Abstract
Computed tomographic based indices of emphysematous lung destruction may highlight differences in disease pathogenesis and further enable the classification of subjects with Chronic Obstructive Pulmonary Disease. While there are multiple techniques that can be utilized for such radiographic analysis, there is very little published information comparing the performance of these methods in a clinical case series. Our objective was to examine several quantitative and semi-quantitative methods for the assessment of the burden of emphysema apparent on computed tomographic scans and compare their ability to predict lung mechanics and function. Automated densitometric analysis was performed on 1094 computed tomographic scans collected upon enrollment into the National Emphysema Treatment Trial. Trained radiologists performed an additional visual grading of emphysema on high resolution CT scans. Full pulmonary function test results were available for correlation, with a subset of subjects having additional measurements of lung static recoil. There was a wide range of emphysematous lung destruction apparent on the CT scans and univariate correlations to measures of lung function were of modest strength. No single method of CT scan analysis clearly outperformed the rest of the group. Quantification of the burden of emphysematous lung destruction apparent on CT scan is a weak predictor of lung function and mechanics in severe COPD with no uniformly superior method found to perform this analysis. The CT based quantification of emphysema may augment pulmonary function testing in the characterization of COPD by providing complementary phenotypic information.
INTRODUCTION
Chronic Obstructive Pulmonary Disease (COPD) is characterized by incompletely reversible airflow obstruction due to small airways remodeling and dynamic expiratory airways collapse secondary to the presence of emphysema. Classically, subjects with COPD were thought to have a preponderance of either emphysema or airways disease (Citation[1]); however, these processes are no longer thought of as mutually exclusive but rather coincident and likely additive with regard to functional impairment (Citation[2]). While current standards for the diagnosis and staging of COPD are based largely upon spirometric measures of lung function (Citation[3]), such metrics are insensitive to the etiology of functional impairment. Because of this, new methods are being sought to augment the characterization of subjects with COPD (Citation[4]). One such tool is analysis of Computed Tomographic (CT) imaging of the lungs.
There are several CT based methods commonly employed in clinical investigation for the assessment of the burden of emphysema, including such techniques as a semi-quantitative visual scoring systems and more automated densitometric analyses (Citation[5], Citation[6], Citation[7], Citation[8], Citation[9], Citation[10], Citation[11]). Prior investigations have reported correlations of such metrics of emphysema and measures of lung function (Citation[12], Citation[13], Citation[14], Citation[15]). The literature also suggests that CT imaging may aid in the selection of subjects for therapeutic interventions such as surgical lung volume reduction (Citation[16], Citation[17]).
Questions remain, however, regarding the selection of an optimal method for CT scan analysis and the impact of using these different techniques on correlative studies to lung function and mechanics. Data from a large cohort of well-characterized subjects enrolled in the National Emphysema Treatment Trial (NETT) will allow examination of the performance and comparability of these methods. Such an examination may also provide insight into the remarkable heterogeneity of the pulmonary processes present in COPD. Aspects of this analysis have been published in abstract form (Citation[18]).
METHODS
During active patient enrollment in the NETT, 1218 patients underwent functional and radiographic testing after rehabilitation and prior to randomization (Citation[16]). Of these, 1094 of these patients had CT scans and corresponding pre-randomization pulmonary function tests that were used in this investigation. Post-randomization data was excluded from this analysis. Subjects were included in the NETT if they had CT scan evidence of bilateral emphysema, a post-bronchodilator TLC and RV of ≥ 100% and 150% respectively and a post-bronchodilator FEV1 of ≤ 45% predicted. Detailed inclusion and exclusion criteria for study enrollment can be found Supplementary Appendix 1 of the primary outcomes publication (http://www.nejm.org) (Citation[16]).
CT scans
Imaging protocols were established for single slice spiral CT scanners (General Electric, Siemens, and Picker). Using an equivalent standard or “lung” reconstruction algorithm for each brand of scanner, volumetric imaging data was collected without intravenous contrast using a collimation of 5–8 mm, pitch of 1.7:1, 50% overlap, a field of view (FOV) defined by the outer ribs at their widest dimension, and 120 to 140 kVp. Study subjects were instructed to perform 3 to 4 hyperventilatory breaths immediately prior to imaging. The CT scans were then acquired in a cranial to caudal direction with the subject holding their breath at full inflation (TLC).
Computed tomographic based measures of emphysema were performed using Hounsfield Unit thresholds of −950, −930, −910, −850, and −810 and a software package developed by the University of Iowa (Pulmonary Analysis Software Suite). These measures were calculated for both the whole and regional lung fields. In the latter case, the lung was divided into equal upper, middle, and lower thirds based upon cranial-caudal height. Additional densitometric measures used for this investigation include the mean lung density (HU) and the HU threshold below which 15% of the lung voxels fall (Perc15) (Citation[19]).
A second set of high-resolution CT scan images was obtained contemporaneously with the conventional scans. Radiologists visually scored these image sets using a 5-point ordinal system applied to each of 3 regions in each lung defined as the lung apex to the top of the aortic arch (upper zone), aortic arch to the right inferior pulmonary vein (mid zone), and the right inferior pulmonary vein to the most caudal extent of the lungs (lower zone) (Citation[16]). “Zero” was defined as normal or having no emphysema, 1 as mild or 1–25% emphysema, 2 as moderate with 26–50% emphysema, 3, marked with 51–75% emphysema and 4 as severe with > 75% emphysema. This was performed on the left and right lung with a total possible score of 24 if all 6 regions consisted of > 75% emphysema. The sum of these regional scores for each subject was used for correlation studies.
Pulmonary function tests
Pulmonary function test data included spirometry, lung volumes, and diffusing capacity for carbon monoxide. Each of the 17 clinics participating in the trial used their own pulmonary function lab following American Thoracic Society guidelines (Citation[20], Citation[21]). Post-bronchodilator values of lung function were used for this analysis.
Lung mechanics
At the time of subject randomization, a subset of 225 subjects underwent placement of an esophageal balloon catheter to estimate pleural pressure (Citation[22]). Static transpulmonary pressures at total lung capacity were reported as static lung recoil.
Statistical analysis
Univariate measures of pulmonary function, the percent of emphysema on the CT scans, the sum of the radiologist scores, and the measures of lung mechanics were treated as continuous data. Pearson correlation coefficients for each of the Hounsfield Unit thresholds and measures of pulmonary function were assessed. p values <.001 (Bonferroni adjusted α′ = 0.05/50 = 0.001) were considered to be statistically significant. SAS Version 8.2 (Cary, NC) was used for statistical analysis.
RESULTS
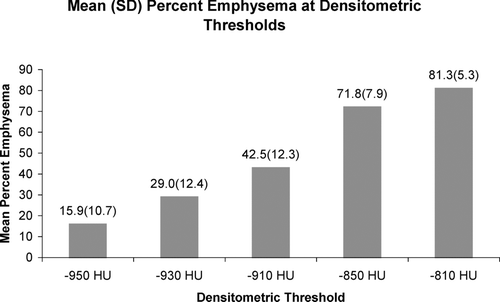
Patient characteristics at time of enrollment are provided in . A total of 1094 CT scans with corresponding pulmonary function tests were available for analysis in all categories except for the percent-predicted DLCO. illustrates the effect of HU threshold selection on the percent emphysema in the cohort. As shown, the percentage of lung designated as emphysematous increased with less negative HU thresholds. The mean lung density in the cohort was −864 ± 21 HU and the mean HU threshold below which 15% of the voxels fell was –946 ± 18 HU. Finally, the mean total lung score for the visual assessment of emphysema was 16.5 (± 3.8), which corresponds to an average score of 3 (50–75%) for each of the six lung zones assessed.
Table 1 Characteristics of subjects including post rehabilitation, post bronchodilator pulmonary function measurements and corresponding pre rehabilitation CT scans (n = 1094 unless specified)
provides univariate correlations of measures of pulmonary function to percent emphysema as defined by a range of densitometric HU thresholds. Pearson correlation coefficients and their corresponding p values are shown. Weak but statistically significant correlations were found between measures of lung function and the burden of emphysema at all thresholds with some exceptions. These included all HU defined measures of emphysema and the FVC % predicted, the HU of −810 and the DLCO % predicted, and both the FEV1 % predicted and the FEV1/FVC ratio using a threshold of −950 HU. provides correlations between pulmonary function and the radiologist scoring system, mean lung density and Perc15. Modest but statistically significant correlations are seen with all measures of lung function except for the Forced Vital Capacity (FVC). and illustrate the relationship of percent emphysema on CT scan and the FEV1and DLCO expressed as percent predicted. For the regression of percent emphysema on FEV1 and DLCO percent predicted, the coefficients of determination were 0.036 and 0.078, explaining approximately 4 and 8 percent of the variability in those measures, respectively.
Figure 2 Relationship of FEV1 percent predicted and percent emphysema using a density threshold of −910 HU.
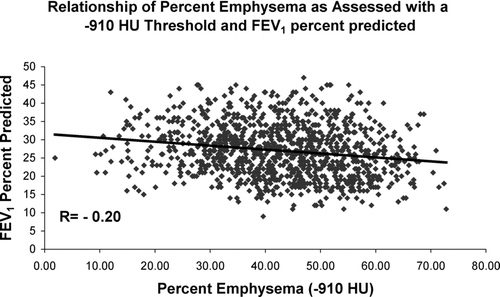
Figure 3 Relationship of DLCO percent predicted and percent emphysema using a density threshold of −910 HU.
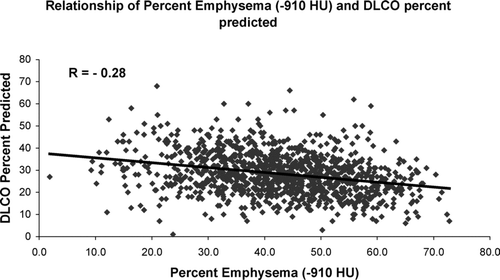
Table 2. a Univariate correlations of measures of pulmonary function and percent emphysema as defined by a range of HU density mask thresholds
Table 2. b Univariate correlations of measures of lung function and the CT burden of emphysema as assessed by the radiologist visual score, and objective measures of mean lung density and the Hounsfield Unit threshold that defines the lowest 15% of lung field attenuation (perc15)
Additional investigation was performed with a HU of −910 to define regional (upper and lower lung zone) emphysema. Using these measures, there was no difference in the strength of the correlation between either the upper or lower zone emphysema and the FEV1 % predicted (R = −0.14, p < 0.0001; R = −0.15, p < 0.0001, respectively). Lower zone emphysema was a stronger predictor of the FEV1/FVC ratio (R = −0.21, p < 0.0001 and R = −0.11, p = 0.0001, respectively) while upper zone emphysema was more strongly correlated with a subject's diffusing capacity expressed as a percent predicted (R = −0.27, p < 0.0001; R = −0.11, p = 0.0005).
Finally, in an attempt to directly compare the various methods of objectifying the CT based burden of emphysema, univariate regression was performed between each of the densitometric measures of emphysema (HU thresholds, perc15, and mean lung density) and the radiologist scoring system. The strongest correlation between the densitometric thresholds and the radiologist score occurred with a HU threshold of −930 (R = 0.37, p < 0.0001). There was an inverse relationship observed between the visually determined burden of emphysema and both the perc15 (R = −0.34, p < 0.0001) and the mean lung density (R = −0.27, p < 0.0001).
In the subset of 225 subjects with corresponding lung mechanics measurements, a HU threshold of −910, the mean lung density, Perc15, and radiologist visual scoring system were used to explore the relationship of the burden of emphysema and lung static recoil at total lung capacity. Subject characteristics for the cohort with lung mechanics data were similar to that of the group as a whole and are provided in . There was no significant relationship between any of these metrics and lung static recoil as assessed with an esophageal balloon (R = 0.03, p = 0.67; R = −0.05, p = 0.48; R = −0.03, p = 0.62; R = 0.05, p = 0.48 for a HU threshold of −910, mean lung density, Perc15, and the radiologist scoring system respectively). The relationship of the visual scoring system and lung static recoil is further depicted visually in .
Figure 4 Relationship of lung static recoil as measured with an esophageal balloon and the Radiologist Visual Scoring System.
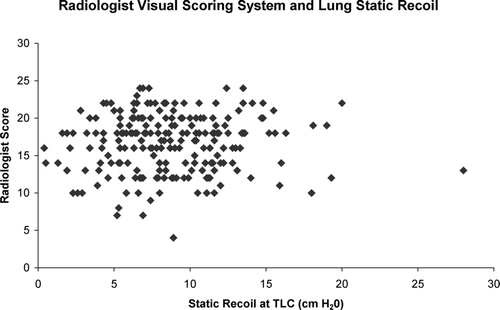
Table 3 Characteristics of subjects that had pulmonary function measurements, corresponding CT scans, and lung mechanics measurements at time of enrollment
DISCUSSION
In this investigation, several methods were employed to assess the burden of emphysema apparent on CT scan including semi-quantitative visual interpretation and objective densitometric measurements. These were found to be of modest correlative value for selected univariate measures of pulmonary function but poorly correlated of the lung static recoil. With all methods, the strongest correlations were found with those lung function tests that measured lung hyperinflation: RV and TLC. Correlations of a similar magnitude were found with the DLCO. Weaker correlations were found with the spirometric measures of function FEV1,FVC, and FEV1/FVC with the weakest being the FVC percent predicted. Regional measures of emphysema proved to be no better than whole lung measures of emphysema for correlative analysis however as shown previously, lower lung zone emphysema was more strongly associated with measures of airflow obstruction while upper zone disease was more strongly correlated with measures of gas exchange (Citation[23]).
A range of HU thresholds (−810 to −950) were employed in the densitometric quantification of emphysema including the −910 HU believed to be an optimal choice for analysis given the reconstruction of the scans in this investigation (Citation[6]). While visual inspection of , reveals a trend of increasing correlation coefficients with less negative HU thresholds, the strength of these correlations was modest in all cases. Interestingly, the trend in the strength of correlations between the HU thresholds and the DLCO was opposite that of all other univariate measures with the magnitude of the coefficients of determination (R2) increasing with more negative HU values. The reason for this observed trend is unclear.
Similar correlative studies were performed with measures of pulmonary function and the HU threshold that defined the lowest 15% of lung volume, the Perc15. While it is essentially the reciprocal to the HU thresholding (rather than having the HU threshold determine the percent emphysema, the percent emphysema defines the HU threshold), it may offer some advantages over conventional densitometric thresholding. Prior publications have suggested that in certain regions of the lung density histogram, densitometric thresholding may be insensitive to the burden of emphysema, a property not shared by the Perc15 (Citation[24]). Because of this, the Perc15 may be more sensitive for the detection of disease progression and may be a more useful tool for correlative studies (Citation[19]). In this investigation, the Perc15 was comparable to densitometric thresholding in its ability to predict lung function with the univariate coefficients of determination being closest to those found with a HU threshold of −950.
The final automated densitometric measure of emphysema employed in this investigation was the Mean Lung Density. While prior investigations have reported correlations between the MLD and measures of lung function, this metric has significant limitations. It is quite sensitive to image noise and has been found to be less sensitive to changes in lung density than other automated methods (Citation[25]). Because of this, it is not an optimal tool for the assessment of parenchymal disease progression. In the present investigation, MLD was found to be of similar predictive value for measures of lung function and did not clearly outperform the Perc15 or the densitometric thresholding methods.
The visual grading of HRCT images by the study radiologists proved to be inferior to densitometric analysis for correlation with most measures of lung function. These results must be interpreted with some caution since the visual scoring was performed on the higher resolution images while the automated densitometric methods utilized the standard clinical reconstructions. As reported with densitometric thresholding methods, this difference in reconstruction algorithms is systematic in its influence on the burden of emphysema apparent on the images (Citation[26]).
Higher-resolution reconstruction algorithms have been reported to increase the fraction of emphysematous lung for a given Hounsfield Unit threshold. Consistent with this observation is the higher mean burden of emphysema determined by visual as compared to densitometric methods. This effect should, however, be systematic and therefore should not significantly influence analysis. It is more likely that the subjectivity of the visual quantification methods, specifically the inter-observer variability in scoring had a significant impact on the strength of these correlative measures.
The lack of a correlation between the burden of emphysema apparent on CT scan and static recoil supports previous observations made by Baldi et al., and may in part be due to the limitations of the currently available imaging technology (Citation[27]). While the standard anatomic definition of emphysema is one of permanent dilation and destruction of the terminal airspaces due to loss of lung parenchyma (Citation[28]), CT imaging lacks the resolution to directly detect and differentiate such microscopic processes as centrilobular (CLE) and panlobular emphysema (PLE).
Further, it is unclear if the macroscopic pattern of parenchymal destruction apparent on the CT image assessed by automated methods can be used to infer the presence and quantity of these distinct pathologic conditions. Finally, both visual and objective methods for assessing the burden of emphysema do not differentiate emphysematous destruction of the lung tissue and over-expanded lung tissue due to gas trapping. Whereas one is a direct result of parenchymal destruction, the other reflects regional expiratory airflow obstruction that may not be representative of the aggregate burden of lung disease. Such a lack of distinction may in part be responsible for the weak predictive value of “emphysema” and lung function in this study. Additional work is needed to investigate and clarify the ability of quantitative CT scan analysis to predict the presence of these conditions. Given recent published data on the relationship of small airways disease and CLE, one solution may the measurement of the airway wall thicknesses (Citation[29]).
A surprising finding in this investigation is the wide range of emphysema on CT scan in a study cohort that was selected to have emphysema as their predominant manifestation of lung disease. For example, using a threshold of −910HU to define airspace disease, the burden of emphysema present in the study subjects ranged from 10 to over 70% of the lung. A similar finding is shown when examining the relationship of lung function and percent emphysema (). Again, in this investigation, a subject with an FEV1 of 25% predicted had between 10 and 70% of their lung defined as emphysema. Such a finding suggests that CT image analysis may be complementary to the phenotyping data obtained through pulmonary function testing. Additional investigation is required to determine if such factors as the type of emphysema, centrilobular or panlobular, and CT based quantitative measures of airway disease would provide further discriminatory data in this cohort.
While the size of the cohort is larger than any previously reported for such an analysis, the relative homogeneity of the lung function within the cohort limits the conclusions that can be drawn. As is outlined in the NETT inclusion criteria, this group consists of subjects with severe ventilatory obstruction and emphysema on CT scan. A second limitation to the investigation is the densitometric tools themselves. Simple dichotomization of the lung into a fraction of healthy and diseased tissue does not differentiate bullous and more homogeneous disease and may not adequately address their unique mechanical impact.
Acknowledging these limitations, while there may be an optimal HU threshold for comparison to histologic specimens, selection of a specific densitometric method for analysis appears to have a limited impact on the strength of the correlations to measures of lung function. Both densitometric analysis and visual inspection of the CT scans proved to be of weak predictive value for function in this investigation. They did however convey potentially valuable information on the burden of parenchymal disease in COPD not readily quantifiable with standard lung function testing. Computed Tomographic imaging is widely available, safe, and can simultaneously provide useful clinical and research based information. The application of such an imaging technique as a biomarker for disease characterization and as an intermediate endpoint for interventional studies requires further studies into the methodology of disease quantification.
NETT CREDIT ROSTER
Source of funding
The National Emphysema Treatment Trial (NETT) is supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (CMS; formerly the Health Care Financing Administration); and the Agency for Healthcare Research and Quality (AHRQ).
Members of the NETT research group
Office of the Chair of the Steering Committee, University of Pennsylvania, Philadelphia, PA: Alfred P. Fishman, MD (Chair); Betsy Ann Bozzarello; Ameena Al-Amin.
Clinical centers
Baylor College of Medicine, Houston, TX: Marcia Katz, MD (Principal Investigator); Carolyn Wheeler, RN, BSN (Principal Clinic Coordinator); Elaine Baker, RRT, RPFT; Peter Barnard, PhD, RPFT; Phil Cagle, MD; James Carter, MD; Sophia Chatziioannou, MD; Karla Conejo-Gonzales; Kimberly Dubose, RRT; John Haddad, MD; David Hicks, RRT, RPFT; Neal Kleiman, MD; Mary Milburn-Barnes, CRTT; Chinh Nguyen, RPFT; Michael Reardon, MD; Joseph Reeves-Viets, MD; Steven Sax, MD; Amir Sharafkhaneh, MD; Owen Wilson, PhD; Christine Young PT; Rafael Espada, MD (Principal Investigator 1996–2002); Rose Butanda (1999–2001); Minnie Ellisor (2002); Pamela Fox, MD (1999–2001); Katherine Hale, MD (1998–2000); Everett Hood, RPFT (1998 B 2000); Amy Jahn (1998–2000); Satish Jhingran, MD (1998–2001); Karen King, RPFT (1998–1999); Charles Miller III, PhD (1996–1999); Imran Nizami, MD (Co-Principal Investigator, 2000–2001); Todd Officer (1998–2000); Jeannie Ricketts (1998–2000); Joe Rodarte, MD (Co-Principal Investigator 1996–2000); Robert Teague, MD (Co-Principal Investigator 1999–2000); Kedren Williams (1998–1999).
Brigham and Women's Hospital, Boston, MA: John Reilly, MD (Principal Investigator); David Sugarbaker, MD (Co-Principal Investigator); Carol Fanning, RRT (Principal Clinic Coordinator); Simon Body, MD; Sabine Duffy, MD; Vladmir Formanek, MD; Anne Fuhlbrigge, MD; Philip Hartigan, MD; Sarah Hooper, EP; Andetta Hunsaker, MD; Francine Jacobson, MD; Marilyn Moy, MD; Susan Peterson, RRT; Roger Russell, MD; Diane Saunders; Scott Swanson, MD (Co-Principal Investigator, 1996–2001).
Cedars-Sinai Medical Center, Los Angeles, CA: Rob McKenna, MD (Principal Investigator); Zab Mohsenifar, MD (Co-Principal Investigator); Carol Geaga, RN (Principal Clinic Coordinator); Manmohan Biring, MD; Susan Clark, RN, MN; Jennifer Cutler, MD; Robert Frantz, MD; Peter Julien, MD; Michael Lewis, MD; Jennifer Minkoff-Rau, MSW; Valentina Yegyan, BS, CPFT; Milton Joyner, BA (1996–2002).
Cleveland Clinic Foundation, Cleveland, OH: Malcolm DeCamp, MD (Principal Investigator); James Stoller, MD (Co-Principal Investigator); Yvonne Meli, RN,C (Principal Clinic Coordinator); John Apostolakis, MD; Darryl Atwell, MD; Jeffrey Chapman, MD; Pierre DeVilliers, MD; Raed Dweik, MD; Erik Kraenzler, MD; Rosemary Lann, LISW; Nancy Kurokawa, RRT, CPFT; Scott Marlow, RRT; Kevin McCarthy, RCPT; Pricilla McCreight, RRT, CPFT; Atul Mehta, MD; Moulay Meziane, MD; Omar Minai, MD; Mindi Steiger, RRT; Kenneth White, RPFT; Janet Maurer, MD (Principal Investigator, 1996–2001); Terri Durr, RN (2000–2001); Charles Hearn, DO (1998–2001); Susan Lubell, PA-C (1999–2000); Peter O'Donovan, MD (1998–2003); Robert Schilz, DO (1998–2002).
Columbia University, New York, NY in consortium with Long Island Jewish Medical Center, New Hyde Park, NY: Mark Ginsburg, MD (Principal Investigator); Byron Thomashow, MD (Co-Principal Investigator); Patricia Jellen, MSN, RN (Principal Clinic Coordinator); John Austin, MD; Matthew Bartels, MD; Yahya Berkmen, MD; Patricia Berkoski, MS, RRT (Site coordinator, LIJ); Frances Brogan, MSN, RN; Amy Chong, BS, CRT; Glenda DeMercado, BSN; Angela DiMango, MD; Sandy Do, MS, PT; Bessie Kachulis, MD; Arfa Khan, MD; Berend Mets, MD; Mitchell O = Shea, BS, RT, CPFT; Gregory Pearson, MD; Leonard Rossoff, MD; Steven Scharf, MD, PhD (Co-Principal Investigator, 1998–2002); Maria Shiau, MD; Paul Simonelli, MD; Kim Stavrolakes, MS, PT; Donna Tsang, BS; Denise Vilotijevic, MS, PT; Chun Yip, MD; Mike Mantinaos, MD (1998–2001); Kerri McKeon, BS, RRT, RN (1998–1999); Jacqueline Pfeffer, MPH, PT (1997–2002).
Duke University Medical Center, Durham, NC: Neil MacIntyre, MD (Principal Investigator); R. Duane Davis, MD (Co-Principal Investigator); John Howe, RN (Principal Clinic Coordinator); R. Edward Coleman, MD; Rebecca Crouch, RPT; Dora Greene; Katherine Grichnik, MD; David Harpole, Jr., MD; Abby Krichman, RRT; Brian Lawlor, RRT; Holman McAdams, MD; John Plankeel, MD; Susan Rinaldo-Gallo, MED; Sheila Shearer, RRT; Jeanne Smith, ACSW; Mark Stafford-Smith, MD; Victor Tapson, MD; Mark Steele, MD (1998–1999); Jennifer Norten, MD (1998–1999).
Mayo Foundation, Rochester, MN: James Utz, MD (Principal Investigator); Claude Deschamps, MD (Co-Principal Investigator); Kathy Mieras, CCRP (Principal Clinic Coordinator); Martin Abel, MD; Mark Allen, MD; Deb Andrist, RN; Gregory Aughenbaugh, MD; Sharon Bendel, RN; Eric Edell, MD; Marlene Edgar; Bonnie Edwards; Beth Elliot, MD; James Garrett, RRT; Delmar Gillespie, MD; Judd Gurney, MD; Boleyn Hammel; Karen Hanson, RRT; Lori Hanson, RRT; Gordon Harms, MD; June Hart; Thomas Hartman, MD; Robert Hyatt, MD; Eric Jensen, MD; Nicole Jenson, RRT; Sanjay Kalra, MD; Philip Karsell, MD; Jennifer Lamb; David Midthun, MD; Carl Mottram, RRT; Stephen Swensen, MD; Anne-Marie Sykes, MD; Karen Taylor; Norman Torres, MD; Rolf Hubmayr, MD (1998–2000); Daniel Miller, MD (1999–2002); Sara Bartling, RN (1998–2000); Kris Bradt (1998–2002).
National Jewish Medical and Research Center, Denver, CO: Barry Make, MD (Principal Investigator); Marvin Pomerantz, MD (Co-Principal Investigator); Mary Gilmartin, RN, RRT (Principal Clinic Coordinator); Joyce Canterbury; Martin Carlos; Phyllis Dibbern, PT; Enrique Fernandez, MD; Lisa Geyman, MSPT; Connie Hudson; David Lynch, MD; John Newell, MD; Robert Quaife, MD; Jennifer Propst, RN; Cynthia Raymond, MS; Jane Whalen-Price, PT; Kathy Winner, OTR; Martin Zamora, MD; Reuben Cherniack, MD (Principal Investigator, 1997–2000).
Ohio State University, Columbus, OH: Philip Diaz, MD (Principal Investigator); Patrick Ross, MD (Co-Principal Investigator); Tina Bees (Principal Clinic Coordinator); Jan Drake; Charles Emery, PhD; Mark Gerhardt, MD, PhD; Mark King, MD; David Rittinger; Mahasti Rittinger.
Saint Louis University, Saint Louis, MO: Keith Naunheim, MD (Principal Investigator); Robert Gerber, MD (Co-Principal Investigator); Joan Osterloh, RN, MSN (Principal Clinic Coordinator); Susan Borosh; Willard Chamberlain, DO; Sally Frese; Alan Hibbit; Mary Ellen Kleinhenz, MD; Gregg Ruppel; Cary Stolar, MD; Janice Willey; Francisco Alvarez, MD (Co-Principal Investigator, 1999–2002); Cesar Keller, MD (Co-Principal Investigator, 1996–2000).
Temple University, Philadelphia, PA: Gerard Criner, MD (Principal Investigator); Satoshi Furukawa, MD (Co-Principal Investigator); Anne Marie Kuzma, RN, MSN (Principal Clinic Coordinator); Roger Barnette, MD; Neil Brister, MD; Kevin Carney, RN, CCTC; Wissam Chatila, MD; Francis Cordova, MD; Gilbert D'Alonzo, DO; Michael Keresztury, MD; Karen Kirsch; Chul Kwak, MD; Kathy Lautensack, RN, BSN; Madelina Lorenzon, CPFT; Ubaldo Martin, MD; Peter Rising, MS; Scott Schartel, MD; John Travaline, MD; Gwendolyn Vance, RN, CCTC; Phillip Boiselle, MD (1997–2000); Gerald O = Brien, MD (1997–2000).
University of California, San Diego, San Diego, CA: Andrew Ries, MD, MPH (Principal Investigator); Robert Kaplan, PhD (Co-Principal Investigator); Catherine Ramirez, BS, RCP (Principal Clinic Coordinator); David Frankville, MD; Paul Friedman, MD; James Harrell, MD; Jeffery Johnson; David Kapelanski, MD; David Kupferberg, MD, MPH; Catherine Larsen, MPH; Trina Limberg, RRT; Michael Magliocca, RN, CNP; Frank J. Papatheofanis, MD, PhD; Dawn Sassi-Dambron, RN; Melissa Weeks.
University of Maryland at Baltimore, Baltimore, MD in consortium with Johns Hopkins Hospital, Baltimore, MD: Mark Krasna, MD (Principal Investigator); Henry Fessler, MD (Co-Principal Investigator); Iris Moskowitz (Principal Clinic Coordinator); Timothy Gilbert, MD; Jonathan Orens, MD; Steven Scharf, MD, PhD; David Shade; Stanley Siegelman, MD; Kenneth Silver, MD; Clarence Weir; Charles White, MD.
University of Michigan, Ann Arbor, MI: Fernando Martinez, MD (Principal Investigator); Mark Iannettoni, MD (Co-Principal Investigator); Catherine Meldrum, BSN, RN, CCRN (Principal Clinic Coordinator); William Bria, MD; Kelly Campbell; Paul Christensen, MD; Kevin Flaherty, MD; Steven Gay, MD; Paramjit Gill, RN; Paul Kazanjian, MD; Ella Kazerooni, MD; Vivian Knieper; Tammy Ojo, MD; Lewis Poole; Leslie Quint, MD; Paul Rysso; Thomas Sisson, MD; Mercedes True; Brian Woodcock, MD; Lori Zaremba, RN.
University of Pennsylvania, Philadelphia, PA: Larry Kaiser, MD (Principal Investigator); John Hansen-Flaschen, MD (Co-Principal Investigator); Mary Louise Dempsey, BSN, RN (Principal Clinic Coordinator); Abass Alavi, MD; Theresa Alcorn, Selim Arcasoy, MD; Judith Aronchick, MD; Stanley Aukberg, MD; Bryan Benedict, RRT; Susan Craemer, BS, RRT, CPFT; Ron Daniele, MD; Jeffrey Edelman, MD; Warren Gefter, MD; Laura Kotler-Klein, MSS; Robert Kotloff, MD; David Lipson, MD; Wallace Miller, Jr., MD; Richard O = Connell, RPFT; Staci Opelman, MSW; Harold Palevsky, MD; William Russell, RPFT; Heather Sheaffer, MSW; Rodney Simcox, BSRT, RRT; Susanne Snedeker, RRT, CPFT; Jennifer Stone-Wynne, MSW; Gregory Tino, MD; Peter Wahl; James Walter, RPFT; Patricia Ward; David Zisman, MD; James Mendez, MSN, CRNP (1997–2001); Angela Wurster, MSN, CRNP (1997–1999).
University of Pittsburgh, Pittsburgh, PA: Frank Sciurba, MD (Principal Investigator); James Luketich, MD (Co-Principal Investigator); Colleen Witt, MS (Principal Clinic Coordinator); Gerald Ayres; Michael Donahoe, MD; Carl Fuhrman, MD; Robert Hoffman, MD; Joan Lacomis, MD; Joan Sexton; William Slivka; Diane Strollo, MD; Erin Sullivan, MD; Tomeka Simon; Catherine Wrona, RN, BSN; Gerene Bauldoff, RN, MSN (1997–2000); Manuel Brown, MD (1997–2002); Elisabeth George, RN, MSN (Principal Clinic Coordinator 1997–2001); Robert Keenan, MD (Co-Principal Investigator 1997–2000); Theodore Kopp, MS (1997–1999); Laurie Silfies (1997–2001).
University of Washington, Seattle, WA: Joshua Benditt, MD (Principal Investigator), Douglas Wood, MD (Co-Principal Investigator); Margaret Snyder, MN (Principal Clinic Coordinator); Kymberley Anable; Nancy Battaglia; Louie Boitano; Andrew Bowdle, MD; Leighton Chan, MD; Cindy Chwalik; Bruce Culver, MD; Thurman Gillespy, MD; David Godwin, MD; Jeanne Hoffman; Andra Ibrahim, MD; Diane Lockhart; Stephen Marglin, MD; Kenneth Martay, MD; Patricia McDowell; Donald Oxorn, MD; Liz Roessler; Michelle Toshima; Susan Golden (1998–2000).
Other participants
Agency for Healthcare Research and Quality, Rockville, MD:Lynn Bosco, MD, MPH; Yen-Pin Chiang, PhD; Carolyn Clancy, MD; Harry Handelsman, DO.
Centers for Medicare and Medicaid Services, Baltimore, MD: Steven M Berkowitz, PhD; Tanisha Carino, PhD; Joe Chin, MD; JoAnna Baldwin; Karen McVearry; Anthony Norris; Sarah Shirey; Claudette Sikora; Steven Sheingold, PhD (1997–2004).
Coordinating Center, The Johns Hopkins University, Baltimore, MD: Steven Piantadosi, MD, PhD (Principal Investigator); James Tonascia, PhD (Co-Principal Investigator); Patricia Belt; Amanda Blackford, ScM; Karen Collins; Betty Collison; Ryan Colvin, MPH; John Dodge; Michele Donithan, MHS; Vera Edmonds; Gregory L. Foster, MA; Julie Fuller; Judith Harle; Rosetta Jackson; Shing Lee, ScM; Charlene Levine; Hope Livingston; Jill Meinert; Jennifer Meyers; Deborah Nowakowski; Kapreena Owens; Shangqian Qi, MD; Michael Smith; Brett Simon, MD; Paul Smith; Alice Sternberg, ScM; Mark Van Natta, MHS; Laura Wilson, ScM; Robert Wise, MD.
Cost Effectiveness Subcommittee:Robert M. Kaplan, PhD (Chair); J. Sanford Schwartz, MD (Co-Chair); Yen-Pin Chiang, PhD; Marianne C. Fahs, PhD; A. Mark Fendrick, MD; Alan J. Moskowitz, MD; Dev Pathak, PhD; Scott Ramsey, MD, PhD; Steven Sheingold, PhD; A. Laurie Shroyer, PhD; Judith Wagner, PhD; Roger Yusen, MD.
Cost Effectiveness Data Center, Fred Hutchinson Cancer Research Center, Seattle, WA: Scott Ramsey, MD, PhD (Principal Investigator); Ruth Etzioni, PhD; Sean Sullivan, PhD; Douglas Wood, MD; Thomas Schroeder, MA; Karma Kreizenbeck; Kristin Berry, MS; Nadia Howlader, MS.
CT Scan Image Storage and Analysis Center, University of Iowa, Iowa City, IA: Eric Hoffman, PhD (Principal Investigator); Janice Cook-Granroth, BS; Angela Delsing, RT; Junfeng Guo, PhD; Geoffrey McLennan, MD; Brian Mullan, MD; Chris Piker, BS; Joseph Reinhardt, PhD; Blake Robinswood; Jered Sieren, RTR; William Stanford, MD.
Data and Safety Monitoring Board: John A. Waldhausen, MD (Chair); Gordon Bernard, MD; David DeMets, PhD; Mark Ferguson, MD; Eddie Hoover, MD; Robert Levine, MD; Donald Mahler, MD; A. John McSweeny, PhD; Jeanine Wiener-Kronish, MD; O. Dale Williams, PhD; Magdy Younes, MD.
Marketing Center, Temple University, Philadelphia, PA: Gerard Criner, MD (Principal Investigator); Charles Soltoff, MBA.
Project Office, National Heart, Lung, and Blood Institute, Bethesda, MD: Gail Weinmann, MD (Project Officer); Joanne Deshler (Contracting Officer); Dean Follmann, PhD; James Kiley, PhD; Margaret Wu, PhD (1996–2001).
Other acknowledgments
Arthur Gelb, MD, Lakewood Regional Medical Center, Lakewood, CA
The authors would like to thank Janice Cook-Granroth at the University of Iowa for her work generating the CT data used in this analysis. Drs Washko, Criner, Mohsenifar, Sciurba, Sharafkhaneh, Make, and Reilly report no conflict of interest. Dr. Hoffman is the developer of a commercially available image analysis software package, Vida. Dr. Washko was supported by NIH Grant Number T32 HL007633 and NHLBI #U10HL074428.The National Emphysema Treatment Trial (NETT) was supported by contracts with the National Heart, Lung, and Blood Institute (N01HR76101, N01HR76102, N01HR76103, N01HR76104, N01HR76105, N01HR76106, N01HR76107, N01HR76108, N01HR76109, N01HR76110, N01HR76111, N01HR76112, N01HR76113, N01HR76114, N01HR76115, N01HR76116, N01HR76118, and N01HR76119), the Centers for Medicare and Medicaid Services (CMS; formerly the Health Care Financing Administration); and the Agency for Healthcare Research and Quality (AHRQ).
REFERENCES
- Black L F, Hyatt R E, Stubbs S E. Mechanism of expiratory airflow limitation in chronic obstructive pulmonary disease associated with 1 -antitrypsin deficiency. Am Rev Respir Dis 1972; 105(6)891–899
- Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Pare P D, Hogg J C, Mishima M. Computed tomographic measurements of airway dimensions and emphysema in smokers. Correlation with lung function. Am J Respir Crit Care Med 2000; 162(3 Pt 1)1102–1108
- Pauwels R A, Buist A S, Calverley P M, Jenkins C R, Hurd S S. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med 2001; 163(5)1256–1276
- Croxton T L, Weinmann G G, Senior R M, Wise R A, Crapo J D, Buist A S. 2003. Clinical research in chronic obstructive pulmonary disease: needs and opportunities. Am J Respir Crit Care Med 2003; 167(8)1142–1149
- Gevenois P A, de Maertelaer V, De Vuyst P, Zanen J, Yernault J C. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1995; 152(2)653–657
- Muller N L, Staples C A, Miller R R, Abboud R T. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest 1988; 94(4)782–787
- Hayhurst M D, MacNee W, Flenley D C, Wright D, Mc Lean A, Lamb D, Wightman A J, Best J. Diagnosis of pulmonary emphysema by computerised tomography. Lancet 1984; 2(8398)320–322
- Gould G A, MacNee W, Mc Lean A, Warren P M, Redpath A, Best J J, Lamb D, Flenley D C. CT measurements of lung density in life can quantitate distal airspace enlargement–an essential defining feature of human emphysema. Am Rev Respir Dis 1988; 137(2)380–392
- Park K J, Bergin C J, Clausen J L. Quantitation of emphysema with three-dimensional CT densitometry: comparison with two-dimensional analysis, visual emphysema scores, and pulmonary function test results. Radiology 1999; 211(2)541–547
- Bankier A A, De Maertelaer V, Keyzer C, Gevenois P A. Pulmonary emphysema: subjective visual grading versus objective quantification with macroscopic morphometry and thin-section CT densitometry. Radiology 1999; 211(3)851–858
- Guenard H, Diallo M H, Laurent F, Vergeret J. Lung density and lung mass in emphysema. Chest 1992; 102(1)198–203
- Kinsella M, Muller N L, Abboud R T, Morrison N L, DyBuncio A. Quantitation of emphysema by computed tomography using a “density mask” program and correlation with pulmonary function tests. Chest 1990; 97(2)315–321
- Sanders C, Nath P H, Bailey W C. Detection of emphysema with computed tomography. Correlation with pulmonary function tests and chest radiography. Invest Radiol 1988; 23(4)262–266
- Heremans A, Verschakelen J A, Van fraeyenhoven L, Demedts M. Measurement of lung density by means of quantitative CT scanning. A study of correlations with pulmonary function tests. Chest 1992; 102(3)805–811
- Gould G A, Redpath A T, Ryan M, Warren P M, Best J J, Flenley D C, MacNee W, Lung C T. density correlates with measurements of airflow limitation and the diffusing capacity. Eur Respir J 1991; 4(2)141–146
- Fishman A, Martinez F, Naunheim K, Piantadosi S, Wise R, Ries A, Weinmann G, Wood D E. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med 2003; 348(21)2059–2073
- Coxson H O, Whittall K P, Nakano Y, Rogers R M, Sciurba F C, Keenan R J, Hogg J C. Selection of patients for lung volume reduction surgery using a power law analysis of the computed tomographic scan. Thorax 2003; 58(6)510–514
- Washko G, Criner G, Mohsenifar Z, Sciurba F, Sharafkhaneh A, Make B, Hoffman E, Reilly J, F. T. N. R. Group. Assessing Pulmonary Function with CT Scanning in Patients with Emphysema [Abstract]. Proc Am Thorac Soc 2005; 2: A259
- Stolk J, Ng W H, Bakker M E, Reiber J H, Rabe K F, Putter H, Stoel B C. Correlation between annual change in health status and computer tomography derived lung density in subjects with alpha1-antitrypsin deficiency. Thorax 2003; 58(12)1027–1030
- American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med 1995; 152(3)1107–1136
- American Thoracic Society. Single-breath carbon monoxide diffusing capacity (transfer factor). Recommendations for a standard technique—1995 update. Am J Respir Crit Care Med 1995; 152(6 Pt 1)2185–2198
- Milic-Emili J, Mead J, Turner J M, Glauser E M. Improved technique for estimating pleural pressure from esophageal balloons. J Appl Physiol 1964; 19: 207–211
- Parr D G, Stoel B C, Stolk J, Stockley R A. Pattern of emphysema distribution in alpha1-antitrypsin deficiency influences lung function impairment. Am J Respir Crit Care Med 2004; 170(11)1172–1178
- Dowson L J, Guest P J, Hill S L, Holder R L, Stockley R A. High-resolution computed tomography scanning in alpha1-antitrypsin deficiency: relationship to lung function and health status. Eur Respir J 2001; 17(6)1097–1104
- Stoel B C, Bakker M E, Stolk J, Dirksen A, Stockley R A, Piitulainen E, Russi E W, Reiber J H. Comparison of the sensitivities of 5 different computed tomography scanners for the assessment of the progression of pulmonary emphysema: a phantom study. Invest Radiol 2004; 39(1)1–7
- Boedeker K L, McNitt-Gray M F, Rogers S R, Truong D A, Brown M S, Gjertson D W, Goldin J. G.J.G. Emphysema: effect of reconstruction algorithm on CT imaging measures. Radiology 2004; 232(1)295–301
- Baldi S, Miniati M, Bellina C R, Battolla L, Catapano G, Begliomini E, Giustini D, Giuntini C. Relationship between extent of pulmonary emphysema by high-resolution computed tomography and lung elastic recoil in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164(4)585–589
- Saetta M, Shiner R J, Angus G E, Kim W D, Wang N S, King M, Ghezzo H, Cosio M G. Destructive index: a measurement of lung parenchymal destruction in smokers. Am Rev Respir Dis 1985; 131(5)764–769
- Kim W D, Ling S H, Coxson H O, English J C, Yee J, Levy R D, Pare P D, Hogg J C. The association between small airway obstruction and emphysema phenotypes in chronic obstructive pulmonary disease. Chest 2007; 131(5)1372–1378