Abstract
Chronic Obstructive Pulmonary Disease (COPD) is the fourth-leading cause of chronic morbidity and mortality in North America and its burden continues to increase. Tiotropium has been shown to reduce exacerbations, hospitalizations, symptoms, and improve health-related quality of life in patients with COPD. Its effect on mortality and its effects relative to long-acting beta-agonists (LABAs), however, remain unknown. To examine the association between tiotropium use compared to LABA use on all-cause mortality in older patients with COPD, a longitudinal, population-based cohort study was conducted in Ontario, Canada. Subjects were individuals 65 years and older discharged from hospital with a diagnosis of COPD between January 1, 2003 and March 31, 2006. The hazard of receiving a prescription for tiotropium compared to a long-acting beta-agonist on all-cause mortality at 180 days post-hospital discharge, controlling for a number of potential confounders, was eliminated. Data from 7218 eligible patients were analyzed. Of these, 1046 (14.5%) died in the follow-up period. Patients who received tiotropium were 20% less likely to die than those receiving a long-acting beta-agonist (hazard ratio 0.80, 95% confidence interval 0.70 to 0.93). In conclusion, in older patients recently discharged from hospital for COPD, receiving tiotropium was found to be associated with reduced mortality at 6 months compared to receiving a long-acting beta-agonist. This result suggests that tiotropium might also be associated with decreased mortality compared to no treatment at all. Randomized placebo-control trials are needed to confirm these findings.
INTRODUCTION
Chronic Obstructive Pulmonary Disease (COPD is the fourth-leading cause of chronic morbidity and mortality in North America and its burden continues to increase (Citation[1], Citation[2], Citation[3]). Despite numerous advances in the management of COPD, the only interventions that have clearly demonstrated to improve mortality remain smoking cessation, vaccination, and lung volume reduction surgery, and oxygen therapy in some individuals (Citation[2], Citation[4]).
In the last several years, new medications have demonstrated efficacy in improving outcomes for patients with COPD. One of these, tiotropium (Pfizer Incorporated, New York, New York, USA), is a long-acting inhaled anticholinergic medication that has been shown to reduce exacerbations, hospitalizations, symptoms, and improve health-related quality of life and it is therefore rapidly becoming the standard of care for patients with COPD (Citation[2], Citation[5]). Its long-term effects on mortality are still unknown (Citation[1], Citation[2], Citation[6]). A randomize controlled trial (RCT) would be the best methodology to use to answer this question, but recent large scale RCTs studying tiotropium were not designed, nor powered to examine mortality as an outcome so it will be some time before patients and clinicians will obtain answers this way (Citation[7], Citation[8]).
Besides being relatively quicker to conduct, observational studies have the advantage of being able to determine the effectiveness, not just the efficacy, of a medication in real-world conditions. Therefore, in order to provide an estimate of the mortality effects of tiotropium in real world conditions, we conducted a large, retrospective, longitudinal population-based cohort study of high risk, older people with COPD recently discharged from hospital. To avoid selection bias, instead of comparing tiotropium with no treatment, we compared tiotropium with a class of medications with similar indications and risk of adverse events: long-acting beta-agonists (LABA) (Citation[2]). Since inhaled corticosteroids (ICS) have been found to influence the effects of both tiotropium and LABA, the current study also looked at the relative effects of these medications stratified for ICS use.
METHODS
Subjects
All Ontario residents more than or equal to 65 years of age who were discharged from hospital with a primary diagnosis of COPD between January 1, 2003 and March 31, 2006 as per the Ontario version of the Canadian Institute of Health Information (CIHI) hospital discharge database were included. Patients were identified using the International Classification of Disease, 10th Revision (ICD-10) codes J41(simple and mucopurulent chronic bronchitis), J42 (unspecified chronic bronchitis), J43 (emphysema), and J44 (other chronic obstructive pulmonary disease).
Data sources
The CIHI discharge abstract database contains clinical administrative data relating to the health care services provided to patients by all hospital facilities in Ontario. A most responsible discharge diagnostic code field of COPD has been previously validated against chart abstraction (Citation[9], Citation[10]).
The Ontario Drug Benefit (ODB) database contains prescription medication information on all patients the Ontario government provides prescription medication to for a nominal fee or free of charge depending on income level. This includes all individuals more than or equal to 65 years of age.
The Ontario Health Insurance Plan (OHIP) physicians' claims database provides information on outpatient visits for all Ontario residents.
Finally, the Ontario Registered Persons database captures all-cause mortality information for Ontario residents including their date of death. Linkage of the above 3 databases with this database (and thus with each other) was performed through unique identifiers given to all Ontario residents. Since Ontario provides universal health care coverage for all its residents (approximately 12 million individuals) regardless of ability to pay, the final linked database included virtually all eligible subjects in the province. Ethics approval was obtained through the formal ethics review process at the Institute for Clinical Evaluative Sciences.
Study design
A longitudinal cohort design was used. Subjects entered into the cohort on the date they filled a prescription for a long-acting bronchodilator (tiotropium or LABA) within 90 days of being discharged from hospital with a most responsible diagnosis of COPD. Subjects who filled prescriptions for more than one class of long-acting bronchodilator (tiotropium and a LABA) were excluded. The end of patient observation time was defined as when the patient died or 180 days after the discharge date, whichever was earliest. Patients who died within 7 days of the prescription date were excluded to permit a reasonable opportunity for the medications to take effect. They were also excluded if they were transferred or discharged to another acute care or chronic hospital as outpatient drug information was not available for these patients. Failure was defined as death.
Outcome variables
The main outcome of interest was the hazard ratio of all-cause mortality of treatment with tiotropium compared to treatment with LABA within 180 days of the discharge date, after adjusting for other important covariables. One hundred eighty days was chosen to minimize the effect of medications being discontinued and/or group crossover which has been noted to occur with high frequency in COPD patients (Citation[7]). It was also chosen to increase the probability the deaths were due to COPD and because patients' health was probably most fragile and therefore death most likely closer to discharge. Finally, since ICS are believed to promote the effects of long-acting bronchodilators, the primary analysis was repeated with stratification for ICS use (Citation[7], Citation[11]). A secondary analysis was also done looking at outcomes at 365 days.
Covariables
A number of covariables that might influence outcomes in COPD and thus survival were controlled for in the analysis. They were determined using fields from the linked dataset described. A Charlson Index Score, modified for use in administrative databases, was calculated for each patient to determine a level of comorbidity (Citation[12], Citation[13]). A number of surrogate markers were used to adjust for disease severity including: receiving other COPD medications within 90 days of the index hospitalization (appropriate antibiotics, inhaled short-acting beta-2-agonists, inhaled short-acting anti-cholinergic medication, ICS, oral corticosteroids, and methylxanthines), use of emergency room and outpatient services within 1 year prior to the index hospitalization, and 1 or more specialist (physicians with additional training to manage COPD) visits within 1 year prior to the index hospitalization. These variables were also controlled for because of their potential beneficial effects. Lung volume reduction surgery was included as a covariable because of its potential favourable outcome. Demographic factors such as age, sex, and socioeconomic status and other potential influencing factors such as beta-antagonist use and length of index hospital stay were also considered (Citation[14], Citation[15]). Socioeconomic status divisions were based on patients' income quintile which was approximated using their postal codes. Quintile 1 representing the highest and quintile 5, the lowest (Citation[16]).
Statistical analysis
Study outcomes were analyzed using the Student's two-tailed t-test for continuous variables, Wilcoxon's Rank Sum test for non-normally distributed variables, and the chi square test for categorical variables. Survival rates between patients receiving tiotropium and LABA were compared using a Cox proportional hazards model in order to adjust for the effect of the covariables described above. All covariables were forced in the final model because of their clinical potential to be determinants of outcomes in COPD (Citation[2]). All statistical tests were two tailed in nature and a p value ≤ 0.05 was considered statistically significant. Statistical analyses were performed using SAS 9 software (SAS Institute Inc., Cary, North Carolina, USA).
RESULTS
There were 15,703 patients aged 65 years or older discharged from hospitals in Ontario, Canada with a diagnosis of COPD between January 1, 2003 and March 31, 2006 who filled prescriptions for long-acting bronchodilators within 90 days of discharge. Of these, 223 (0.01%) were excluded because they were transferred or discharge to another acute care or chronic hospital, 8122 (51.7%) were excluded because they filled prescriptions for both tiotropium and a LABA, and 140 (0.01%) were excluded because they died within 7 days of filling their prescription. This left 7218 patients in the final cohort. Of these, 3018 (41.8%) filled a prescription for tiotropium and the rest filled a prescription for a LABA. There were 1046 (14.5%) deaths within 180 days of discharge ().
Table 1 Characteristics of older patients with COPD who were treated with a long-acting beta-agonists or tiotropium after discharge
On average, compared to patients who filled a prescription for LABA, those who filled a prescription for tiotropium were slightly older and more likely to be male. They were also less likely to have seen a specialist or have visited a doctor's office or emergency room in the preceding year and were less likely to have filled a prescription for a number of COPD medications. They were more likely to have filled a prescription for a beta-blocker.
Controlling for age and sex, there was no significant difference in mortality between the LABA and tiotropium groups (hazard ratio 0.89, 95% confidence interval 0.79 to 1.01). Controlling for all covariables, patients using tiotropium were found to be 20% less likely to die at 180 days than patients using LABA (hazard ratio 0.80, 95% confidence interval 0.70 to 0.93); (see and ).
Table 2 Adjusted hazard ratio of various factors on all-cause mortality in patients with COPD who were treated with long-acting beta-agonists or tiotropium after discharge
Figure 1 Kaplan-Meier graph displaying probability of survival in patients with chronic obstructive pulmonary disease receiving tiotropium and long-acting beta-agonists.
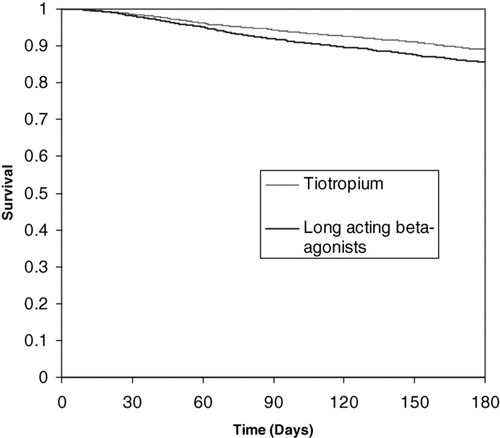
There were 1653 (55.8%) patients receiving tiotropium and 3726 (88.7%) patients receiving a LABA who also received an ICS. Compared to the entire cohort, these patients had slightly less comorbidity and received slightly more short acting anticholinergic medication, short acting beta-adrenergic medication, and oral corticosteroids (data not shown). Controlling for age and sex, those subject receiving tiotropium and an ICS were 27% less likely to die than those receiving LABA and an ICS (hazard ratio 0.73, 95% confidence interval 0.61 to 0.86). For subjects not on ICS, there was no significant association found between type of long-acting bronchodilator received and mortality when age and sex were controlled for (hazard ratio 0.86, 95% confidence interval 0.67 to 1.01).
Controlling for all covariables, patients receiving tiotropium and an ICS were 25% less likely to die than those receiving LABA and an ICS (hazard ratio 0.75, 95% confidence interval 0.63 to 0.90) while for patients not receiving an ICS there continued to be no apparent association (hazard ratio 0.90, 95% confidence interval 0.70 to 1.15).
The analyses were repeated considering mortality at 365 days. Controlling for all covariables, patients using tiotropium were found to be 15% less likely to die at 365 days than patients using LABA (hazard ratio 0.85, 95% confidence interval 0.76 to 0.95). Those receiving ICS still appeared to derive a mortality benefit from tiotropium (hazard ratio 0.85, 95% confidence interval 0.74 to 0.97) while those not receiving ICS did not (hazard ratio 0.84, 95% confidence interval 0.68 to 1.02) ().
Table 3 Adjusted hazard ratio of tiotropium compared to long-acting beta-agonists on all-cause mortality at 180 days and 365 days in patients with COPD who received and did not receive inhaled corticosteroids after discharge
Finally, in further secondary analyses, mortality hazard ratios of tiotropium use compared to LABA use at 180 days stratified by a number of factors were produced (). Although there was some variation in the magnitude of the association and the degree of significance, tiotropium use consistently appeared to be associated with improved survival in every strata.
Table 4 Adjusted hazard ratio of tiotropium compared to long-acting beta-agonists on all-cause mortality at 180 days stratified by various factors
DISCUSSION
This longitudinal, retrospective cohort study demonstrated that elderly patients hospitalized with a diagnosis of COPD who filled a prescription for tiotropium on discharge were 20% less likely to die before 180 days than those who filled a prescription for LABA. This mortality benefit was also found at 365 days and when only patients receiving ICS were considered. This the first study to directly compare mortality between these two long-acting bronchodilators. These findings are consistent with previous randomized controlled trials that found that treatment with tiotropium resulted in significantly greater bronchodilation than treatment with a LABAs (Citation[17], Citation[18]). Possible hypotheses as to why there would be lower mortality in the tiotropium group are that, in COPD, anticholinergic medications are more effective than beta-agonists, they are relatively safer than beta-agonists, and/or patients are more compliant with them than beta-agonists (Citation[19], Citation[20]).
There are 3 possible explanations for the mortality benefit seen with tiotropium in this study. The first is that LABAs are associated with relatively increased mortality compared to tiotropium. Although an absolute increased risk of death has been observed with LABA use in individuals with asthma, this has not been observed in individuals with COPD (Citation[21]). On the contrary, previous studies—including one large RCT—have found that LABA use leads to less hospitalization, fewer exacerbations, and improved lung function compared to placebo. These same trials have not demonstrated an absolute increased incidence of death nor any other significant adverse events due to LABA; however, this does not rule out the possibility that a relative increased risk of death compared to tiotropium might exist (Citation[22], Citation[23]).
The second possible explanation is that tiotropium is associated with decreased all-cause mortality compared to LABA. Since, as mentioned above, mortality with LABA appears no worse than placebo, it follows that tiotropium would also be associated with decreased mortality compared to placebo. If this is the case, our study is the first to demonstrate that tiotropium decreases mortality. As such, it extends the findings of previous studies that have shown that tiotropium decreases hospitalizations, decreases exacerbations, improves health-related quality of life, and reduces symptoms in patients with COPD. It is not consistent, however, with a recent meta-analysis that found little association between tiotropium use and all-cause mortality. This might be because that study focused on stable COPD patients who had less to gain than the frail, older patients in the current study (Citation[5]).
Finally, the third possible explanation is that one or more biases made it appear that tiotropium was more effective than LABA. As mentioned above, selection bias and survival bias can both be a problem in observational, retrospective studies. In the current study, care was taken to avoid their influence (Citation[24], Citation[25]). Selection bias is bias due to differences in outcome from unmeasured prognostically important baseline differences. To avoid this bias the treatment of interest—tiotropium—was compared to a second, known treatment of similar clinical indication and risk (Citation[24]). In other words, these medications would be prescribed by clinicians interchangeably, as is recommended in COPD guidelines, without bias as to which type of patient gets which type of medication (Citation[6]).
Survival bias typically occurs when a common time point is used to define the beginning of follow-up for patients that do and do not receive treatment if, in the treatment group, the time from the beginning follow-up to the patients' actual treatment initiation is not recognized as unexposed survival time. This bias was avoided by using the initiation of treatment for each patient as the beginning of follow-up so that only the time that patients were on treatment was counted as survival time.
Despite the fact that, during the study period, neither tiotropium or LABA were favoured over the other in clinical practise because of known superior effectiveness or risk, the cohorts of patient on these medications still appeared to be different in some respects. This leaves room for the possibility that unmeasured confounders, such as oxygen use or smoking status, which were not available in the databases studied, biased the results; however, there are several reasons why we do not believe this was the situation.
First, because if such confounders existed, they should also bias the results of the ICS subgroup analyses and yet an advantage of tiotropium over LABA was not observed in the non-ICS cohorts. Second, because if such confounders existed, they would be highly correlated with disease severity (i.e., current smokers are more likely to have more severe disease) and disease severity was controlled for through other variables, thus the potential confounding effects should have been removed.
Third, because the differences in the cohorts observed can be explained much more easily by factors other than the presence of unmeasured confounders. For example, patients receiving a LABA were more likely that patients receiving tiotropium to receive an ICS. This was most likely because combination LABA/ICS medications are widely available whereas combination tiotropium/ ICS medications are not—not because the individuals in one cohort were more likely, for example, to be on oxygen. Likewise, it was observed that patients receiving a beta-blocker were more likely to be prescribed tiotropium than a LABA. This was most likely because the mechanism of action of tiotropium does not directly counteract the mechanism of the beta-blocker—not because the people on tiotropium were less likely to be, for example, smokers. Thus, it is far from clear that unmeasured confounders did bias the results. Still, the possibility that they did does limit the interpretation of the study results to being hypothesis generating, as most observational studies are, rather than absolutely conclusive.
Subgroup analysis demonstrated that tiotropium was associated with decreased mortality in the presence of, and not in the absence of, an ICS. This is consistent with a recent randomized controlled trial that demonstrated improved lung function, quality of life, and hospitalization rates in patients on tiotropium and an ICS compared to tiotropium alone (Citation[7]). A similar benefit has also been noted with LABA in the presence of ICS suggesting that this might be a characteristic of inhaled long-acting bronchodilator medications in general (Citation[22]).
At 365 days, a significant but smaller mortality benefit was seen. This would be expected, as more patients are would be likely to discontinue their medications or cross-over groups in the longer time period.
In summary, this study suggests that tiotropium use in older patients recently discharged from hospital for COPD reduces mortality at 6 months. This is the first large-scale, population-based study looking at the relationship between tiotropium use and mortality and it appears to support its use after hospitalization for COPD. The study's strengths are its large, inclusive sample and its ability to examine the effect of tiotropium in ‘real-world’ conditions. Its observational, retrospective design should not discount the significance of its findings as such studies have proven valuable in understanding pharmacologic interventions in the past (Citation[26]). COPD is a widespread, debilitating disease with limited options for management. These promising results extend the positive outcomes already noted with tiotropium to include short-term mortality. Altogether, there is now compelling justification for the conduct of more complicated observational studies which use advanced techniques to control for unmeasured confounders and/or a large randomized controlled trial to confirm these results.
REFERENCES
- Celli B R, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23(6)932–946
- Global Initiative for Chronic Obstructive Pulmonary Disease. National Institutes of Health, National Heart, Lung and Blood Institute, Bethesda, MD 2005, Workshop Report, Global Strategy for Diagnosis, Management, and Prevention of COPD. Update Sept.
- National Institutes of Health NHLaBI. Morbidity and Mortality: 2004 Chart Book on cardiovascular, lung, and blood diseases. U.S. Department of Health and Human Services. 2004
- Naunheim K S, Wood D E, Mohsenifar Z, Sternberg A L, Criner G J, DeCamp M M, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery versus medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg 2006; 82(2)431–443
- Barr R G, Bourbeau J, Camargo C A, Ram F S. Inhaled tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005, (2):CD002876
- O'Donnell D E, Aaron S, Bourbeau J, Hernandez P, Marciniuk D, Balter M, et al. State of the Art Compendium: Canadian Thoracic Society recommendations for the management of chronic obstructive pulmonary disease. Can Respir J 2004; 11(Suppl B)7B–59B
- Aaron S D, Vandemheen K L, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: A randomized trial. Ann Intern Med 2007
- Decramer M, Celli B, Tashkin D P, Pauwels R A, Burkhart D, Cassino C, et al. Clinical trial design considerations in assessing long-term functional impacts of tiotropium in COPD: the UPLIFT trial. COPD 2004; 1(2)303–312
- Canadian Institute of Health Information. Data quality of the discharge abstract database following the first year implementation of ICD-10-CA/CCI - Final report. Canadian Institute of Health Information. 2004
- Rawson N S, Malcolm E. Validity of the recording of ischaemic heart disease and chronic obstructive pulmonary disease in the Saskatchewan health care datafiles. Stat Med 1995; 14(24)2627–2643
- Calverley P M, Anderson J A, Celli B, Ferguson G T, Jenkins C, Jones P W, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356(8)775–789
- Charlson M E, Pompei P, Ales K L, MacKenzie C R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40(5)373–383
- Deyo R A, Cherkin D C, Ciol M A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992; 45(6)613–619
- Sin D D, Tu J V. Are elderly patients with obstructive airway disease being prematurely discharged?. Am J Respir Crit Care Med 2000; 161(5)1513–1517
- Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2005, (4):CD003566
- PCCF+ Version 4D User's Guide (Geocodes/PCCF). Automated Geographic Coding Based on the Statistics Canada Postal Code Conversion Files. Statistics Canada, Ottawa 2004, Including Postal Codes to December 2003
- Briggs D D, Jr., Covelli H, Lapidus R, Bhattycharya S, Kesten S, Cassino C. Improved daytime spirometric efficacy of tiotropium compared with salmeterol in patients with COPD. Pulm Pharmacol Ther 2005; 18(6)397–404
- Hodder R, Kesten S, Menjoge S, Viel K. Outcomes in COPD patients receiving tiotropium or salmeterol plus treatment with inhaled corticosteroids. Int J Chron Obstruct Pulmon Dis 2007; 2(2)157–167
- Salpeter S R, Buckley N S, Salpeter E E. Meta-analysis: anticholinergics, but not beta-agonists, reduce severe exacerbations and respiratory mortality in COPD. J Gen Intern Med 2006; 21(10)1011–1019
- Breekveldt-Postma N S, Koerselman J, Erkens J A, Lammers J W, Herings R M. Enhanced persistence with tiotropium compared with other respiratory drugs in COPD. Respir Med 2007; 101(7)1398–1405
- Walters E, Gibson P, Lasserson T, Walters J. Long-acting beta2-agonists for chronic asthma in adults and children where background therapy contains varied or no inhaled corticosteroid. Cochrane Database Syst Rev 2007, (1):CD001385
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003; 361(9356)449–456
- Appleton S, Poole P, Smith B, Veale A, Lasserson T J, Chan M M. Long-acting beta2-agonists for poorly reversible chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006; 3, CD001104
- Stukel T A, Fisher E S, Wennberg D E, Alter D A, Gottlieb D J, Vermeulen M J. Analysis of observational studies in the presence of treatment selection bias: effects of invasive cardiac management on AMI survival using propensity score and instrumental variable methods. JAMA 2007; 297(3)278–285
- Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol 2005; 162(10)1016–1023
- Benson K, Hartz A J. A comparison of observational studies and randomized, controlled trials. N Engl J Med 2000; 342(25)1878–1886