Abstract
A significant proportion of patients with COPD show post-bronchodilator improvement in lung volume even though this response is rarely considered when classifying subjects as having reversible or irreversible airway disease. We studied 266 patients with a clinical and physiological diagnosis of COPD who underwent pulmonary function testing and had their spirometric response to 5 mg salbutamol assessed. After the bronchodilator 125 (47%) patients increased their forced vital capacity by more than the known variability of the test while 60 (23%) showed only a volume response without improvement in expiratory flow. These ‘volume responders’ had greater degrees of airflow obstruction–lower FEV1 (p < 0.001) and FEV1/FVC (p < 0.05)—and a higher residual volume at rest (p = 0.005) with similar degrees of emphysema measured by KCO. Subjects with evidence of greater dynamic airway collapse, assessed by the ratio of early to mid expiratory flow, were less likely to have a flow response but more likely to have a volume response after salbutamol (p < 0.005). This would be compatible with volume response being commoner in patients who exhibit tidal expiratory flow limitation. We suggest that post-bronchodilator absolute change in FVC provides important additional physiological information when interpreting bronchodilator reversibility testing.
INTRODUCTION
Bronchodilator drugs are commonly used in the treatment of COPD and their effectiveness is often assessed by their ability to improve tests of expiratory flow (Citation[1]). Although airflow improves in many cases, this is not a universal finding (Citation[2]). Despite this many patients report symptomatic and functional improvement when using these drugs despite showing little post-bronchodilator change in FEV1 (Citation[3])—probably due to a reduction in static and dynamic hyperinflation (Citation[4]). Consequently, there is still considerable debate as to what constitutes a significant spirometric bronchodilator response, with most guidelines setting different criteria that results in subjects being labeled as bronchodilator responsive and unresponsive, according to potentially arbitrary criteria (Citation[5], Citation[6], Citation[7]).
It is clear that some subjects show relatively greater improvements in tests of volume post bronchodilator and these individuals have been termed “volume responders” (Citation[8], Citation[9], Citation[10]). We hypothesized that changes in lung volume post-bronchodilator would be as common a physiological end point as change in expiratory flow and would identify a different group of patients otherwise labeled unresponsive. In addition we hypothesized that change in forced vital capacity would be an acceptable surrogate for change in other volumes. To test this hypothesis we studied a large unselected group of COPD patients presenting to our laboratory and defined the physiological characteristics of these patients in relation to their volume response to bronchodilatation. Specifically we sought evidence for a role of dynamic airway collapse and closure during forced expiratory maneuvers as a likely mechanism of volume response.
MATERIALS AND METHODS
We retrospectively reviewed the pulmonary function of 266 consecutive patients with chronic obstructive pulmonary disease who attended our laboratory. All patients were attending our clinical respiratory service and had been diagnosed with COPD by a senior respiratory physician. Any patients with a diagnosis of bronchial asthma, bronchiectasis or any other respiratory disease were excluded, as were individuals with both COPD and another co-existent respiratory condition. Age and smoking history were documented with ex-smokers defined as individuals who had quit for 3 months or longer. All subjects had smoked at least 20 pack years of cigarettes.
Patients were asked to omit short-acting inhaled bronchodilators for at least 8 hours, long-acting beta-agonists for at least 12 hours and oral bronchodilators for 24 hours before testing. At the time of the study no patient was taking tiotropium bromide which is known to have an extended action of bronchodilation and might have influenced our data interpretation (Citation[11]). Patients had no history of exacerbation nor had they taken antibiotics or oral corticosteroids in the preceding 6 weeks. Patients taking regular inhaled corticosteroids were included in the study but we excluded any patient taking regular oral corticosteroids for any reason.
All tests were performed in accordance with ATS standards (Citation[12], Citation[13]). Flow volume loops, lung volumes and single breath diffusion capacity for carbon monoxide (DLCO) were measured in the same sequence using a rolling seal spirometer (PK Morgan, Kent, UK). At least 3 acceptable flow volume loops were performed with < 0.2 L between the value of the highest and next highest FEV1 and FVC. The largest forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) were taken. Peak expiratory flow (PEF) and two measures of mid expiratory flow (mean FEF25 − 75 and FEF50) were taken from the loop with the largest sum of FEV1 plus FVC. Slow vital capacity (SVC) was measured on a minimum of 2 occasions until there was < 0.2 L between the highest and next highest measurement. Functional residual capacity (FRC) was measured using the helium equilibration method and immediately followed by one further SVC measurement. Other lung volumes were calculated using the highest SVC. Absolute DLCO and DLCO adjusted for alveolar volume (KCO) were also averaged from 2 acceptable manoeuvres which did not differ from each other by more than 10%.
Bronchodilator responsiveness was assessed by performing spirometry before and 15 minutes after the patient had received 5 mg salbutamol, from 2.5 ml of saline, nebulised in oxygen at a flow rate of 6 litres per minute via a system 22 nebuliser (Medic-Aid, UK). The spirometer used to measure FEV1, FVC and FEV1/FVC ratio was a non-computerised volume-time wedge bellows spirometer (Vitalograph, Buckingham, UK). Bronchodilator response is expressed as an absolute change in milliliters and as a percentage of the baseline value.
The flow and volume response status of the individual patients with the bronchodilator was determined by whether the increase in FEV1 and FVC exceeded that of known variability of the measurement. In keeping with previous data we accepted a change in FEV1 measurement of greater than 160 ml and FVC greater than 330 ml, as being unlikely to have arisen by chance (Citation[14]). Hence, our subjects were classified as volume responders if FVC increased > 330 ml post-bronchodilator and FEV1 by < 160 ml. Flow responders had a FEV1 response of > 160 ml but FVC change of < 330 ml. Flow and volume responders had an increase in FEV1 > 160 ml and FVC of > 330 ml. Non responders had increases in FEV1 < 160 ml and FVC < 330 ml.
Statistics
Data are presented as mean plus standard deviation for whole group data and mean plus standard error of the mean for sub-group data. Data were normally distributed and between group comparisons were made using Students t-tests for paired groups and analysis of variance (ANOVA) for multiple groups. Tukey's Honestly Significant Difference (HSD) was used to assess post-hoc between group comparisons. Statistical analysis was performed using SPSS version 12.0 and significance set at a level of 95% (p < 0.05).
RESULTS
The baseline characteristics of the 266 subjects are shown in . The group had moderate-to-severe COPD, an increased residual volume and mild to moderate reduction in diffusing capacity. Mean change in spirometry after reversibility testing is shown in . FEV1/FVC ratio fell from 0.46 to 0.44 (p < 0.001) compatible with a small increase in degree of airflow obstruction.
Table 1 Baseline characteristics of the 266 COPD subjects quoted as mean (SD)
Table 2 Whole group bronchodilator response to salbutamol quoted as mean (SD)
Sixty (23%) subjects were isolated volume responders, 32 (12%) were isolated flow responders, 65 (24%) were flow and volume responders and 109 (41%) were non-responders. The characteristics of the 4 groups are shown in and post-hoc analysis determining the differences between individual groups is shown in . Volume responders had a lower FEV1 (% predicted), lower FVC (% predicted), lower IC (% predicted) and higher residual volume.
Table 3 Characteristics of groups classified by response to salbutamol quoted as mean (SEM)
Table 4 Post hoc statistically significant differences between the groups defined by response to bronchodilators
We used the ratio of early to mid expiratory maximal flows (PEF/ FEF50) to identify patients with a dynamic airway collapse (15) pattern on their maximum expiratory flow volume loop. We compared this group to subjects with better preserved peak and mid expiratory flows by dividing the population into tertiles. Representative flow/volume curves for each of the three groups (preserved ratio—group 1, intermediate—group 2 and dynamic airway collapse—group 3) are shown in . The flow and volume post-bronchodilator responses and baseline characteristics of these groups are shown in .
Figure 1 Typical examples of the flow volume loops for group 1 (preserved ratio), group 2 (intermediate) and group 3 (dynamic airway collapse).
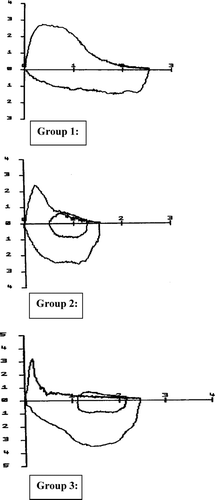
There was significantly lower FEV1 improvement (102 ml vs 153 ml; difference 51 ml; p = 0.004) but a trend towards a greater FVC improvement (396 ml vs 328 ml; difference 68 ml; p = 0.08) in the patients with dynamic airway collapse pattern compared with the group with least evidence of airway collapse. Subjects with the greatest degrees of a dynamic airway collapse had greater airflow obstruction (FEV1 and FEV1/FVC), poorer static lung volumes (FVC and SVC), were more hyperinflated at rest (RV and RV/TLC) and had a greater degree of emphysema (DLCO and KCO).
Table 5 Bronchodilator responsiveness and characteristics of subjects with likely dynamic airway collapse
DISCUSSION
Administering a bronchodilator drug to a patient with stable COPD serves several purposes. It defines the post-bronchodilator FEV1 accurately, which is a better guide to subsequent disease progression than the pre-bronchodilator value (Citation[16]). It also defines patients whose airflow obstruction is abolished by this treatment, an infrequent finding in our study population derived from a hospital out-patient service, although seen in some 20% of patients referred to community spirometric screening in our area(Citation[17]). This concept has been extended to suggest that the magnitude of improvement in spirometry after administration of a bronchodilator would identify patients who are most responsive to treatment. This has not proven to be the case (Citation[3], Citation[4], Citation[18]) and our data help explain why this should be so, as a majority of our patients showed some improvement in either volume and/or flow responses after spirometry. Almost 25% of patients had changes beyond the usual between test reproducibility of the FVC manoeuvre with little accompanying change in FEV1.
High-dose nebulised salbutamol increased FEV1 and FVC on average by an amount close to the between tests reproducibility of these measurements (Citation[14]) and there was no relationship between absolute change in either measure and the baseline value. Overall, these changes were not accompanied by a significant alteration of the FEV1/FVC ratio suggesting that the time constant for lung emptying in these patients did not change post-bronchodilator with the increase in volume being the result of additional lung units with similar mechanical properties contributing to expiration.
We saw evidence of a heterogeneous response with almost 25% of patients exhibiting a significant improvement in FVC with little change in FEV1, slightly more patients showing changes in both volume and flow and only 12% improving FEV1 with a little change in FVC. This latter group had better initial spirometry and a higher peak expiratory flow rate. In this they resembled those patients who showed no identifiable response to bronchodilators although this group also had a significantly greater inspiratory capacity and less gas trapping than subjects who showed an improvement in FVC. This suggests that a different mechanism, possibly involving changes in airway smooth muscle tone, predominates in these two subgroups. In contrast, the two volume responder groups resembled each other with regard to their baseline characteristics, both having a significantly lower FEV1, a greater reduction in inspiratory capacity and an increased residual volume compared to the unresponsive and flow responsive patients alone. In the volume responders the FEV1/FVC ratio predictably tended to worsen after the bronchodilator due to the disproportionate increase in volume relative to flow.
Total lung capacity measured by helium dilution was not different in this subgroup before testing, although it would have been more desirable had these measurements been made by body plethysmography. We saw no significant difference in the degree of emphysema assessed by gas transfer and KCO measurements. Previous work has suggested that volume responders had more CT defined emphysema but our data using KCO as a surrogate measure for emphysema did not support this view.
When we split our population by tertiles based on the peak to mid expiratory flow ratio, a reflection of the shape of the maximum expiratory flow volume envelope, we saw a significantly lower change in flow but a trend towards a greater change in volume in patients with the greatest collapse pattern, supporting the importance of volume change in these patients as the major effect of bronchodilators. Subjects with the greatest ‘collapse’ pattern were significantly more obstructed, had greater degrees of resting hyperinflation and had more emphysema, assessed using KCO. Our data are compatible with a greater volume change in patients breathing close to or under conditions of tidal expiratory flow limitation. Several studies have shown that bronchodilator drugs have a significant effect on inspiratory capacity when EFL is present during stable conditions (Citation[19], Citation[20], Citation[21]) and during exacerbations (Citation[22]) and this occurs without necessarily changing the degree of EFL (Citation[20], Citation[23]).
The reasons for these different patterns of response remain unclear. In a careful mechanistic study Cerveri found little change in small airway calibre where lung volume increased from FRC to TLC in patients who were pure volume responders while patients with a mixed volume and flow response had less severe emphysema on CT scanning (Citation[10]). Whether change in volume alone is beneficial to the patient is less certain as recent work has shown that when volume change occurs without the corresponding improvement in expiratory flows the resulting breathing pattern may impair exercise performance (Citation[24]).
Our study has a number of strengths and weaknesses. We have retrospectively reported standardised data collected in an unselected group of patients with a clinical and physiological diagnosis of COPD. Although we did not have access to data about inhaled corticosteroid use this does not appear to affect the immediate bronchodilator response (Citation[25], Citation[26]). We used salbutamol rather than a combination of salbutamol and ipratropium as in earlier studies where additional effects were seen at both low and high dose combinations (Citation[23], Citation[25]). Our data therefore represent a conservative estimate of the effect of bronchodilators, although the additive effect of combining drugs is relatively modest.
We did not record slow vital capacity as our primary index of volume improvement as this procedure is less standardised than the FVC maneuver. We did not adjust the FEV1/FVC ratios for age as each served as their own control. Had we been able to repeat these tests in our population on a separate occasion we would have been able to establish the reproducibility of this response. Undoubtedly, some patients would have changed responder group. The size of the isolated volume responders makes it unlikely that all of these would have been reclassified.
In summary, we have identified a significant number of symptomatic COPD patients who exhibited an increase in forced vital capacity and assuming a constant total lung capacity, a reduction in residual volume after high dose nebulised salbutamol. Whatever the functional effect of these changes it is clear that bronchodilator drugs affect both lung volumes as well as indices of expiratory flow, a change that becomes more obvious as baseline FEV1 declines. Future studies should identify the reproducibility of these volume-based definitions that appear to be an equally appropriate way of identifying the physiological effect of bronchodilators in symptomatic COPD patients.
REFERENCES
- Miller M R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten C P, Gustafsson P, Jensen R, Johnson D C, MacIntyre N, McKay R, Navajas D, Pedersen O F, Pellegrino R, Viegi G, Wanger J. ATS/ERS Task Force Standardisation of spirometry. Eur Respir J 2005; 26: 319–338
- Nisar M, Harris J E, Pearson M G, Calverley P MA. Acute bronchodilator trials in chronic obstructive pulmonary disease. Am Rev Respir Dis 1992; 146: 555–559
- Hay J G, Stone P, Carter J, Church S, Eyre-Brook A, Pearson M G, Woodcock A A, Calverley P M. Bronchodilator reversibility, exercise performance and breathlessness in stable chronic obstructive pulmonary disease. Eur Respir J 1992; 5: 659–664
- O'Donnell D E, Lam M, Webb K A. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160: 542–549
- American Thoracic Society/European Respiratory Society. 2005, URL: http://www.ersnet.org. Standards for the diagnosis and treatment of people with COPD. Accessed 24 July 2007
- Global Initiative for Chronic Lung Disease (GOLD). 2006, URL: http://www.gold.com. GOLD Workshop Report, 2006 update. Accessed 24 July 2007
- National Institute for Clinical Effectiveness. Chronic Obstructive Pulmonary Disease: Management of chronic obstructive pulmonary disease in adults in primary and secondary care. 2004, Accessed 24 July 2007 URL: http://www.nice.org.uk.
- Ramsdell J W, Tisi G M. Determination of bronchodilation in the clinical pulmonary function laboratory. Chest 1979; 76: 622–662
- Girard W M, Light R W. Should the FVC be considered in evaluating response to bronchodilator. Chest 1983; 84: 87–89
- Cerveri I, Pellegrino R, Dore R, Corsico A, Fulgoni P, van de Woestijne K P, Brusasco V. Mechanisms for isolated volume response to a bronchodilator in patients with COPD. J Appl Physiol 2000; 88: 1989–1995
- van Noord J A, Bantje T A, Eland M E, Korducki L, Cornelissen P J. A randomised controlled comparison of tiotropium nd ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study Group. Thorax 2000; 55: 289–294
- American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med 1995; 152: 1107–1136
- American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique, 1995 update. Am J Respir Crit Care Med 1995; 152: 2185–2198
- Tweeddale P M, Alexander F, McHardy G JR. Short-term variability in FEV1 and bronchodilator responsiveness in patients with obstructive ventilatory defects. Thorax 1987; 42: 487–490
- Calverley P M, Koulouris N G. Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology. Eur Respir J 2005; 25: 186–99
- Hansen E F, Phanareth K, Laursen L C, KokJensen A, Dirksen A. Reversible and irreversible airflow obstruction as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159: 1267–1271
- Walker P P, Mitchell P, Diamantea F, Warburton C J, Davies L. Effect of Primary Care Spirometry on the Diagnosis and Management of COPD. Eur Respir J 2006; 28: 945–952
- Belman M J, Botnick W C, Shin J W. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 153: 967–975
- Pellegrino R, Brusasco V. Lung hyperinflation and flow limitation in chronic airway obstruction. Eur Respir J 1997; 10: 543–549
- Tantucci C, Duguet A, Similowski T, Zelter M, Derenne J-P, Milic-Emili J. Effect of salbutamol on dynamic hyperinflation in chronic obstructive disease patients. Eur Respir J 1998; 12: 799–804
- Boni E, Corda L, Franchini D, Chiroli P, Damiani G P, Pini L, Grassi V, Tantucci C. Volume effect and exertional dyspnoea after bronchodilator in patients with COPD with and without expiratory flow limitation at rest. Thorax 2002; 57: 528–532
- Stevenson N J, Walker P P, Costello R W, Calverley P M. Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172: 1510–1516
- Hadcroft J, Calverley P MA. Alternative methods for assessing bronchodilator reversibility in chronic obstructive pulmonary disease. Thorax 2001; 56: 713–720
- Aliverti A, Rodger K, Dellaca R L, Stevenson N, Lo Mauro A, Pedotti A, Calverley P M. Effect of salbutamol on lung function and chest wall volumes at rest and during exercise in COPD. Thorax 2005; 60: 916–924
- Calverley P M, Burge P S, Spencer S, Anderson J A, Jones P W. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax 2003; 58: 659–664
- Anthonisen N R, Wright E C. Bronchodilator response in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986; 133: 814–819