Abstract
Cold air hyperventilation is an indirect challenge (cold air challenge, CACh) with high specificity and low sensitivity in defining asthmatic subjects. A small proportion of chronic obstructive pulmonary disease (COPD) patients present with positive CACh. The aim of this prospective study was to investigate the presence of factors related to cold air challenge (CACh) in COPD patients. Factors examined were FEV1, FEV1/FVC, reversibility after bronchodilation, eosinophils in induced sputum, bronchial hyperresponsiveness to methacholine and the spirometric response to tiotropium compared to placebo. We studied 92 consecutive COPD patients in order to retrieve 15 CACh positive (+) patients. Fifteen COPD patients with negative CACh [CACh(−)], randomly selected from the initial group, were added in order to retrieve a group of 30 patients. Spearman's correlation coefficient was used in order to evaluate possible significant correlations between CACh values and study parameters. Sixteen percent of our subjects presented CACh(+). CACh values were repeatable with an intraclass correlation coefficient between the two measurements 0.980 (95% CI 0.940–0.993). The only significant correlation observed was between Δ FEV1 after CACh [ΔCFEV1] and trough FEV1 values post tiotropium inhalation (r2 = 0.62, p < 0.0001). When we analyzed the response to tiotropium in the 2 separate groups we found that patients with CACh(+) presented significantly lower values of trough FEV1 compared to those with CACh(−). In conclusion, a small proportion of COPD patients present with bronchial hyperresponsiveness to CACh. The only parameter related to CACh (+) in our study was a smaller bronchodilating effect of tiotropium.
INTRODUCTION
Bronchial hyperresponsiveness (BHR) is a hallmark of the pathophysiology of asthma and is related to the underlying inflammation, the disease severity and the response to treatment (Citation[1]). The prevalence and the underlying mechanism for BHR in Chronic Obstructive Pulmonary Disease (COPD) are not clearly established. It is still not clear whether it represents part of the pathophysiology of the disease or just an accompanying epiphenomenon (Citation[2]). Cold air hyperventilation is an indirect challenge (cold air challenge, CACh) with high specificity and low sensitivity for the recognition of asthmatic subjects (Citation[3]). A small proportion of COPD patients present with positive CACh [CACh(+)]; however, no specific underlying factors related to this indirect BHR have been identified (Citation[4], Citation[5]). The vagal tone and the function of the muscarinic (M) receptors may play a role in BHR, given that a dysfunction of the M2 receptors could lead to bronchoconstriction through the suppression of the negative feedback of acetylcholine, whereas the increased function of the M3 receptors could also provoke bronchoconstriction through the increased release of acetylcholine (Citation[6]). Anticholinergic drugs act by inhibiting the neurotransmitter acetylcholine which is released from the parasympathetic nerves in the vagus.
The aim of this prospective study was to investigate the presence of factors related to cold air challenge (CACh) in COPD patients. Factors examined were FEV1, FEV1/FVC, reversibility after bronchodilation, eosinophils in induced sputum, bronchial hyperresponsiveness to methacholine and the spirometric response to tiotropium compared to placebo. The latter evaluation was based on the pharmacological properties of tiotropium, a long acting bronchodilator which causes short term suppression of the M2 receptors and long term suppression of the M3 receptors (Citation[7]).
METHODS
Patients
Patients' characteristics are summarized in . We studied 92 patients with COPD, all male, current smokers, diagnosed according to the GOLD guidelines (Citation[8]). None of our patients had atopy, as assessed by the low IgE levels and negative skin prick tests to 6 common aeroallergens. All patients had been recently diagnosed with COPD for the first time and were not receiving any specific treatment, besides short acting bronchodilators (either beta-agonists and anticholinergics or their combination) on demand. They were all in stable condition and had no evidence of exacerbation for at least 4 weeks prior to the study.
Table 1 Subjects' characteristics
The final number of patients was the total number of COPD patients who underwent isocapnic cold air challenge in order to retrieve 15 patients with positive test [CACh(+)]. Only patients with mild-to-moderate COPD were included (Citation[8]), in order to be in agreement with the instructions of the accepted baseline lung function before challenges (Citation[9]). Fifteen COPD patients with negative CACh [CACh(−)], randomly selected from the initial group, were added in order to retrieve a group of 30 patients. The characteristics of the two subgroups are also presented in .
Study protocol details
In the group of the 30 patients, simple spirometry was performed for the assessment of post bronchodilator FEV1 (% of the predicted value), post bronchodilator FEV1/FVC ratio, and degree of reversibility after bronchodilation. Additional tests included sputum induction for the measurement of eosinophils, assessment of serum eosinophilic cationic protein (ECP), methacholine challenge testing. Additionally, the spirometric response to tiotropium was examined after inhalation of a single dose of 18 μ g of tiotropium bromide with subsequent measurements of FEV1. The latter assessment was controlled with the administration of placebo to the same patients in a random order.
The timetable for the assessment of these parameters had as follows: Spirometry, sputum induction and serum ECP measurement were performed on day 1. On day 2 methacholine challenge test was performed. On day 3 reversibility of airway obstruction was performed. On days 4 and 5 tiotropium or placebo inhalation and FEV1 measurements on two separate days were performed. Seven days after metacholine challenge a cold air challenge was performed. On each separate day simple spirometry was performed before the measurements in order to ensure that patients presented similar values of FEV1. All patients were in stable condition throughout the study.
The differentiation of COPD patients with reversibility of airway obstruction from asthmatics was done as previously described (8, 10). The diagnosis of COPD in these patients was made by two experienced clinicians (K.K. and T.K.) that were not aware of the subsequent results at the time of the evaluation of the patients.
All subjects were recruited from the outpatient clinics of the Army General Hospital of Athens and the clinics of IKA Neas Ionias during a period of 18 months. The Scientific Committee of the hospital approved the study protocol and all participants provided written informed consent.
Cold air challenge
CACh was performed according to an established protocol as a single step 4-minutes isocapnic hyperventilation test, as previously described (Citation[11]). Cold dry air was produced by a commercially available respiratory heat exchange system (RHES Jaeger Wuerzburg Germany). After the measurement of pre-challenge FEV1 (in liters) subjects hyperventilated absolutely dry air of −17°C at 75% of their maximal voluntary ventilation for 4 minutes. The correct level of hyperventilation was maintained by having the subject compete with a target balloon. A CO2-analyser continuously monitored the CO2 concentration in the expired air and CO2 was added to the inspired air in order to keep the subject eucapnic.
Three minutes after the end of the procedure, a second measurement of FEV1 was performed. The observed difference in the value of FEV1 was expressed as a percentage % difference from baseline (Δ CFEV1). The repeatability of the method was evaluated within 4 weeks from the initial measurement in the 30 selected subjects. For this method a ΔCFEV1of minus 9% or greater defines airway hyperresponsiveness (Citation[11]).
Methacholine challenge
Bronchial hyperresponsiveness to methacholine was measured by a rapid methacholine inhalation test for the determination of PD20, as previously described for histamine (Citation[12]). Briefly non-cumulative doses of metacholine were 0.025, 0.1, 0.15, 0.2, 0.3, 0.4, 0.4, 0.4 and 0.8 mg administered at one minute intervals until the provocative dose of metacholine causing a 20% fall in FEV1 (PD20). PD20 was determined by linear interpolation on a semi-logarithmic scale. Challenge was performed according to the American Thoracic Society guidelines (Citation[9]).
Lung function
Pulmonary function and reversibility tests were performed with a dry spirometer (Vica-test, Model VEP2; Mijnhardt; Rotterdam, Holland). FEV1and FVC were measured according to the American Thoracic Society criteria (Citation[13]). Reversibility test was performed according to the GOLD guidelines (Citation[8]). Briefly Patients should not have taken inhaled short-acting bronchodilators in the previous 6 hours. FEV1 was measured before the administration of 400 μ g of salbutamol via a spacer device and 10–15 minutes after administration post-bronchodilation FEV1 was evaluated (Citation[8]).
Sputum induction and processing
Sputum induction and analysis was performed as previously described (Citation[14]). An induction procedure using inhalation of an aerosol of hypertonic saline solution (3.5%) generated by a DeVilbiss ultrasonic nebulizer (2696 Somerset PA, USA) was chosen. At least 2 mL of sputum were collected in a sterile container. The person who did the differential cell counts in sputum (G.P.) was not aware of the clinical and functional status of the patients or the results of challenge.
ECP measurement
The concentrations of ECP in the serum were determined by a radioimmunoassay with a lower limit of detection of 2.0 μ g/L (Pharmacia ECP RIA; Pharmacia; Uppsala, Sweden).
Tiotropium inhalation
A single dose of 18 μ g of tiotropium or placebo (dry powder device like tiotropium) was administered in the subjects of the two subgroups between 0900 and 1000 am. The inhalation of tiotropium or placebo was administered to the patients in a random order on 2 consecutive days by an experienced nurse who was blinded to the medication. Before inhalation the absolute values of FEV1 were recorded in order to ensure that they were comparable to the baseline measurements of the evaluation of the subjects.
At 2, 3 hours, 1 hour before drug administration (23 hours) and immediately before the next drug administration (23 hours and 55 minutes) after the initial inhalation of tiotropium or placebo, the absolute values of FEV1 were recorded and the difference noticed was expressed as ΔTFEV1 (for tiotropium) or ΔPFEV1(for placebo) in liters. The predose FEV1 (trough FEV1) was calculated as the mean value of the two predose measurements (Citation[15]). Patients were asked not to use any extra bronchodilators during the procedure. Patients were also asked to record any bronchodilator usage before tiotropium/placebo assessments with no usage to be reported in either treatment regimens.
Smoking was not permitted 2 hours before the start of the procedure and 2 hours before measuring the trough FEV1 when they returned to the hospital the following morning. Smoking abstinence was ensured by close supervision of the patients during those periods. All measurements were performed under the supervision of an experienced nurse and one of the investigators (S.L.) who were blinded to the cold air challenge result (+ or −CACh).
Statistical analysis
Data are expressed as mean (SD) with ranges in parenthesis. For comparisons of values between two groups, Mann-Whitney U-tests (for skewed data: CACh, Δ FEV1 after CaCh, FEV1/FVC, eosinophils, ECP) and unpaired t-tests (for normally distributed data: age, BMI, FEV1% pred, PD20, reversibility) were used. Spearman's rank correlation coefficient was used in order to evaluate possible significant correlations between CACh values and study parameters. For the assessment of repeatability of cold air challenge testing after 4 weeks, the intraclass correlation coefficient with 95% confidence intervals (95% CI) was calculated. A p value < 0.05 was considered significant.
RESULTS
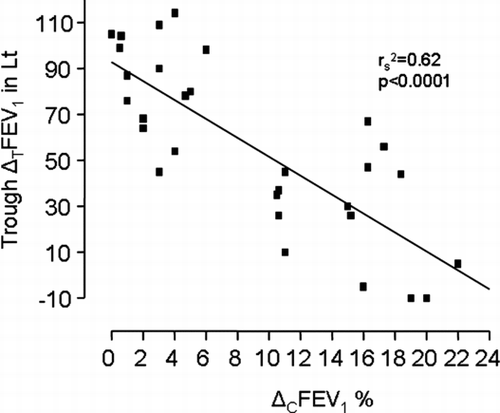
Fifteen of the total of 92 patients (16%) presented with positive CACh. Patients with CACh(+) had significantly higher values of ΔCFEV1 compared to those randomly selected with CACh(−) (15(4), range 10.5–22% vs. 2.65(2), range 0–6% respectively, p < 0.0001, )
Repeatability of CACh
Cold air challenge testing was repeated in the 30 selected subjects after 4 weeks, presenting excellent repeatability in both groups. Identical results regarding the positive or negative interpretation of test were found in all thirty subjects tested. Patients with CACh(+) on baseline and 4 weeks later presented Δ CFEV115.3(4)% vs. 14.9(3.5)% change from baseline respectively (p = 0.3). Patients with CACh(−) on baseline and 4 weeks later presented ΔCFEV1 2.65(2.0)% vs. 2.95(2.0)% change from baseline respectively (p = 0.2).The intraclass correlation coefficient between the two sets of measurements was 0.980 (95% CI 0.940−0.993). For the rest of the analysis we used the first value of Δ FEV1.
Correlations
Major correlation data are summarized in . Briefly, the only significant correlation was observed between the ΔCFEV1 and the ΔTFEV1 (in L) 24 hours post tiotropium inhalation (rs2 = 0.62, p < 0.0001, , ). No other significant correlation was observed.
Table 2 Correlations between ΔCFEV1and the parameters that might affect its values in the 30 study subjects of both groups.
Comparisons between the 2 groups
BHR to methacholine
No statistically significant difference was observed regarding the PD20 to methacholine between the CACh(+) and the CACh(−) groups [0.53(0.12) range 0.41–0.91 μ g vs. 0.52(0.06) range 0.44–0.67 μ g, respectively, p = 0.2 )].
Table 3 Study variables in the 2 subgroups
Reversibility of airway obstruction
No statistically significant difference was observed regarding the reversibility of airway obstruction after inhalation of a short acting β2-agonist between the CACh(+) and the CACh(−) groups [6(4.5) range 0–14 % of the baseline value of FEV1 vs. 7.5(3) range 3–15% of the baseline value of FEV1, respectively, p = 0.5 ()].
Sputum eosinophils—serum ECP
Two patients from the group with CACh(+) had 2% eosinophils in induced sputum, whereas in the group CACh(−) one patient had 3% eosinophils. None of the other patients had eosinophils in induced sputum. None of the patients with eosinophils presented significant reversibility of airway obstruction. Serum ECP did not differ significantly between the CACh(+) and the CACh(−) groups [5.5(2.5) range 2.6–10.5 IU/mL vs. 5.9(3) range 2.3–11.4 IU/mL, respectively, p = 0.4, ].
Spirometric values
No statistically significant difference in FEV1 % predicted was observed between the CACh(+) and the CACh(−) groups [67.5(5) range 60–78% vs. 69(5.5) range 60–78%, respectively, p = 0.26 ()]. Similar results were observed for the FEV1/FVC ratio [63.5(5) range 54–70% vs. 67(4) range 60–70%, respectively, p = 0.8 ()].
Tiotropium inhalation in the two separate groups
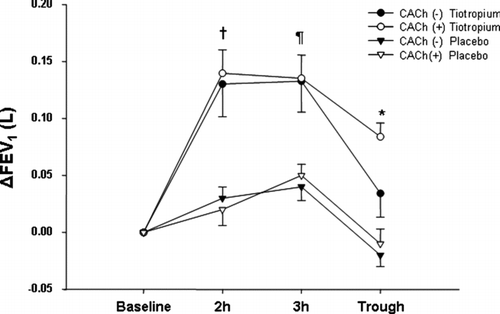
No statistically significant difference in ΔTFEV1was observed at 2 and 3 hours after tiotropium inhalation between the CACh(+) and the CACh(−) groups [0.130 (0.02) range 0.090–0.190 liters vs. 0.140 (0.020) range 0.110–0.170 liters, respectively, p = 0.1 at 2 hours; 0.130 (0.020) range 0.090–0.175 liters vs. 0.135 (0.020) range 0.090–0.165 liters, respectively, p = 0.25 at 3 hours, . Regarding the trough Δ TFEV1, patients with CACh(+) presented significantly lower values compared to those with CACh(−) [0.030(0.020) range −0.010–0.070 liters vs. 0.085 (0.020) range 0.045–0.110 liters, p < 0.0001, . The inhalation of placebo had no significant bronchodilating effect in either group; additionally, the Δ PFEV1 values were lower than the ones measured after tiotropium at any of the time points (p < 0.0001 for all measurements compared to tiotropium, ).
Figure 2 Tiotropium inhalation in patients with CACh (+) (filled circles) and CACh (−) (open circles). The Δ FEV1 did not differ significantly in patients with CACh (+) at 2 and 3 hours after the inhalation compared to those with CACh (−) (p = 0.9, and p = 0.27, respectively). Patients with CACh (−) had significantly higher values 24 hours post-inhalation Δ FEV1, compared to those with CACh (+) (*p = 0.01). The inhalation of placebo had no significant bronchodilating effect in patients with CACh (+) (filled triangles) and CACh (−) (open triangles), and the Δ FEV1 values were lower than the ones measured after tiotropium at all time points.
DISCUSSION
In the present study we have shown that a small proportion of patients with COPD present with BHR to CACh and that BHR to CACh is repeatable after four weeks. We did not identify any related factors for this type of BHR apart from a smaller bronchodilating effect after the inhalation of tiotropium bromide.
Previous studies in COPD patients have shown that a small proportion of patients present with CACh(+), whereas a higher percentage of patients present with positive methacholine challenge which has been related to the underlying degree of airflow obstruction (Citation[4], Citation[5]). The main differences of the present study compared to the previous ones is the larger number of subjects studied, the data about the repeatability of the procedure, and the selection of pharmacological response to tiotropium as a possible contributing factor for CACh-related BHR.
The reversibility of airway obstruction has been extensively studied in COPD with confusing results, which have been mainly attributed to the large within subject variability and the low repeatability of the measurements (Citation[16],Citation[17]). However, despite the fact that reversibility represents a hallmark of asthma pathophysiology, there are no data confirming its parallel course with BHR in COPD (Citation[18]). Similar results are presented in our study, where bronchodilator reversibility does not seem to be related to the CACh results. COPD patients with BHR to AMP showed an increased number of sputum eosinophils (Citation[19]). Likewise, the polymorphism of the receptor of IL-4, an eosinophil-chemoattractant cytokine, is significantly related to the development of BHR in COPD (Citation[20]). However, the data from the present study did not identify eosinophils as the cellular parameter that could be responsible for the presence of BHR—either in CACh or methacholine challenge—in order to separate the two distinct groups.
An interesting finding of the current study is the absence of significant correlations between CACh, methacholine BHR and the parameters expressing airway obstruction, and this is in contrast to previous studies (Citation[21]). This might be attributed to the fact that a small increase in the thickness of an airway as a result of the chronic inflammatory process may markedly augment the increase in airway resistance induced by a bronchoconstrictor without being affected or related to the baseline lung function measurement (Citation[22]).
Airway smooth muscle expresses abundant muscarinic M2 and M3 receptors, roughly in a 4:1 ratio. Despite their lower expression levels, M3 receptors represent the primary subtype responsible for acetylcholine-mediated bronchoconstriction in bronchial and tracheal smooth muscle (Citation[23], Citation[24]). Tiotropium is a long acting bronchodilator which causes short-term suppression of the M2 receptors and long-term suppression of the M3 receptors (Citation[7]). Based on our results, we hypothesize that M2 receptors are probably working properly, since there is no difference regarding the bronchodilative effect of tiotropium within 2 to 3 hours after administration for both methacholine and CACh BHR. These results are partially confirmed by a previous study, where nasal cold air challenge with simultaneous administration of an M2 receptor agonist prevented the cold air-induced bronchoconstriction, indicating normal function of M2 receptors (Citation[25]).
A critical point for the assessment of CACh-related BHR may possibly be the functional status of M3 receptors. A hyperactivity of M3 receptors could lead to increased and/or prolonged release of acetylcholine that eventually results in further bronchoconstriction. In our study we showed that the bronchodilative effect of tiotropium, as expressed by the trough ΔTFEV1, is reduced in relation to Δ CFEV1values after CACh. This might be indirectly attributed to the different functional status of M3 receptors according to the degree of CACh related BHR. Our data are partially supported by previous in vitro studies, where bronchoconstrictive responses were totally abolished in mice lacking M3 receptors (Citation[26]). Additionally, the pathogenesis of airway hyperresponsiveness is related to excessive effect of M3 muscarinic receptors on the airway smooth muscle (Citation[27]) and, finally, antigen-induced bronchial hyperresponsiveness does not appear to depend upon M2-receptor dysfunction (Citation[28]). The absence of significant differences after the inhalation of placebo further confirms our hypothesis.
A previous study raised the issue of absence of short-term spirometric response to tiotropium in some patients with COPD (Citation[29]). A practical issue arising from the present study is that it may provide a possible contributing factor for the above issue, since patients with CACh(+) presented smaller bronchodilative response after tiotropium inhalation.
A potential study limitation is the simplicity of CACh protocol used in the present study. Unlike other protocols used in athletes or patients, the current protocol included a hyperpnoea of a relatively short duration and single pre-and post-measurements of FEV1. A protocol with multiple FEV1 measurements may have resulted in decreasing the plausible variability of FEV1 in study subjects and the final patients' misclassification. However we believe that we overcome these plausible limitations by presenting repeatability data as well as by emphasizing the reproducibility of baseline FEV1 values in all study measurements.
In conclusion, we have confirmed that a small proportion of COPD patients present with bronchial hyperresponsiveness to isocapnic cold air challenge. The only parameter related to CACh(+) was a smaller bronchodilating effect after the inhalation of tiotropium bromide. Further studies are warranted to clarify the role of bronchial hyperresponsiveness to cold air in COPD.
REFERENCES
- Grootendorst D C, Rabe K F. Mechanisms of bronchial hyperreactivity in asthma and COPD. Proc Am Thorac Soc 2004; 1: 77–87
- Wise R A. The relationship between airways hyperreactivity and COPD. Eur Respir Rev 2002; 12: 386–389
- Joos G F, O'Connor B, Anderson S D, Chung F, Cockcroft D W, Dahlen B, Di Maria G, Foresi A, Hargreave F E, Holgate S T, Inman M, Lotvall J, Magnussen H, Polosa R, Postma D S, Riedler J. ERS Task Force Indirect airway challenges. Eur Respir J 2003; 21: 1050–1068
- Ramsdale E H, Roberts R S, Morris M M, Hargreave F E. Differences in repsonsiveness to hyperventilation and methacholine in asthma and chronic bronchitis. Thorax, 40: 422–426
- Ramsdale E H, Morris M M, Roberts R S, Hargreave F E. Bronchial responsiveness to methacholine in chronic bronchitis: relationship to airflow obstruction and cold air responsiveness. Thorax 1984; 39: 912–918
- Costello R W, Jacoby D B, Fryer A D. Pulmonary neuronal M2 muscarinic receptor function in asthma and animal models of hyperreactivity. Thorax 1998; 53: 613–618
- Panning C A, De Bisschop M. Tiotropium: an inhaled, long-acting anticholinergic drug for chronic obstructive pulmonary disease. Pharmacotherapy 2003; 23: 183–189
- Global Initiative for Chronic Obstructive Lung Disease. July, 2005, www.goldcopd.com Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease. NHLBI/WHO workshop report. Bethesda, National Heart, Lung and Blood Institute, April 2001; Update of the Management Sections, GOLD website Date updated
- American Thoracic Society. Guidelines for methacholine and exercise challenge testing. Am J Respir Crit Care Med 1998; 161: 309–329
- Kostikas K, Gaga M, Papatheodorou G, Karamanis T, Orphanidou D, Loukides S. Leukotriene B4 in exhaled breath condensate and sputum supernatant in patients with COPD and asthma. Chest 2005; 127: 1553–1559
- Modl M, Eber E, Steinbrugger B, Weinhanl E, Zach M S. Comparing methods for assessing bronchial responsiveness in children: single step cold air challenge, multiple step cold air challenge, and histamine provocation. Eur Respir J 1995; 8: 1742–1747
- Zervas E, Loukides S, Papatheodorou G, Psathakis K, Tsindiris K, Panagou P, Kalogeropoulos N. Magnesium levels in plasma and in erythrocytes before and after histamine challenge. Eur Respir J 2000; 16: 621–625
- American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med 1995; 152: 1107–1136
- Pizzichini E, Pizzichini M M, Leigh R, Djukanovic R, Sterk P J. Safety of sputum induction. Eur Respir J 2002, Suppl 37: 9–18s
- Vincken W, van Noord J A, Greefhorst A P, Bantje T A, Kesten S, Korducki L, Cornelissen P J, Dutch/Belgian Tiotropium Study Group. Improved health outcomes in patients with COPD during 1 yr's treatment with tiotropium. Eur Respir J 2002; 19(2)209, 216
- Calverley P M, Burge P S, Spencer S, Anderson J A, Jones P W. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax 2003; 58: 659–664
- Anthonisen N R, Lindgren P G, Taskhin D P, Kanner R E, Scanlon P D, Connet J E, Lung Health Study Research Group. Bronchodilator response in Lung Health Study over 11 years. Eur Respir J 2005; 26: 45–51
- Wise R A, Kanner R E, Lindgren P, Connet J E, Altose M D, Enright P L, Taskhin D P. The effect of smoking intervention and an inhaled bronchodilator on airways reactivity in COPD. Chest 2003; 124: 449–458
- Rutgers S R, Kerstjens H A, Timens W, Tzanakis N, Kauffman H F, Postma D S. Airway inflammation and hyperresponsiveness to adenosine 5′-monophosphate in chronic obstructive pulmonary disease. Clin Exp Allergy 2000; 30: 657–662
- He J Q, Connett J E, Anthonisen N R, Sanford A J. Polymorphisms in the IL-13, IL-13RA1 and IL-4RA genes and rate of decline in lung function in smokers. Am J Respir Cell Mol Biol 2003; 28: 379–385
- Martin J G, Duguet A, Eidelman D H. The contribution of airway smooth muscle to airway narrowing and airway hyperresponsiveness in disease. Eur Respir J 2000; 16: 349–354
- Wiggs B R, Busken C, Paré P, James A, Hogg J C. A model of airway narrowing in asthma and in chronic obstructive airways disease. Am Rev Respir Dis 1992; 145: 1251–1258
- Gosens R, Zaagsma J, Meurs H, Halayko A J. Muscarinic receptor signalling in the pathophysiology of asthma and COPD. Respir Res 2006; 7: 73
- Fryer A D, Jacoby D B. Muscarinic receptors and control of airway smooth muscle. Am J Respir Cri Care Med 1998; 158: S154–S160
- On L S, Boonyongsunchai P, Webb S, Davies L, Calverley P M, Costello R W. Function of pulmonary neuronal M2 muscarinic receptors in stable Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 2001; 163: 1320–1325
- Struckmann N, Schwering S, Wiegand S, Gschnell A, Yamada M, Kummer W, Wess J, Haberberger R V. Role of muscarinic receptor subtypes in the constriction of peripheral airways: studies on receptor-deficient mice. Mol Pharmacol 2003; 64: 1444–1451
- Kadota H, Kuwahara M, Nishibata R, Mikami H, Tsubone H. Effect of M2 and M3 muscarinic receptors on airway responsiveness to carbachol in bronchial-hypersensitive (BHS) and bronchial-hyposensitive (BHR) guinea pigs. Exp Anim 2001; 50: 49–58
- Patel H J, Douglas G J, Herd C M, Spina D, Giembycz M A, Barnes P J, Belvisi M G, Page C P. Antigen-induced bronchial hyperresponsiveness in the rabbit is not dependent on M(2)-receptor dysfunction. Pulm Pharmacol Ther 1999; 12: 245–255
- Tashkin D, Kesten S. Long-term treatment benefits with tiotropium in COPD patients with and without short-term bronchodilator responses. Chest 2003; 123(5)1441–1449