Abstract
In COPD patients, tachypnea should increase (dynamic) hyperinflation by shortening expiratory time. We developed a method to evaluate the time course and degree of dynamic hyperinflation during metronome-paced tachypnea. Fourteen patients with stable COPD (FEV1 43 ± 13% predicted) were studied. Inspiratory capacity (IC) was measured breathing through a flow transducer. Subjects paced their respiratory rate (fR) at 20/min, 30/min and 40/min for 60-second periods in response to audible tones generated by a computer. IC measurements were obtained at baseline and after 30 and 60 seconds at each fR. End-tidal carbon dioxide was monitored and fR was allowed to return to baseline between periods of tachypnea. Tachypnea produced reductions in IC of 200 ± 240 ml, 380 ± 330 ml and 540 ± 300 ml after 30 seconds at 20/min, 30/min and 40/min, respectively. IC reduction at 60 seconds was similar to 30 seconds for each fR. In patients with moderate-to-severe COPD, the dynamic hyperinflation induced by metronome-paced tachypnea was shown to occur rapidly and be complete by 30 seconds for a given fR. Controlled increments in fR produced stepwise increases in dynamic hyperinflation. This standardized method could be a useful and easier method of assessing dynamic hyperinflation in COPD patients before and after therapeutic interventions.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a disease characterized by progressive airflow obstruction, that when advanced results in breathlessness, exercise impairment, and reduced quality of life. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) stages the severity of COPD by the degree of expiratory airflow limitation based on the reduction in FEV1 as a percentage of its reference value (Citation[1]). However, FEV1 has been shown to be a poor predictor of clinical symptoms and exercise tolerance (Citation[2], Citation[3]).
There is now substantial evidence that dynamic hyperinflation occurs in patients with COPD and contributes to their breathlessness and exercise impairment. Repeated measurements of inspiratory capacity (IC) have been used to track dynamic hyperinflation (DH) during exercise in COPD (Citation[4]), and a decline in IC with exercise has been shown to be a good predictor of decreased exercise ability and increased exertional dyspnea (Citation[5], Citation[6]). Because total lung capacity (TLC) is believed to remain constant with exercise (Citation[7]), a reduction in IC reflects an increased end-expiratory lung volume (EELV) and thus DH.
Given that exercise-induced DH is a good predictor of exertional symptoms in COPD patients, it can serve as a useful tool for assessing the impact of different interventions on COPD pathophysiology (Citation[5], Citation[8], Citation[9], Citation[10], Citation[11]). Unfortunately, in the clinical setting, exercise testing is sometimes impractical because it requires expensive equipment, and may be difficult or uncomfortable for patients.
Exercise worsens hyperinflation by increasing the ventilatory requirement and also, importantly, by increasing respiratory frequency, thus inversely reducing expiratory time. Exactly which of these factors is more important in determining the degree of DH is unclear. However, any cause of tachypnea, such as anxiety or hypoxemia, should predictably induce dynamic hyperinflation in a similar manner. Tachypnea can be paced at pre-determined rates by a metronome, such as an audible tone at varying temporal frequencies produced by a computer. In COPD patients, 20 seconds of metronome-paced tachypnea has been shown to produce DH to a degree similar to that produced by exercise for a given respiratory frequency (fR) (Citation[8]). Increasing rates of paced tachypnea (20/min, 30/min, 40/min) for 30 seconds duration causes a corresponding stepwise decline in IC (Citation[12]). However, to our knowledge, the time course of DH for longer durations of metronome paced tachypnea in COPD patients has not been examined.
We hypothesized that there would be a graded increase in the degree of hyperinflation in response to increased rates of metronome-paced tachypnea. We also sought to characterize the time-course of metronome-paced tachypnea at various controlled respiratory frequencies. In 14 clinically stable patients with COPD, we monitored the degree of DH by measuring IC at baseline and after 30 and 60 seconds of metronome-paced tachypnea at fR 20/min, 30/min, and 40/min.
METHODS
Patients
Male and female patients at least 40 years old were recruited. Inclusion criteria required a clinical history consistent with COPD according to the recent ATS/ERS criteria (Citation[13]) and subjects had to be current or previous smokers. Patients were also required to have pulmonary function testing (including diffusing capacity and body plethysmography) with evidence of airflow obstruction (FEV1/FVC < 70%, FEV1 < 80% predicted) done within 6 months of the study.
Potential subjects were excluded if they had restrictive physiology (TLC < 80% predicted) or had a history of chronic pulmonary disease other than COPD. In addition, patients were excluded if they had a recent history (within 6 months) of myocardial infarction, heart failure, or cardiac dysrhythmia requiring treatment.
Study design
This study was approved by the UCLA IRB and all subjects gave written informed consent before any study procedure was undertaken. Enrolled subjects were studied in the Exercise Physiology Research Laboratory at UCLA during a single visit. Subjects were not required to discontinue any of their usual medications as a part of this study.
Measurement of inspiratory capacity
Patients breathed through a conventional flow transducer (Vmax metabolic cart; VIASYS Healthcare, Yorba Linda, CA) with nose-clips applied in the seated position. IC was measured in accordance with recent ATS/ERS guidelines (Citation[14]). For baseline IC measurements, tidal breathing was monitored and when the end-expiratory lung volume appeared stable for at least 3 tidal cycles, the patient was instructed to inspire to TLC. During metronome paced tachypnea, patients were instructed to inspire to TLC after 30 and 60 seconds. The IC was determined to be the difference between the TLC and the average of the three preceding end-expiratory lung volume measurements.
Metronome-paced tachypnea
The metronome was first demonstrated to subjects to ensure that they could hear the tones and familiarize themselves with the pacing protocol. Then, while breathing through the flow transducer, two reproducible IC measurements (within 200 ml) were measured to establish a baseline value of IC.
Subjects then increased their respiratory rate (fR) to 20 breaths per minute for 60 seconds in response to audible signals generated by the computer. The metronome produced tones for alternating inspiration and expiration, or 40 equally spaced tones per minute for fR 20/min. We chose this approach because preliminary attempts showed that patients found it easier to entrain their breathing frequency to two tones per breathing cycle rather than one. Subjects were allowed to regulate their own tidal volumes. After 30 seconds, subjects were prompted to perform an IC maneuver and then immediately return to paced breathing. Subjects were again prompted to perform the IC maneuver after 60 seconds, and then allowed to resume normal tidal breathing. End-tidal carbon dioxide tensions were monitored for the duration of testing and allowed to return to baseline along with fR before continuing with the protocol.
The protocol was then repeated for paced tachypnea at fR 30/min and again for fR 40/min with interval repeat measures of baseline IC. We recorded the time interval between the completion of metronome paced tachypnea at fR 20/min and the re-measure of baseline IC. This time interval was also recorded after metronome pacing at fR 30/min.
Statistical analysis
Results are expressed as means ± SD unless otherwise indicated. The mean IC values for baseline measurements were compared using a repeated measures ANOVA analysis. The IC values after metronome-paced tachypnea were compared to the initial baseline and to each other accounting for repeated measures and were corrected for multiple comparisons with the Bonferroni method. In an exploratory analysis, baseline demographic and physiologic data were examined using univariate linear regression to evaluate independent variables that may predict DH induced by metronome-paced tachypnea. Analyses were carried out using JMP IN version 5.1 for windows from the SAS Institute.
RESULTS
Baseline characteristics of the study population
Baseline characteristics of the 14 COPD patients tested are shown in . Of the 14 subjects, 13 (93%) were men. Their ages ranged from 59 to 82 years. By GOLD criteria, COPD was moderate in 5 patients, severe in 7 patients, and very severe in two patients. Nearly half of the subjects had hyperinflation at baseline; 5 with a TLC greater than 120% predicted, and one additional patient had a baseline IC/TLC ratio of less than 25%. Although there are no current standard equations for predicting normal spirometric IC, others have suggested the predicted TLC minus the predicted FRC as a reasonable method of deriving this value (Citation[15]).
Table 1 Patient characteristics
Dynamic Hyperinflation Induced by Metronome-Paced Tachypnea
All 14 patients studied were able to perform and tolerated the metronome-paced tachypnea protocol after minimal instruction and practice. The mean respiratory rate at baseline was 18.6 ± 6.5 breaths per minute. There were no significant differences among the four baseline IC measurements (repeated measures ANOVA p = 0.73). At each fR, the degree of DH appeared to be complete by 30 seconds. The performance of an IC maneuver after 30 seconds did not disrupt adherence to the metronome pacing over the subsequent 30 seconds prior to the 60-second maneuver. There was no significant difference between IC measurements performed at 30 and 60 seconds for any fR (, ).
Figure 1 Time course and degree of dynamic hyperinflation produced by metronome paced tachypnea. There is a graded decline in IC corresponding to the graded increase in fR. For each fR the decline in IC occurs by 30 seconds and does not decline further by 60 seconds. The IC returns to baseline between episodes of paced tachypnea. The plot's horizontal line represents the median, the box encompasses the 25th to 75th percentile and the error bars encompass the 10th to the 90th percentile. Repeated Measures ANOVA P < 0.001. * p < 0.05 compared to Baseline 1. **P < 0.05 compared to Baseline 1 and fR 20/min (30s). *** p < 0.05 compared to Baseline 1, fR 20/min (30s and 60s), and fR 30/min (30s and 60s).
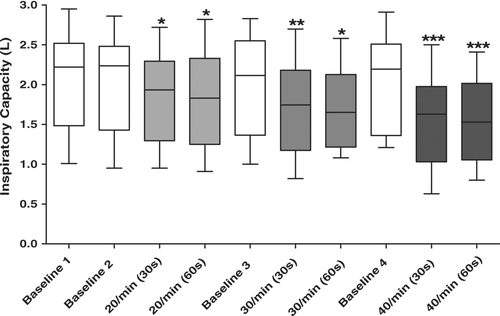
Table 2 Inspiratory capacity differences over time and between frequencies of respiration
With each stepwise increase in the rate of paced tachypnea, there was a corresponding reduction in mean IC (). After correction for multiple comparisons, the IC remained significantly lower after each paced fR compared to baseline. In addition, the IC after paced tachypnea at 40 breaths per minute was significantly reduced compared to 30 breaths per minute and 20 breaths per minute. After 30 seconds of tachypnea at 30 breaths per minute, the IC was also significantly reduced compared to 20 breaths per minute (, ).
The mean time interval between paced tachypnea at fR 20/min and the re-measure of baseline IC was 120 ± 51 seconds (Range = 41 to 233). The mean time interval between paced tachypnea at fR 30/min and the re-measure of baseline IC was 134 ± 50 seconds (Range = 40 to 219). Respiratory rates and end-tidal CO2 tensions measured just prior to each baseline IC measurement were similar (). End-tidal CO2 tensions declined predictably with tachypnea but to a reasonable limited degree ().
Table 3 Respiratory rate and PETCO2 at baseline and following 60 seconds of metronome-paced tachypnea
Correlation Between Baseline Characteristics and Dynamic Hyperinflation
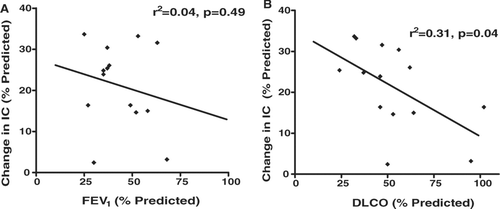
In univariate linear regression analyses, only diffusing capacity correlated significantly with DH. A lower diffusing capacity (DLCO % predicted) predicted greater DH, or a greater decline in IC expressed as a percentage of the predicted IC (for 30 secs at 40/min) (). FEV1 % predicted correlated poorly with changes in IC during metronome paced tachypnea (). Measures of hyperinflation at baseline (static hyperinflation) were also poor predictors of DH with metronome paced tachypnea (IC/TLC r2 = 0.05, p = 0.43; IC % predicted r2 = 0.12, p = 0.24).
Figure 2 (A) Correlation between FEV1 percent predicted and change in inspiratory capacity (IC) percent of predicted. (B) Correlation between DLCO percent predicted and change in inspiratory capacity (IC) percent of predicted.
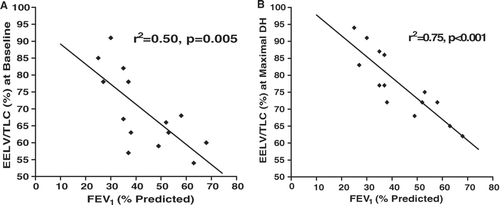
The EELV was calculated at by subtracting the IC from the TLC. In contrast to the lack of correlation between baseline FEV1 % predicted and changes in IC with paced tachypnea (DH), the FEV1 % predicted correlated well with EELV expressed as a percent of TLC at baseline (static hyperinflation). This relationship strengthened with the addition of DH during metronome paced tachypnea, with the strongest correlation when subjects were metronome paced at 40 breaths per minute for 30 seconds ().
DISCUSSION
This study of 14 patients with COPD has confirmed the ability of short periods of metronome-paced tachypnea to induce dynamic hyperinflation. We developed our protocol based on the technique described by Gelb et al. (Citation[8], Citation[16]) but chose to precisely control fR at fixed rates rather than an approximate doubling of resting breathing frequency. We also extended the investigation to study the time course and degree of DH in response to graded increases in respiratory rate produced by metronome pacing up to 60 seconds. We have confirmed that a graded increase in fR produces a corresponding graded decline in IC, and thus a graded increase in EELV. In this regard, our findings are similar to those reported by Fujimoto et al. (Citation[12]). However, to our knowledge, this study is the first to demonstrate that the degree of dynamic hyperinflation (DH) produced by a specific paced fR is essentially complete by 30 seconds.
DH can be mathematically modeled as being dependant on three factors: the elastic recoil of the respiratory system, airway resistance and exhalation time. Admittedly, DH is seen in various circumstances where there is increased minute ventilation. This could be the hyperpnea of exercise, where minute ventilation is matched to metabolic requirement (at least during mild and moderate exercise) or conditions of hyperventilation such as voluntary hyperventilation or the hyperventilation that occurs in response to hypoxemia or metabolic acidosis. Our conviction is that it is not the hyperventilation itself that primarily determines DH but the increased respiratory rate which shortens the time available for exhalation with a given airway resistance and elastic recoil. We did not coach patients to maintain a constant tidal volume and this may be a weakness of our protocol. However, we hypothesized that controlled changes in respiratory rate would be informative in relation to the time course and degree of DH. Our results support this assertion.
Ultimately, the diminished IC and inability to expand tidal volume results in increased elastic work of breathing and manifests as dyspnea and impaired exercise tolerance (Citation[5], Citation[17], Citation[18]). Serial IC measurements have been shown to be a reliable and reproducible way to track dynamic hyperinflation during exercise (Citation[5], Citation[15]). In contrast to FEV1, exercise induced DH has been shown to correlate well with symptoms, exercise tolerance and disability in COPD (Citation[5], Citation[18]). Changes in exercise-induced DH are also a sensitive marker of changes in COPD symptoms in response to various treatments (Citation[9], Citation[19], Citation[20], Citation[21], Citation[22], Citation[23]). Because cardiopulmonary exercise testing facilities may not be readily available to many physicians managing patients with COPD, an alternative simple method to reliably detect DH would be clinically useful.
Other studies have evaluated IC changes with metronome-paced tachypnea and determined the method to be a good measure of DH (Citation[8], Citation[12], Citation[16]). However, none of these studies have examined both the time course and degree of hyperinflation in response to various respiratory frequencies in a systematic manner. We believe that the appropriate use of this method as tool for monitoring the effect of interventions in COPD requires further refinement and standardization of the technique of metronome-paced tachypnea.
In addition to confirming the ability of metronome-paced tachypnea to induce DH in COPD patients, the present study was also designed to better characterize the time course of development of DH, and to evaluate the degree of DH induced by various respiratory frequencies. We showed the degree of hyperinflation is increased with metronome paced tachypnea and that the highest fR (40 breaths/min) produced the greatest degree of hyperinflation. This relationship between the respiratory frequency and the degree of DH reaffirms the belief that interventions which help to slow the respiratory rate, such as breathing retraining, supplemental oxygen and exercise training, should have a beneficial effect on the degree of DH (Citation[21], Citation[23], Citation[24]).
While patients tolerated metronome-paced tachypnea for up to 60 seconds, our study showed that there is no additional hyperinflation experienced beyond 30 seconds. This novel finding provides interesting insight into the mechanism of DH. The fact that DH seems to result in attainment of equilibrium between inspired and expired volumes at a faster respiratory rate and higher lung volumes within 30 seconds, indicates that the effect of the shortened expiratory time must be counter-balanced by other factors. The most important of these factors is likely to be the increased elastic recoil at the higher lung volumes. There could be a smaller contributory factor that curtails DH, from widening of the airways and reduced airway resistance at higher lung volumes. Our finding that DH does not worsen between 30 and 60 seconds has interesting clinical implications. First and foremost, our findings illustrate how quickly the abnormalities of respiratory system mechanics worsen with any increase in respiratory rate. They help explain why COPD patients who experience anxiety or desaturation rapidly become symptomatic and cease exertion. Future studies evaluating DH in COPD patients using a metronome pacing protocol could justifiably apply a respiratory rate of 40/min and limit the periods of tachypnea to 30 seconds.
Due to the fact that our study protocol dictated that the rate of metronome pacing be increased in a stepwise manner, it is possible that the greater decline in IC seen at higher respiratory rates may be due to an additive effect of DH induced by the previous periods of tachypnea. To minimize this potential effect, we monitored respiratory rates to ensure that they had returned to baseline levels before moving on to the next step. For this reason, the interval between tests was not standardized, but on average, patients had returned to baseline after approximately 2 minutes. Baseline IC measurements were repeated before initiating new periods of tachypnea and these IC measurements were not significantly different than the initial baseline measurements of IC. Hence, we found that, in the same manner that DH developed quickly and was essentially complete at a given respiratory rate within 30 seconds, so too did DH resolve rapidly within a matter of minutes. This finding also indicates that there was no cumulative effect of sequential periods of tachypnea on lung volumes using this protocol. Once again, there are important clinical implications, in that when COPD patients develop DH they can expect rapid resolution if respiratory rate can be controlled at lower rate. This important finding provides a scientific basis to techniques such as pursed-lip breathing that have been traditionally used in pulmonary rehabilitation program and are aimed at slowing respiratory rate.
The degree of DH experienced in our study sample was variable between subjects. In an unplanned analysis, we explored possible associations between baseline characteristics and the development of DH during metronome-paced tachypnea. As might be expected, based on its poor correlation with COPD symptoms, FEV1 correlated poorly in univariate analysis with the changes in IC seen with metronome-paced tachypnea. However, FEV1 did have an association with EELV, expressed as a percent of TLC, at both baseline and with tachypnea. While the change in IC represents DH, the EELV/TLC after metronome-paced tachypnea is a measure that encompasses both static and dynamic hyperinflation. If confirmed in other studies, this finding is consistent with expiratory flow limitation being an important contributory cause of hyperinflation, while additional variables such as reduced lung elastic recoil also determine the extent to which hyperinflation is present at rest and with tachypnea.
In our study, DLCO % predicted was inversely associated with the reduction in IC (expressed as a percent of the predicted IC), where a lower DLCO predicted a greater dynamic reduction in IC with paced tachypnea. A similar relationship has also been reported in exercise-induced DH where a low DLCO was associated with faster rates of DH, that is DH occurring earlier in exercise (Citation[15]). A low DLCO is likely a marker of a more emphysematous clinical profile with greater lung compliance and less elastic recoil. This loss of lung elastic recoil appears to amplify the effects of reduced expiratory time with increasing tachypnea, and thus results in a greater degree of DH.
A potential limitation of the present study is that we chose to monitor, rather than control for, end-tidal CO2. We recognize the potential for hypocapnea-induced bronchoconstriction with this method. However, in addition to keeping the protocol simple, an advantage of not controlling end-tidal CO2 is that our protocol more closely models natural tachypnea induced by hypoxia or anxiety. In these situations, hypocapnea may also be an important clinical and pathophysiological factor. Overall, the mean changes in end-tidal CO2 with metronome-paced tachypnea that we observed over 60 seconds were minor.
We also recognize that the duty cycle (TI/TTOT) in our protocol of metronome-paced tachypnea is not entirely physiologic. Specifically, the metronome protocol used for this study produces two equally spaced tones per breath corresponding to inspiration and expiration. During initial testing, patients found it easier to synchronize their breathing to two tones compared to a single tone per breathing cycle. For simplicity, we chose to equally space the inspiratory and expiratory tones producing a duty cycle equal to 0.50. At rest, a typical duty cycle for patients with severe COPD ranges from 0.30 to 0.35, and for normal subjects 0.36 to 0.39 (Citation[25]). In a separate study examining duty cycle at maximal exercise, the mean TI/TTOT for COPD patients was 0.41, and in normal subjects was 0.47 (Citation[26]). Therefore, for a given fR, the expiratory time during metronome-paced tachypnea would be shorter than during exercise. This might result in a greater degree of DH at a given fR for metronome-paced tachypnea compared to exercise. Despite the theoretical variation from the physiology of natural tachypnea, we believe that this protocol for metronome-paced tachypnea is a reasonable and simple tool for studying DH.
In conclusion, we have confirmed the ability of metronome-paced tachypnea to induce DH in COPD patients and that the degree of DH is increased with increasing respiratory rates. Furthermore, we have shown that DH occurs quickly and is not further increased beyond 30 seconds of tachypnea. Also, DH resolves quickly within a similar time period. These findings provide important insights into the mechanisms of hyperinflation in COPD patients and have important clinical implications as already discussed. We recommend that future studies using metronome-paced tachypnea to induce DH should aim for a fixed respiratory rate of 40 breaths per minute for a period of 30 seconds, however this approach remains to be validated against results obtained at the same respiratory rate during exercise. This metronome pacing protocol is simple and can be reproduced in a variety of clinical settings. Given its simplicity and the fact that DH is an important therapeutic target in COPD, metronome-paced tachypnea could be a useful tool for assessing the impact of different interventions on dynamic hyperinflation in COPD patients.
REFERENCES
- Rabe K F, Hurd S, Anzueto A, Barnes P J, Buist S A, Calverley P, et al. Global Strategy for the Diagnosis, Management, and Prevention of COPD – 2006 Update. Am J Respir Crit Care Med 2007; 176(6)532–555
- Hay J G, Stone P, Carter J, Church S, Eyre-Brook A, Pearson M G, et al. Bronchodilator reversibility, exercise performance and breathlessness in stable chronic obstructive pulmonary disease. Eur Respir J 1992; 5(6)659–664
- Cooper C B. The connection between chronic obstructive pulmonary disease symptoms and hyperinflation and its impact on exercise and function. Am J Med 2006; 119: 21–31, (10 Suppl 1)
- Dodd D S, Brancatisano T, Engel L A. Chest wall mechanics during exercise in patients with severe chronic air-flow obstruction. Am Rev Respir Dis 1984; 129(1)33–38
- O'Donnell D E, Lam M, Webb K A. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 158: 1557–1565, (5 Pt 1)
- O'Donnell D E, Webb K A. Breathlessness in patients with severe chronic airflow limitation. Physiologic correlations. Chest 1992; 102(3)824–831
- Yan S, Kaminski D, Sliwinski P. Reliability of inspiratory capacity for estimating end-expiratory lung volume changes during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997; 156(1)55–59
- Gelb A F, Gutierrez C A, Weisman I M, Newsom R, Taylor C F, Zamel N. Simplified detection of dynamic hyperinflation. Chest 2004; 126(6)1855–1860
- Maltais F, Hamilton A, Marciniuk D, Hernandez P, Sciurba F C, Richter K, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest 2005; 128(3)1168–1178
- O'Donnell D E, Lam M, Webb K A. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 160(2)542–549
- O'Donnell D E, Voduc N, Fitzpatrick M, Webb K A. Effect of salmeterol on the ventilatory response to exercise in chronic obstructive pulmonary disease. Eur Respir J 2004; 24(1)86–94
- Fujimoto K, Yoshiike F, Yasuo M, Kitaguchi Y, Urushihata K, Kubo K, et al. Effects of bronchodilators on dynamic hyperinflation following hyperventilation in patients with COPD. Respirology 2007; 12(1)93–99
- Celli B R, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23(6)932–946
- Miller M R, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J 2005; 26(2)319–338
- O'Donnell D E, Revill S M, Webb K A. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 164(5)770–777
- Gelb A F, Taylor C F, McClean P A, Shinar C M, Rodrigues M T, Gutierrez C A, et al. Tiotropium and simplified detection of dynamic hyperinflation. Chest 2007; 131(3)690–695
- Celli B, ZuWallack R, Wang S, Kesten S. Improvement in resting inspiratory capacity and hyperinflation with tiotropium in COPD patients with increased static lung volumes. Chest 2003; 124(5)1743–1748
- Diaz O, Villafranca C, Ghezzo H, Borzone G, Leiva A, Milic-Emil J, et al. Role of inspiratory capacity on exercise tolerance in COPD patients with and without tidal expiratory flow limitation at rest. Eur Respir J 2000; 16(2)269–275
- Belman M J, Botnick W C, Shin J W. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996; 153(3)967–975
- Martinez F J, de Oca M M, Whyte R I, Stetz J, Gay S E, Celli B R. Lung-volume reduction improves dyspnea, dynamic hyperinflation, and respiratory muscle function. Am J Respir Crit Care Med 1997; 155(6)1984–1990
- O'Donnell D E, D'Arsigny C, Webb K A. Effects of hyperoxia on ventilatory limitation during exercise in advanced chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163(4)892–898
- O'Donnell D E, Fluge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 2004; 23(6)832–840
- Porszasz J, Emtner M, Goto S, Somfay A, Whipp B J, Casaburi R. Exercise training decreases ventilatory requirements and exercise-induced hyperinflation at submaximal intensities in patients with COPD. Chest 2005; 128(4)2025–2034
- Somfay A, Porszasz J, Lee S M, Casaburi R. Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patients. Eur Respir J 2001; 18(1)77–84
- Bloch K E, Li Y, Zhang J, Bingisser R, Kaplan V, Weder W, et al. Effect of surgical lung volume reduction on breathing patterns in severe pulmonary emphysema. Am J Respir Crit Care Med 1997; 156: 553–560, (2 Pt 1)
- Clarenbach C F, Senn O, Brack T, Kohler M, Bloch K E. Monitoring of ventilation during exercise by a portable respiratory inductive plethysmograph. Chest 2005; 128(3)1282–1290