonary function variables throughout the trial.
Underlying contributors to airflow impairment can differ markedly in subjects with COPD, including the extent of airflow impairment at baseline, the degree of reversibility, and the severity of airway inflammation as reflected by the use of corticosteroid therapy at study entry. Accordingly, bronchodilator efficacy was assessed among subgroups stratified by these and other features. Neither gender nor age was an important modifier of response to arformoterol. The role of racial or ethnic differences in the response to arformoterol could not be adequately analyzed given the small number of non-Caucasian subjects in these subgroups. As illustrated in , the degree of bronchodilation efficacy was inversely related to baseline FEV1 impairment, with greater response evident in subjects with more profound airway function compromise (equivalent to GOLD stages III [severe] and IV [very severe]). Substantial efficacy relative to placebo was seen in all reversibility subgroups, even in subjects with the least airflow reversibility at baseline. Finally, the degree of improvement in airway response did not vary markedly when stratified by baseline steroid therapy.
The use of LABAs in asthma has been associated with increases in life-threatening asthma exacerbations and asthma-related deaths, especially when used over long duration and as monotherapy (Citation[15], Citation[16]). However, a recently published 3-year study in COPD patients showed no increase in the occurrence of deaths and exacerbations among patients receiving the LABA salmeterol versus placebo (Citation[17]). In fact, the point estimates suggested a decrease in the number of deaths in some of the LABA-containing treatment arms. The current studies were 12 weeks in duration and not designed to detect differences in infrequent but severe safety outcomes. As such, inferences about these outcomes cannot be made with certainty. Nonetheless, the frequency of serious respiratory exacerbations in these trials was similar in all active treatment groups and not greater than in subjects receiving placebo (i.e., those receiving only rescue albuterol and supplemental ipratropium bromide).
Of note, 5 deaths were observed among arformoterol-treated subjects. However, preexisting cardiovascular conditions and co-morbidities other than malignancy did not exclude COPD subjects from participating in these trials. No dose-response relationship was apparent for the occurrence of deaths or serious adverse events and participating investigators assessed the deaths as unlikely related to arformoterol. Moreover, the adverse event profile of arformoterol (including respiratory adverse events) was similar to both placebo and salmeterol, with the exception of dose-related increases in the β -mediated adverse events of nervousness, tremor, and insomnia. A description of the safety results from the 2 trials will be the subject of separate reports.
Nebulization may be a preferred route of administration for those COPD patients who are unable to use a single-breath actuation device, despite the practical limitations of nebulizer systems (Citation[18]). As an agent administered by means of tidal breathing over several minutes, a nebulized formulation may provide an otherwise unavailable LABA treatment option for these COPD patients. Data from these trials demonstrated effective bronchodilation of all 3 arformoterol doses including the lowest dose evaluated (15 μ g BID). Effective bronchodilation was observed among all subject subgroups, especially those with more severe degrees of baseline airway function compromise. We conclude that arformoterol represents an effective long-acting, nebulized option for the maintenance treatment of bronchoconstriction in patients with COPD.
ACKNOWLEDGMENTS
The authors thank Amy Wilson, Ph.D., for her assistance with the development of this manuscript and the investigators who participated in these trials: M. Angeli Adamczyk, John R. Adams, Tahir Ahmed, Victor F. Almufdi, Arnold B. Alper, Pamela J. Amelung, Theodore R. Amgott, Luis E. Angles, Marc L. Benton, Jonathan A. Bernstein, Bruce T. Bowling, Wesley R. Bray, Shari A. Brazinsky, Michael A. Brown, William W. Busse, Francisco J. Candal, Gene L. Colice, Clinton N. Corder, Jonathan Corren, Bruce C. Corser, Peter J. Costantini, Edward C. Cullen, Eleuterio P. Delfin, Naresh A. Dewan, Randy G. Dotson, Steven L. Duckor, David Elkayam, Debra S. Eriksen, Richard H. Fei, Barry K. Feinstein, Eric L. Fernandez, Charles M. Fogarty, William P. Fukuda, Walter N. Gaman, Stuart M. Garay, Glenn M. Giessel, Ronald Gilman, Gregory M. Gottschlich, Gary I. Greenwald, James E. Greenwald, Robert E. Grubbe, Frank C. Hampel, Jr., James B. Harris III, Roger Hatharasinghe, Alan M. Heller, David C. Henke, Delmer W. Henninger Jr., Charles B. Herring, Robert Ian Hewlett, Frederick C. Hiller, E. Walter. Hood, James D. Hoyt, Thomas M. Hyer, Benjamin Interiano, Jeffrey J. Kaladas, Lawrence D. Kaplan, Mitchell G. Kaye, Amy J. Keenum, Edward M. Kerwin, Kenneth T. Kim, Sylvia K. Knowlton, Nelson Kopyt, Craig F. LaForce, Eric A. Lang, Robert J. Lapidus, Michael Lawrence, Dennis K. Ledford, Theodore M. Lee, Robert D. Lesser, Bernard E. Levine, Stephen R. Lewis, Mark K. Lindley, James H. Lott, III, Timothy A. Lucas, Donald M. Matthees, Bernard A. Michlin, William S. Mullican, Robert A. Nathan, Anjuli S. Nayak, Harold S. Nelson, Michael J. Noonan, Diane M. Normandin, Richard M. Nowak, Rohit G. Patel, Vandana A. Patel, James L. Pearle, Stephen J. Pollard, Keith J. Popovich, Paul H. Ratner, Albert J. Razzetti, Jeffrey Rehm, John C. Rodrigues, Stanley A. Rosenberg, Bradley B.F. Sakran, Emil J. Schelbar, Nathan D. Schultz, Jeffrey S. Schwartz, Joram S. Seggev, Jon Shapiro, Eric T. Shore, John S. Sibille, Thomas M. Siler, Greg M. Silver, Stuart J. Simon, Wayne D. Sinclair, Sudeep Singh, Emil M. Skobeloff, Lewis J. Smith, William B. Smith, Selwyn Spangenthal, George E. Stewart II, James R. Taylor, Mark A. Wentworth, Jan H. Westerman, Douglas Young, and Jose J. Zayas.
Support for this study provided by Sepracor Inc., Marlborough, MA. Drs. Hanrahan and Sciarappa are full-time employees of Sepracor Inc. Dr. Baumgartner was a full-time employee of Sepracor Inc. at the time the trials were conducted. Drs. Hanania, Calhoun, and Sahn participated as investigators in these studies. Dr. Hanania is also a member of the Sepracor Arformoterol Advisory Board.
Results presented in abstract/poster form at the September 2006 European Respiratory Society Congress in Munich.
REFERENCES
- Mannino D M. Chronic obstructive pulmonary disease: definition and epidemiology. Respir Care 2003; 48: 1185–1191
- American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. AmJ of Respir Crit Care Med 1995; 152: S77–S121
- Pauwels R A, Buist A S, Calverley P M, Jenkins C R, Hurd S S, GOLD Scientific Committee. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. AmJ of Respir Crit Care Med 2001; 163: 1256–1276
- O'Donohue W J, the National Association for Medical Direction of Respiratory Care (NAMDRC) Consensus Group. Guidelines for the use of nebulizers in the home and at domiciliary sites. Chest 1996; 109: 814–820
- European Respiratory Task Force. European Society Guidelines on the use of nebulizers. Eur RespirJ 2001; 18: 228–242
- Trofast J, Osterberg K, Kallstrom B L, Waldeck B. Steric aspects of agonism and antagonism at beta-adrenoceptors: synthesis of and pharmacological experiments with the enantiomers of formoterol and their diastereomers. Chirality 1991; 3: 443–450
- Handley D A, Senanayake C H, Dutczak W, Benovic J L, Walle T, Penn R B, Wilkinson H S, Tanoury G J, Andersson R G, Johansson F, Morley J. Biological actions of formoterol isomers. J. Pulm Pharmacol Ther 2002; 15: 135–145
- Zhang X Y, Zhu F X, Olszewski M A, Robinson N E. Effects of enantiomers of β2-agonists on ACh release and smooth muscle contraction in trachea. AmJ Physiol 1998; 274: L32–38
- Schmidt D, Kallstrom B L, Waldeck B, Branscheid D, Magnussen H, Rabe K F. The effect of the enantiomers of formoterol on inherent and induced tone in guinea-pig trachea and human bronchus. Naunyn Schmiedebergs Arch Pharmacol 2000; 361: 405–409
- World Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects, http://www.wma.net/e/policy/b3.htm
- Crapo R O, Morris A H, Gardner R M. Reference spirometric values using techniques and equipment that meet ATS recommendations. Am Rev Respir Dis 1981; 123: 659–664
- Bestall J C, Paul E A, Garrod R, Jones P W, Wedzicha J A. Usefulness of the Medical Research Council (MRC) dyspnea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54: 581–586
- Brookmeyer R, Crowley J. A confidence interval for the median survival time. Biometrics 1982; 38: 29–41
- Donohue J F. Minimal clinically important differences in COPD lung function. COPD: J Chronic Obstructive Pul Dis 2005; 2: 111–124
- Nelson H S, Weiss S T, Bleecker E R, Yancey S W, Dorinsky P M, SMART Study Group. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest 2006; 129: 15–26
- Salpeter S R, Buckley N S, Ormiston T M, Salpeter E E. Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med 2006; 144: 904–912
- Calverley P MA, Anderson J A, Celli B, Ferguson G T, Jenkins C, Jones P W, Yates J C, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. New EngJ Med 2007; 356: 775–789
- Dolovich M B, Ahrens R C, Hess D R, Anderson P, Dhand R, Rau J L, Smaldone G C, Guyatt G. American College of Chest Physicians; American College of Asthma, Allergy, and Immunology. Device selection and outcomes of aerosol therapy: evidence-based guidelines. Chest 2005; 127: 335–371