Abstract
Spirometry is necessary to diagnose and assess severity of COPD, but is used infrequently. Therapy with inhaled medications can improve COPD outcomes, but are not without risks. The use of spirometry may help mitigate the therapy risks if treatment is appropriate based on spirometry results. Before determining benefits of spirometry use, it is important to examine use of medications and the use of spirometry. Our objective was to characterize the association between the use of spirometry and respiratory medications in newly diagnosed COPD. This is a retrospective, longitudinal study using data from the Department of Veterans Affairs. We identified patients with a new diagnosis of COPD (index date). Spirometry use was measured two years before to six months after the index date. Respiratory medications were measured within one year following the index date. The association between spirometry and medication use was evaluated using logistic regressions and stratified by quintiles of the propensity scores for the probability of having had spirometry performed. A total of 81,162 patients were included and 30.8% had a spirometry performed. Patients with spirometry were more likely to have been dispensed an inhaled corticosteroid (AOR = 1.22 (95% CI, 1.11–1.36) to 1.61 (1.45–1.79)), long-acting beta-agonists (AOR = 1.41(1.25–1.58) to 1.63(1.45–1.83)), and ipratropium bromide (AOR = 1.25(1.16–1.35) to 1.64 (1.49–1.81)) across quintiles. Patients with spirometry were more likely to have medications added. The use of spirometry around a new diagnosis of COPD was associated with higher likelihood of using and adding respiratory medications after diagnosis
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is the most common respiratory related cause of death and disability in the United States (Citation[1]). By definition, airflow obstruction must be present in order to classify an individual as having COPD and this can only be accurately assessed by spirometry. Nonetheless, it has been demonstrated that the routine use of spirometry is limited with only a third of newly diagnosed individuals with COPD actually have spirometry to confirm the diagnosis (Citation[2], Citation[3]). Spirometry is important, in part, because at most 15% of patients who smoke develop COPD and previous studies suggest that history and physical finding are neither sensitive nor specific for diagnosing COPD (Citation[4],Citation[5],Citation[6],Citation[7],Citation[8],Citation[9]). Thus, many patients with a diagnosis are being medically managed without this evaluation of airways obstruction through spirometry. Recently, the National Committee for Quality Assurance adopted the use of spirometry for patients with a new diagnosis of COPD as a performance measure for the Healthcare Effectiveness Data and Information Set (HEDIS)®, a tool used by more than 90% of health plans in the United States to measure performance on important dimensions of care and service (Citation[10]).
Beyond accurate diagnosis, the classification of disease severity relies heavily on spirometry measures and recommendations for pharmacologic treatment from international organizations also rely heavily on disease severity based on spirometry measures (Citation[11],Citation[12],Citation[13]). Appropriate therapy with inhaled medications have been demonstrated to reduce symptoms, improve health-related quality of life, and decrease the rate of acute exacerbations (Citation[14],Citation[15],Citation[16],Citation[17],Citation[18],Citation[19],Citation[20],Citation[21],Citation[22],Citation[23],Citation[24],Citation[25],Citation[26],Citation[27]). However, there is growing evidence that pharmacotherapy used in COPD poses some risks (Citation[14], Citation[15], Citation[28],Citation[29],Citation[30],Citation[31],Citation[32]). The use of spirometry may help mitigate the risks of therapy if therapy is appropriate based on spirometry results. Before determining the benefit to using spirometry it is important to determine if there are differences in medication use based on the use of spirometry.
Studies evaluating the association of spirometry and respiratory medication use in newly diagnosed COPD are limited. For example, a Canadian study found that introduction of screening spirometry into primary care practices influenced physician management 15% of the time, especially when unsuspected and severe airways obstruction was present (Citation[33]). A study from the United Kingdom showed that providing open-access spirometry resulted in an increase in the use of anticholinergics, long-acting beta-agonists, and inhaled corticosteroids in patients with newly diagnosed COPD based on a subgroup analysis (Citation[34]). As appropriate therapy with inhaled medications has been demonstrated to improve outcomes, these data suggest that spirometry may either influence or be a marker of higher quality care.
The objective of this analysis was to characterize the association of spirometry performed within the time frame determined by the Hedis© criteria and respiratory medication use within a year following a new diagnosis of COPD.
MATERIALS AND METHODS
This study was approved by the Human Studies Subcommittee of the Hines VA Hospital and the Institutional Review Board at Northwestern University.
Subjects
We used the 2006 Healthcare Effectiveness Data and Information Set (HEDIS®) COPD spirometry performance measure to define a VA cohort for this study (Citation[10]). Using VA administrative data we identified patients with a diagnosis of COPD (International Classification of Diseases, Ninth Revision (ICD-9) 491.x, 492.x, 496) between July 1, 2003 and June 30, 2004. The date of the diagnosis is referred to as the index date. To be included, patients must have been 42 years or older by December 31, 2003 and were required to have at least one non-COPD healthcare visit once a year for two years prior to the index date. Patients were excluded if they had a COPD-related visit during the two years prior to the index date and/or died within six months after the index date.
Spirometry
Spirometry exams were identified through Current Procedural Terminology (CPT) codes () if performed two years before to six months after the index date based on HEDIS® criteria. A clinic stop code (104) specific to pulmonary function testing in the VA was also identified during the same time period. If any of these codes were present, patients were categorized as having received spirometry.
Table 1 Patient characteristics stratified by the probability of having had spirometry performed and use of spirometry
Respiratory medications
Respiratory medications were obtained from VA pharmacy databases and included the following: inhaled corticosteroids (ICS), long-acting beta-agonist (LABA), ipratropium bromide (IPRA), and short-acting beta-agonist (SABA). Medication data were collected for one year prior and one year following the index date.
Demographics and other characteristics
Patient characteristics used as covariates in the analysis included age, marital status, region, and co-morbidities. Co-morbidities were identified using ICD-9 codes from inpatient and outpatient data during the two years prior to the index date. Comorbidities identified include the following: asthma, diabetes mellitus, hypertension, depression, mental health, heart disease, collagen vascular disease, alcoholism, substance abuse, osteoarthritis, BPH, cancer, and other lung disease. Other lung disease includes bronchiectasis, pulmonary hypertension, cor pulmonale, and/or pneumoconiosis. VA healthcare utilization measures including the number of primary care contacts, pulmonary specialist contacts, hospitalizations, and emergency department visits were obtained during the two years prior to the index date, averaged per annum, and used as covariates in the analysis.
Statistical analysis
A propensity score for the use of spirometry was obtained using baseline covariates (age, marital status, comorbidities, healthcare utilization, and region). Patients who could not be matched effectively by propensity score were not included in the analysis. This was true for those with a propensity score greater than 0.55. In comparing those with and without spirometry, the number of patients without spirometry and a propensity score > 0.55 was 3,145 and those with spirometry and a propensity score > 0.55 was 9,417. Identifying a comparable group when the propensity score was > 0.55 was not possible and therefore those with propensity scores higher than 0.55 were excluded from the analysis. The main reason for this imbalance was that the majority of those with a spirometry and a propensity score > 0.55 had a pulmonary specialist visit whereas the group without spirometry had less patients with a pulmonary specialist visit and thus less likely to have a propensity score > 0.55.
For those included in the analysis, the patients were divided into quintiles based on the propensity score or probability of having a spirometry performed with quintile five being the group with characteristics most associated with the likelihood of receiving spirometry (Citation[35]). Once stratified into quintiles, the characteristics were compared and statistically different or unbalanced covariates were identified. Percentages were used to describe categorical variables and means and standard deviations were used to describe continuous variables. Comparisons between the groups with and without spirometry were made with χ2 tests for categorical variables and t-tests for continuous variables.
Within each quintile, the association between spirometry use and respiratory medication use was evaluated in logistic regression models adjusting for unbalanced covariates within each quintile. Medications were considered to have been added if a patient was not using that medication prior to the index date, then using it after the index date. If a patient was using a medication prior to the index date and not using it after the index date, the medications were considered to have been discontinued. Statistical analysis was performed using STATA version 9.2 (StataCorp., College Station, TX). The level of significance used was a P-value of 0.05.
Sensitivity analysis
Although we did not have the results of the spirometry test, we considered whether patients with a diagnosis of COPD and spirometry consistent with the diagnosis would yield different results. To account for this, we performed a sensitivity analysis including only those with a second diagnosis of COPD within a nine-month period following the latter of the index date or spirometry date if spirometry was performed as a proxy for those that had a spirometry consistent with a diagnosis of COPD. This group was compared to those without spirometry who had a second diagnosis of COPD within a nine-month period following the index date. The logistic regression models were again adjusted for unbalanced covariates within the quintiles.
Also, to account for the possibility of a medication being used prior to spirometry, we ran a second sensitivity analysis only including medications that were dispensed in the 180 days to 360 days after the index date; therefore within 180 days after all spirometry. This is a time frame after which all spirometry would have been complete. The logistic regression models were again adjusted for unbalanced covariates within the quintiles.
RESULTS
There were 93,724 patients who fulfilled initial inclusion criteria and of those 36.7% had spirometry performed during the study period. A total of 12,562 patients were excluded because of insufficient overlap in their propensity scores. Of the 81,162 patients with newly diagnosed COPD included in the analysis, a total of 24,976 (30.8%) patients had a spirometry performed. The characteristics of those with and without spirometry by propensity score quintile are shown in .
The percentage of patients receiving medications prior to and after the index date are shown in . Overall, 17,595 (21.7%) patients had no COPD medications in the year prior to the index date then had at least one medication in the post-period. Of those who had spirometry, 6,181 (24.8%) were in this category compared to 11,414 (20.3%) in the group without spirometry (P < 0.001). In the pre-period, there were 4,312 (5.3%) patients who were receiving at least one medication then had no medications in the post period. In the spirometry group, 1,588 (6.4%) were in this category compared to 2,724 (4.9%) in the group without spirometry (P < 0.001).
Table 2 Patients receiving medications before and after the index date
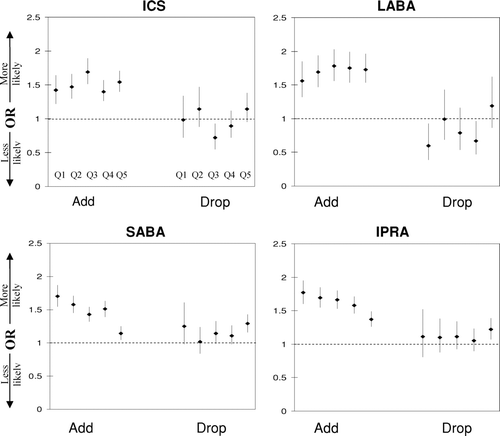
The adjusted odds ratios (AOR) of medication use for patients with spirometry compared to those without spirometry in the pre and post index date are shown in for each of the quintiles. Within one year after the index date, the AOR for patients with spirometry use ranged from 1.22 (95% CI, 1.11–1.36) to 1.61 (95% CI, 1.45–1.79) for ICS use, 1.41(95% CI, 1.25–1.58) to 1.63(95% CI, 1.45–1.83) for LABA use, 1.25(95% CI, 1.16–1.35) to 1.64(95% CI, 1.49–1.81) for IPRA use, and 1.06(95% CI, 0.98–1.14) to 1.65(95% CI, 1.50–1.81) for SABA use across the quintiles. The AOR for adding and discontinuing medications are shown in . The AOR for adding a medication for patients with spirometry compared to those without spirometry ranged from 1.40(95% CI, 1.26–1.57) to 1.69(95% CI, 1.51–1.89) for ICS, 1.56(95% CI, 1.32–1.85) to 1.78(95% CI, 1.56–2.03) for LABA, 1.37(95% CI, 1.26–1.49) to 1.77(95% CI, 1.60–1.95) for IPRA, and 1.14(95% CI, 1.05–1.25) to 1.70(95% CI, 1.55–1.87) for SABA across the quintiles. The likelihood of adding a medication was similar across each of the quintiles for ICS and LABA; however for SABA and IPRA the highest likelihood of adding a medication was seen in the first quintile (SABA = 1.70; IPRA = 1.77) and the lowest was seen in the fifth quintile (SABA = 1.14; IPRA = 1.37). For most of the comparisons there was no association between spirometry and discontinuing a medication.
Figure 1 Adjusted odds ratios of medication use one year prior to (Pre) and after (Post) the index date for those who have ever had a spirometry 720 days prior to 180 days after the index date for quintiles one to five, from left to right. Quintile 1 is the group with the least probability of having spirometry. ICS = inhaled corticosteroid; LABA = long-acting beta agonist; SABA = short-actiing berta agonist; IPRA = ipratropium; Q = quintile. The logistic regression models (LRM) were adjusted for the following unbalanced covariates. Quintile one: age, marital status, depression, mental health, alcohol abuse, cancer, primary care, emergency department, and hospital contacts. Quintile two: age, emergency department and hospital contacts. Quintile three was balanced. Quintile four: age, heart disease, substance abuse, and primary care contacts. Quintile five: age, marital status, asthma, hypertension, alcohol abuse, substance abuse, primary care and pulmonary contacts. The LRM for medication use after the index date was also adjusted for medications prior to the index date if not balanced within the propensity scores quintiles. These included ICS, LABA and no medication use in quintile one, ICS in quintile three, and ICS, LABA, SABA, IPRA and no medication use in quintile five.
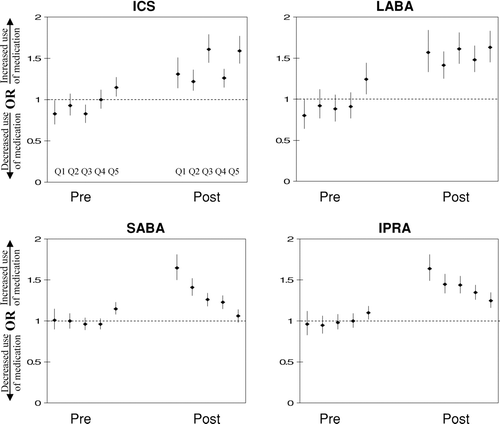
Figure 2 Adjusted odds ratios for adding (Add) and discontinuing (Drop) medications for those who have ever had a spirometry 720 days prior to 180 days after the index date for quintiles one to five, from left to right. Quintile 1 is the group with the least probability of having spirometry. ICS = inhaled corticosteroid; LABA = long-acting beta agonist; SABA = short-actiing beta agonist; IPRA = ipratropium; Q = quintile. The logistic regression models were adjusted for the unbalanced covariates. (Same as in except for medications).
Sensitivity analysis
When including only those with a second diagnosis of COPD, there were 35,556 patients in the analysis and of those 11,647 (32.8%) had spirometry. Within one year after the index date, the AOR for patients with spirometry compared to those without spirometry were similar to the original analysis. The AOR ranged from 1.37 (95% CI, 1.21–1.56) to 1.80 (95% CI, 1.58–2.06) for ICS use, 1.51(95% CI, 1.30–1.74) to 1.75(95% CI, 1.52–2.02) for LABA use, 1.45(95% CI, 1.32–1.60) to 1.81(95% CI, 1.60–2.05) for IPRA use, and 1.35(95% CI, 1.20–1.51) to 1.67(95% CI, 1.47–1.89) for SABA use across the quintiles. The adjusted OR for adding a medication for patients with spirometry compared to those without spirometry ranged from 1.70(95% CI, 1.44–2.01) to 2.13(95% CI, 1.87–2.43) for ICS, 1.90(95% CI, 1.62–2.23) to 2.13(95% CI, 1.83–2.48) for LABA, 1.60(95% CI, 1.44–1.78) to 2.14(95% CI, 1.89–2.44) for IPRA, and 1.35(95% CI, 1.21–1.52) to 1.95(95% CI, 1.72–2.21) for SABA across the quintiles. Patients with spirometry were similar to or less likely to discontinue a medication compared to those without spirometry across the quintiles.
The second sensitivity analysis included only respiratory medications that were dispensed in the 180 to 360 days after the index date, after which all spirometry would have been complete. The AOR ranged from 1.24 (95% CI, 1.08–1.42) to 1.53 (95% CI, 1.36–1.72) for ICS use, 1.49 (95% CI, 1.28–1.73) to 1.65 (95% CI, 1.47–1.86) for LABA use, 1.24 (95% CI, 1.16–1.32) to 1.65(95% CI, 1.50–1.82) for IPRA use, and 1.14(95% CI, 1.07–1.22) to 1.62(95% CI, 1.48–1.77) for SABA use across the quintiles. A trend similar to the original results was seen for IPRA and SABA.
DISCUSSION
This study shows that spirometry which improves diagnostic accuracy and aids in determining severity in COPD, is associated with medication management patterns that differ compared to those without spirometry.
Our results suggest patients who received spirometry had important differences in prescribing practices and these findings may have potentially important clinical implications. Those who had a spirometry performed were more likely to be using LABA and ICS compared to those without spirometry. For SABA and IPRA, there is a trend of decreasing odds ratios from Quintiles 1 to 5. As seen in , the proportion of patients with comorbid asthma and other lung disease increases from Quintile 1 to Quintile 5. Also there is increasing healthcare utilization of primary care services from Quintile 1 to Quintile 5. As seen in , prior to the index date, the proportion of patients using short acting bronchodilators increases from Quintile 1 to Quintile 5 (SABA from 14.5% to 54%; IPRA 9.5% to 29.5%). Taken together, Quintile 5 appears to include a sicker group of patients with more comorbid lung disease than Quintile 1 with an increasing trend from Quintile 1 to 5. As Quintile 5 has a high proportion of patients on short acting bronchodilators prior to the index date there is less room to denote a difference in those with and without spirometry. Also, this may be consistent with more surprising findings in the group less likely to receive spirometry. We found that the group less likely to have spirometry (Quintile 1) may be the group with more unexpected findings leading to greater medication dispensing differences between those with and without spirometry, relative to the group more likely to receive spirometry (Quintile 5). Therefore, spirometry may improve diagnostic accuracy more in the group less likely to receive spirometry than in the group more likely to have spirometry performed, leading to larger differences in medication use for the spirometry group compared to the no spirometry group. Many individuals who did not have spirometry continued to receive medications that they may not have needed, potentially increasing their risk of complications unnecessarily. In future, it is important to determine if this pattern of care is associated with differences and potentially improved outcomes.
It is unclear why physicians do not perform spirometry routinely in patients with suspected or newly diagnosed COPD, but many of the reasons may have direct effects on the quality of care delivered to these individuals. For example, physicians may lack the knowledge of guidelines for spirometry use around a new diagnosis of COPD (Citation[36]). Physicians may also be reluctant to order a test if they are unsure how to interpret the results, which is consistent with greater spirometry use by pulmonologists compared to primary care physicians in patients with a diagnosis of COPD (37–39). When primary care physicians were asked about the reasons for not performing spirometry, the most common response was uncertainty about the impact of the test (Citation[38]). These studies suggest that greater effort is needed to educate physicians about the utility of spirometry for the diagnosis and proper management of COPD.
Regardless of why spirometry is not routinely performed, our study indicates that patients with spirometry are more likely to be using respiratory medications and more likely to have a new medication added after diagnosis. Even after balancing for patient characteristics and healthcare utilization and adjustment for unbalanced variables within the regression model, this association persisted. Within the VA system, we found geographic variation in acute exacerbations of COPD that were not explained by patient factors and climate (Citation[40]). If provider variability determines spirometry use, which in turns is associated with differences in management, this variability needs to be addressed to improve quality of care for COPD. Accurate diagnosis with spirometry and subsequent appropriate management with reduced provider variability could lead to betters outcomes such as acute exacerbations, hospitalizations, and mortality for patients with COPD.
Our study does have some limitations. First, non-VA healthcare use was not included in the analysis. However, in a previous study within the VA there were only 4% of patients with newly diagnosed COPD who may have been misclassified as not having undergone spirometry when Medicare data was used (Citation[3]). Also, Medicare drug benefits did not go into effect until January 1, 2006, after the study conclusion date; therefore, most Medicare eligible patients at the time of the study were likely receiving medications through the VA. Second, the medication data is indicative of medication dispensed not prescribed. Patients who discontinued medications on their own by not picking up a prescription fill would not show up as having had that medication dispensed even if it was prescribed. Conversely, if a medication is dispensed, it had to have been prescribed initially. Therefore, addition of medications is more physician driven. We could not differentiate physician versus patient initiated discontinuation in medications. In quintile three for ICS and quintile one and four for LABA, patients with spirometry were less likely to discontinue the medication compared to those without spirometry. In the other quintiles, the results are equivocal and not significant. This variation across quintiles may indicate that discontinuations are not closely associated with spirometry and more patient driven. However, the discontinuation patterns should be interpreted with caution due to the small number of patients. Third, it is important to note that this study shows an association between the use of spirometry anytime during the specified period and medication use. We cannot assume causality based on this analysis. It is possible that unmeasured factors are confounding this relationship; however, it is more plausible that physicians who have additional information about the degree of airway obstruction are treating their patients differently than those without this information. Fourth, the results of the spirometry were not available for analysis; therefore, we may have included patients who truly did not have COPD. To account for this, we used a second diagnosis of COPD after the performance of spirometry as a surrogate for confirmed diagnoses. Our sensitivity analysis shows that the results are similar when including only those with a subsequent second diagnosis of COPD after the performance of spirometry. It is not possible to verify a diagnosis of COPD in those without spirometry, but based on our sensitivity analysis, there seems to be comparable proportions with a second diagnosis as the percent with spirometry shows only a 2% difference from the original analysis. Fifth, as we only included patients with an ICD-9 code for COPD, it is possible that patients may have been treated for COPD prior to the index date without an ICD-9 diagnosis. Therefore our results are limited to those fulfilling our inclusion criteria of a new diagnosis for COPD with a two year negative diagnosis history based on ICD-9 codes. Sixth, since the results of the spirometry were not available to assess severity of disease, we included healthcare utilization in the propensity score matching as an indirect measure of disease severity. Finally, we did not have reports of patient symptoms to determine an association with medication use. However, previous studies have shown limited accuracy in physicians diagnosis of COPD when based solely on risk factors, symptoms, and physical exam (Citation[4],Citation[5],Citation[6],Citation[7],Citation[8]).
We found that the use of spirometry around a new diagnosis of COPD was associated with higher likelihood of using and adding respiratory medications after their diagnosis. Although, the use of long-acting beta-agonists and inhaled corticosteroids have been associated with better outcomes, such as decreased rate of COPD exacerbations (Citation[14], Citation[15], Citation[24], Citation[25], Citation[29]), it is unclear whether the observed treatment patterns are associated with similar outcomes in the general population. Closer examination of the outcomes associated with this pattern of care will provide useful guidance to improve the care quality of patients with newly diagnosed COPD.
Acknowledgements
Dr. Lee has received funding for his contribution to the Burden of Obstructive Lung Disease (BOLD) Initiative, which has been funded inpart by uinrestricted educational grants to the Operations Center (www.boldcopd.org) from ALTANA, Aventis, AstraZeneca, Boehringer-Ingelheim, Chiesi, GlacoSmith Kline, Merck, Novartis, Pfizer, Schering-Plough, Sepracor and Univeristy of Kentucky. Dr. Lee has received past research grants from Astra-Zeneca, Pfizer, GlaxoSmithKline, and Boehringer-Ingelheim. Dr. Au received a one time consultant fee from GlaxoSmithKline in the past three years. Min Joo, Todd Lee, David Au, Marian Fitzgibbon, and Kevin Weiss have no other potential conflicts of interests to disclose.
This material is based upon work supported by the HSR&D Service, Center for Management of Complex Chronic Care COE, Hines VA Hospital. The funding agency did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Research for this paper was done in part while Min Joo was a National Research Service Award postdoctoral fellow at the Institute for Healthcare Studies, Feinberg School of Medicine at Northwestern University (Chicago, Illinois) under an institutional award from the Agency for Healthcare Research and Quality. Kevin Weiss was the Director for the Management of Complex Chronic Care, Hines VA Hospital, Hines, IL and the Institute for Healthcare Studies at Northwestern University Feinberg School of Medicine, Chicago, IL when this work was performed
REFERENCES
- National Heart Lung and Blood Institute. Morbidity & Mortality: 2007 Chart Book on Cardiovascular, Lung, and Blood Diseases. National Heart Lung and Blood Institute. 2007, Editor
- Han M K, Kim M G, Mardon R, Renner P, Sullivan S, Diette G B, Martinez F J. Spirometry utilization for COPD: How do we measure up?. Chest 2007; 132(2)403–409
- Lee T A, Bartle B, Weiss K B. Spirometry use in clinical practice following diagnosis of COPD. Chest 2006; 129(6)1509–1515
- Badgett R G, Tanaka D J, Hunt D K, Jelley M J, Feinberg L E, Steiner J F, Petty T L. The clinical evaluation for diagnosing obstructive airways disease in high-risk patients. Chest 1994; 106(5)1427–1431
- Buffels J, Degryse J, Heyrman J, Decramer M. Office spirometry significantly improves early detection of COPD in general practice: the DIDASCO Study. Chest 2004; 125(4)139–149
- Straus S E, McAlister F A, Sackett D L, Deeks J J. Accuracy of history, wheezing, and forced expiratory time in the diagnosis of chronic obstructive pulmonary disease. J Gen Intern Med 2002; 17(9)684–688
- van Schayck C P, van Weel C, Harbers H J, van Herwaarden C L. Do physical signs reflect the degree of airflow obstruction in patients with asthma or chronic obstructive pulmonary disease?. Scand J Prim Health Care 1991; 9(4)232–238
- Straus S E, McAlister F A, Sackett D L, Deeks J J. The accuracy of patient history, wheezing, and laryngeal measurements in diagnosing obstructive airway disease. CARE-COAD1 Group. Clinical Assessment of the Reliability of the Examination-Chronic Obstructive Airways Disease. JAMA 2000; 283(14)1853–1857
- Holleman D R, Jr., Simel D L. Does the clinical examination predict airflow limitation?. JAMA 1995; 273(4)313–319
- National Committee for Quality Assurance (NCQA), HEDIS. Health plan employer data & information set, Vol. 2, Technical specifications. 2005. National Committee for Quality Assurance (NCQA), Washington, DC 2006; 350
- American Thoracic Society and European Respiratory Society. Standards for the diagnosis and management of patients with COPD. 2004, (cited 2007 December 28); Available from: http://www.thoracic.org/sections/copd/resources/copddoc.pdf
- Celli B R, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur RespirJ 2004; 23(6)932–946
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (Updated 2007), (cited 2007 December 16); Available from: http://www.goldcopd.org/Guidelineitem.asp?l1=2&l2=1&intId=989
- Burge P S, Calverley P M, Jones P W, Spencer S, Anderson J A, Maslen T K. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. Br MedJ 2000; 320(7245)1297–1303
- Calverley P M, Anderson J A, Celli B, Ferguson G T, Jenkins C, Jones P W, Yates J C, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med 2007; 356(8)775–789
- Donohue J F, van Noord J A, Bateman E D, Langley S J, Lee A, Witek T J, Jr., Kesten S, Towse L. A 6-month, placebo-controlled study comparing lung function and health status changes in COPD patients treated with tiotropium or salmeterol. Chest 2002; 122(1)47–55
- Gartlehner G, Hansen R A, Carson S S, Lohr K N. Efficacy and safety of inhaled corticosteroids in patients with COPD: a systematic review and meta-analysis of health outcomes. Ann Fam Med 2006; 4(3)253–262
- Jones P W, Bosh T K. Quality of life changes in COPD patients treated with salmeterol. Am J Respir Crit Care Med 1997; 155(4)1283–1289
- Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000; 343(26)1902–1909
- Macie C, Wooldrage K, Manfreda J, Anthonisen N R. Inhaled corticosteroids and mortality in COPD. Chest 2006; 130(3)640–646
- Ram F S, Sestini P. Regular inhaled short acting beta2 agonists for the management of stable chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. Thorax 2003; 58(7)580–584
- Ramirez-Venegas A, Ward J, Lentine T, Mahler D A. Salmeterol reduces dyspnea and improves lung function in patients with COPD. Chest 1997; 112(2)336–340
- Rennard S I, Anderson W, Zu Wallack R, Broughton J, Bailey W, Friedman M, Wisniewski M, Rickard K. Use of a long-acting inhaled beta2-adrenergic agonist, salmeterol xinafoate, in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163(5)1087–1092
- Stockley R A, Chopra N, Rice L. Addition of salmeterol to existing treatment in patients with COPD: a 12 month study. Thorax 2006; 61(2)122–128
- Stockley R A, Whitehead P J, Williams M K. Improved outcomes in patients with chronic obstructive pulmonary disease treated with salmeterol compared with placebo/usual therapy: results of a meta-analysis. Respir Res 2006; 7: 147
- van Noord J A, Aumann J L, Janssens E, Verhaert J, Smeets J J, Mueller A, Cornelissen P J. Effects of tiotropium with and without formoterol on airflow obstruction and resting hyperinflation in patients with COPD. Chest 2006; 129(3)509–517
- Wadbo M, Lofdahl C G, Larsson K, Skoogh B E, Tornling G, Arwestrom E, Bengtsson T, Strom K. Effects of formoterol and ipratropium bromide in COPD: a 3-month placebo-controlled study. Eur RespirJ 2002; 20(5)1138–1146
- Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000; 343(26)1902–1909
- Alsaeedi A, Sin D D, McAlister F A. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomized placebo-controlled trials. Am J Med 2002; 113(1)59–65
- Ernst P, Gonzalez A V, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med 2007; 176(2)162–166
- Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of salmeterol/fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 175(2)144–149
- Lee T A, Weiss K B. Fracture risk associated with inhaled corticosteroid use in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2004; 169(7)855–859
- Dales R E, Vandemheen K L, Clinch J, Aaron S D. Spirometry in the primary care setting: influence on clinical diagnosis and management of airflow obstruction. Chest 2005; 128(4)2443–2447
- Walker P P, Mitchell P, Diamantea F, Warburton C J, Davies L. Effect of primary-care spirometry on the diagnosis and management of COPD. Eur RespirJ 2006; 28(5)945–952
- Rubin D B. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997; 127(8 Pt 2)757–763
- Barr R G, Celli B R, Martinez F J, Ries A L, Rennard S I, Reilly J J, Jr., Sciurba F C, Thomashow B M, Wise R A. Physician and patient perceptions in COPD: the COPD Resource Network Needs Assessment Survey. Am J Med 2005; 118(12)1415
- Bolton C E, Ionescu A A, Edwards P H, Faulkner T A, Edwards S M, Shale D J. Attaining a correct diagnosis of COPD in general practice. Respir Med 2005; 99(4)493–500
- Kaminsky D A, Marcy T W, Bachand M, Irvin C G. Knowledge and use of office spirometry for the detection of chronic obstructive pulmonary disease by primary care physicians. Respir Care 2005; 50(12)1639–1648
- Walters J A, Hansen E, Mudge P, Johns D P, Walters E H, Wood-Baker R. Barriers to the use of spirometry in general practice. Aust Fam Physician 2005; 34(3)201–203
- Joo M J, Lee T A, Weiss K B. Geographic variation in chronic obstructive pulmonary disease exacerbation rates. J Gen Intern Med 2007; 22(11)1560–1565