Abstract
The objective was to evaluate the effect of inhaled corticosteroids on disease progression in smokers with moderate to severe chronic obstructive pulmonary disease (COPD), as assessed by annual computed tomography (CT) using lung density (LD) measurements. Two hundred and fifty-four current smokers with COPD were randomised to treatment with either an inhaled corticosteroids (ICS), budesonide 400 μ g bid, or placebo. COPD was defined as FEV1 ≤ 70% pred, FEV1/FVC ≤ 60% and no reversibility to β2-agonists and oral corticosteroids. The patients were followed for 2–4 years with biannual spirometry and annual CT and comprehensive lung function tests (LFT). CT images were analysed using Pulmo-CMS software. LD was derived from a pixel-density histogram of the whole lung as the 15thpercentile density (PD15) and the relative area of emphysema at a threshold of −910 Hounsfield units (RA-910), and both were volume-adjusted to predicted total lung capacity. At baseline, mean age was 64 years and 64 years; mean number of pack-years was 56 and 56; mean FEV1 was 1.53 L (51% pred) and 1.53 L (53% pred); mean PD15 was 103 g/L and 104 g/L; and mean RA-910 was 14% and 13%, respectively, for the budesonide and placebo groups. The annual fall in PD15 was −1.12 g/L in the budesonide group and −1.81 g/L in the placebo group (p = 0.09); the annual increase in RA–910 was 0.4% in the budesonide group and 1.1% in the placebo group (p = 0.02). There was no difference in annual decline in FEV1 between ICS (−54 mL) and placebo (−56 mL) (p = 0.89). Long-term budesonide inhalation shows a non-significant trend towards reducing the progression of emphysema as determined by the CT-derived 15th percentile lung density from annual CT scans in current smokers with moderate to severe COPD.
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is defined as a “preventable and treatable disease state characterised by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and is associated with an abnormal inflammatory response of the lungs to noxious particles or gases, primarily caused by cigarette smoking”(Citation[1]). It has been shown that airway inflammation in smokers results in alveolar destruction and emphysema (Citation[2]). To date, smoking cessation is the only known intervention that reduces the rate of decline of lung function, as assessed by the forced expiratory volume in 1 second (FEV1). The role of inhaled corticosteroids (ICS) in the management of COPD is debatable. Four major longitudinal trials have examined the effect of ICS on the progression of airway obstruction without finding a statistically significant reduction in the rate of decline of FEV1 (3–6). Nevertheless, there is some evidence that ICS have anti-inflammatory effect in COPD (7–9), although the evidence is not consistent (Citation[10]). The anti-inflammatory effects of ICS may be the mechanism behind the reduction in exacerbation rate and improvement in quality of life observed in patients with moderate to severe COPD and frequent exacerbations (Citation[3], Citation[11]), particularly when combined with a long-acting β 2-agonist (Citation[12], Citation[13], Citation[14], Citation[15]).
Emphysema is a major constituent of lung pathology in COPD and is the major determinant of clinically recognised severe airflow obstruction, as it is relatively uncommon to find severe airway obstruction with little or no emphysema (Citation[16]). Emphysema is characterised by progressive loss of lung tissue, which is replaced by air, resulting in a decrease in the physical density of the lung. This decrease can be assessed by repeated measurements of lung density by computed tomography (CT), i.e., lung densitometry (Citation[17]). CT is the imaging method of choice to assess the extent of emphysema in life and studies have shown that objective quantitation by CT has good correlation with the pathological extent of emphysema (18–20).
Findings from a randomised controlled trial of the protective effect of α1-antitrypsin replacement therapy in patients with deficiency suggested that lung densitometry derived from annual CT scans is more sensitive than FEV1 and the carbon monoxide transfer coefficient (KCO) in monitoring the progression of the disease (Citation[21]). The hypothesis of the current study was that budesonide might slow the progression of emphysema in smokers by its known anti-inflammatory properties. For this purpose, we used lung density measurements derived from annual CT scans, in addition to traditional measures of lung function, to assess the effect of ICS on the progression of emphysema in current smokers with moderate to severe COPD.
MATERIAL AND METHODS
Study population
Patients aged 50–80 years were eligible if they were current smokers with a clinical diagnosis of COPD for not less than 2 years. All patients should have a significant smoking history of at least 10 cigarettes per day during the last 6 months and a previous history of at least 20 pack-years. Ex-smokers were excluded. Baseline lung function criteria were: FEV1 between 35% and 70% of predicted (pre-bronchodilator), and FEV1/forced vital capacity (FEV1/FVC) ≤ 60%. Reversibility of ≥ 12% and 200 mL in FEV1 from baseline values, 15 minutes after inhalation of 1 mg terbutaline or ≥ 15% and 300 mL after 2 weeks on oral prednisolone (25 mg) was an exclusion criterion. Patients were also excluded if they: had any severe concomitant disease; had an exacerbation within 30 days prior to the first visit; received oral steroids for more than 4 weeks within 6 months of the first visit; or were on long-term oxygen therapy. Bronchodilators, mucolytics, and short courses of oral corticosteroids (maximum 3 courses of 4-week duration per year) and antibiotics were allowed during the study. The protocol was approved by the Ethics Committee of Copenhagen Municipality, and patients provided written informed consent.
Study design
This was a randomised, double-blinded, placebo-controlled, parallel-group, single-centre study. Recruitment of patients was carried out between January 1999 and May 2001 from the outpatient respiratory clinic and by advertisement in local newspapers. To avoid inclusion of patients with asthma, patients entered a 2-week run-in period on oral prednisolone (25 mg once daily). Patients with reversibility less than 15% or 300 mL from baseline FEV1 values were then randomly assigned to twice-daily treatment with either 400 μ g of budesonide (Pulmicort Turbuhaler) or placebo. Patients were allocated into either group in a proportion of 1:1 by block randomisation using a random sequence generated by a computer program at AstraZeneca. To maintain blinding all Turbuhalers were of identical appearance. All study medications were provided by AstraZeneca, Sweden. Patients were then followed up for 2–4 years with biannual spirometry and annual comprehensive lung function testing and CT.
The pre-specified primary outcome variable was the change over time in the 15thpercentile density (PD15). Secondary variables were change over time in the relative area of emphysema at a threshold of –910 Hounsfield units (RA-910), FEV1 and diffusion capacity (DLCO) and the number of exacerbations, which was defined as a combination of 2 of the 3 following criteria: increased dyspnea, increased sputum production and change in sputum colour. On the basis of the limited published results concerning longitudinal CT lung density measurements in smokers with COPD(Citation[22]), we estimated that ICS might reduce the annual loss of lung density by 50%.
Assuming 50% drop-out, our sample-size calculations (power of 80%, α of 0.05, two-sided) suggested that a total of 250 patients should be followed by annual CT scans for 3 years. For practical reasons, it proved impossible to include a sufficient number of patients during the first year, and it was decided to prolong the inclusion period and stop the trial, when the lastly included patients had been in the trial for 2 years.
Lung function tests (LFTs)
Lung function testing was performed according to the European Respiratory Society (ERS) recommendations (Citation[23], Citation[24]). Patients visited the respiratory laboratory at the same time of the day whenever possible, and LFTs were performed by trained nurses to minimise measurement errors. Spirometry was done biannually. A pressure compensated flow plethysmograph (SensorMedics Vmax 229, Bilthoven, The Netherlands) was used and calibrated on each day before the study measurements according to the manufacturer's guidelines. Fifteen minutes after bronchodilatation (terbutaline 1 mg), with the patient seated and wearing a nose clip, a slow vital capacity (VC) manoeuvre was obtained, followed by a forced vital capacity (FVC) manoeuvre from which the maximal flow-volume loop and FEV1 were derived.
In addition, static lung volumes (i.e., total lung capacity [TLC] and residual volume [RV]) and the diffusion capacity were measured once a year. TLC and RV were determined using body plethysmography during panting with a frequency of less than 1/sec. Carbon monoxide transfer coefficient (KCO) was measured by the single-breath technique. The diffusion capacity (DLCO) was calculated as the product of KCO and the alveolar volume. The latter was obtained from the dilution of methane (CH4) during the single-breath manoeuvre. At least three technically satisfactory lung function measurements were performed and the largest or mean value was selected depending on the ERS recommendations. Gas volumes are reported with body temperature and pressure saturated (BTPS) corrections, and results are expressed in absolute values and percentage of predicted normal calculated according to European reference equations (Citation[23], Citation[24]).
Computed tomography (CT)
From the beginning of the study in January 1999 to March 2001, CT was performed as volume scans on a Siemens (Somatom Plus 4, Erlangen, Germany) spiral scanner. Patients were scanned at functional residual capacity (FRC). The Scanning parameters were: 10 mm slice thickness at pitch factor 2, 140 kVp and 200 mAs. Images were reconstructed with a high-spatial frequency (hard or sharp) algorithm. From April 2001 onwards, CT was done as a low-dose, multi-slice scan on GE equipment (GE Medical Systems, LightSpeed QX/i, Milwaukee, Wisconsin, USA) with 4 detector rows.
Image acquisition was performed with 5 mm collimation, rotation time 0.8 seconds, with a pitchx = 1.5. The voltage across the X-ray tube was 140 kVp, with tube current of 40 mA. Images were reconstructed using a low-spatial (soft) frequency algorithm and 2.5 mm increment. Both scanners were calibrated weekly for air and at regular intervals with a water phantom. In both scanners, patients were scanned in the supine position in a caudo-cranial direction (z-axis) to avoid breathing artefacts at the level of the diaphragm. Scanning time ranged between 10-30 seconds. No contrast medium was injected. The field of view was 40 cm and the matrix size 512 × 512. Images were stored on a suitable medium in DICOM format (Digital Imaging and COmmunication in Medicine).
Quantitative parameters of CT scans
The analysis software and analysis procedure have been described elsewhere (Citation[25], Citation[26]). In brief, images were analysed by Pulmo-CMS (Medical Imaging Systems, Leiden, The Netherlands), which generates a histogram of the pixel attenuation values of the whole lung. The total lung volume (TLV), 15th percentile density (PD15) and relative area of emphysema below -910 Hounsfield Units (HU) (RA-910) are all parameters derived from the histogram.
PD15 is the cut-off point in the histogram at which 15% of the pixels have lower densities. Based on the conventions of Hounsfield in CT density, where water has a density of 0 HU, the density values can be converted with a fairly close approximation into g/L by adding 1000 (e.g. a 15thpercentile density of -950 HU corresponds to 50 g/L, i.e., 15% of the pixels have a density below 50 g/L). RA-910 is the volume corresponding to pixels with densities < -910 HU, divided by the total lung volume. PD15 and RA-910 are commonly applied measures to assess the severity of emphysema from CT images (Citation[21], Citation[27]), and change over time in these 2 CT parameters was the primary outcome variable of the current study. Changes in lung volume have substantial influence on lung density, which more than doubles from full inspiration to full expiration; therefore, lung density parameters (PD15 and RA-910) were adjusted for the change in inspiration level between scans by statistical modelling (Citation[26]).
Statistical analysis
The product of PD15 and TLV for a given patient was fairly constant and independent of the level of inspiration, and therefore we decided to adjust PD15 to predicted TLC by multiplying PD15 by TLV and dividing by TLC (Citation[26]). Then a possible treatment effect was analysed in a mixed-effects regression model with the volume-adjusted PD15 as outcome variable and a treatment-dependent slope as explanatory variable, type of scanner as covariate and patient level and slope as random factors.
For RA-910 the relationship with TLV was fairly linear, and RA-910 was analysed in a mixed-effects regression model with RA-910 as outcome variable and a treatment-dependent slope as explanatory variable, TLV and type of scanner as covariates and patient level and slope as random factors. Similar models (without TLV) were used for FEV1 and DLCO. To assess the influence of the baseline level of emphysema on annual change in each treatment group and the difference between the groups, a similar model was used including the baseline value of PD15 and RA-910 in the respective models. Data from patients, who had stopped smoking during the trial were censored at the date of smoking cessation, and a two-sided p < 0.05 was considered statistically significant.
RESULTS
Patient characteristics
Patient characteristics at baseline are shown in . A total of 278 individuals were enrolled, of whom 254 patients fulfilled the inclusion and exclusion criteria and were randomised. Of the 254 patients randomised, 127 received budesonide and 127 placebo (). All patients were of Caucasian origin. The mean age of the participants was 64 years (range 50–80 years) at entry. There were 79 men and 48 women in the budesonide group and 69 men and 58 women in the placebo group. During the study period, 55 patients (43%) withdrew from the budesonide group and 62 (49%) from the placebo group mainly, because of deterioration in COPD, other adverse events or treatment with inhaled or systemic steroids (). The difference in the withdrawal rate was not statistically significant. In the budesonide group, 26 patients (21%) withdrew because of adverse events, compared to 27 patients (21%) in the placebo group. Patients in both treatment groups were heavy smokers with a mean smoking history of 56 pack-years. The tobacco consumption was similarly large in both groups during the study period (). The treatment groups were well matched for age, sex, smoking history, history of COPD, baseline lung function and baseline CT lung density ().
Table 1 Patient demographics at baseline in the budesonide and placebo groups
Table 2 Median numbers of packs smoked between visits in the budesonide and the placebo groups
All participants were advised to stop smoking and group counselling in smoking cessation was offered. This effort resulted in smoking cessation by 36 patients (14%), 18 in the budesonide group and 18 in the placebo group. There were 10 deaths during the study period, 5 in each treatment group. Incidentally, the annual scans revealed a neoplastic pulmonary nodule in 12 patients (5%). These findings have been reported elsewhere (Citation[28]).
Changes in CT lung density
The relative numbers of scans performed on the two scanners (i.e., Siemens and GE) were similar in the two treatment groups, that is 135 and 228 for the budesonide group and 113 and 200 for the placebo group (p = 0.76). The number of patients, who underwent 2, 3, 4 and 5 scans is shown in and . The annual decline (time trend) in CT lung density, corresponding to the rate of progression of emphysema, was highly significant in the placebo group. The annual decline in PD15 in the placebo group was −1.81 g/L (95% CI −2.55 to −1.14, p < 0.0001) and in the budesonide group −1.12 g/L, and the difference was 0.69 g/L (95% CI −0.11 to 1.49 g/L, p = 0.09) ().
Figure 2 Annual decline in PD15 (A) and annual increase in RA-910 (B) as average of the treatment groups. The continuous line represents the budesonide group and the stippled line the placebo group. The bars represent the standard error of the mean. The figures below the graph refer to the number of patients who underwent 2, 3, 4 and 5 CT scans in the budesonide and the placebo groups, respectively.
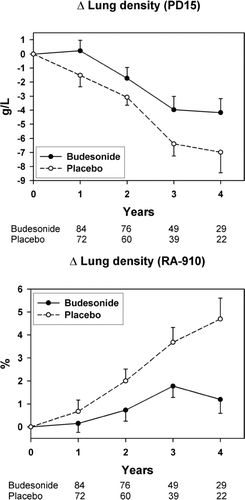
The annual increase in RA-910 in the placebo group was 1.1% (95% CI 0.6 to 1.7, p < 0.0001) and in the budesonide group 0.4%, and the difference was −0.74% (95% CI −1.4 to −0.1%, p = 0.02) (). The slope of change of PD15 of the whole population over time was independent of the baseline PD15 value and there was no significant difference between the two treatment groups. For RA-910, the annual change was slower in patients with mild disease (p = 0.01), and this was common for the two treatment arms (p = 0.32).
Changes in FEV1 and DLCO
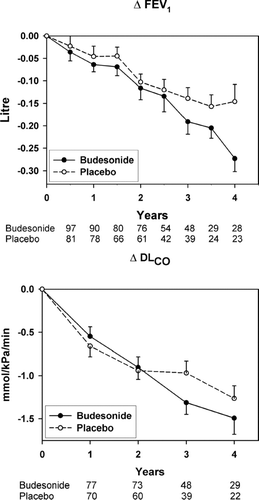
There was no improvement in mean post-bronchodilator FEV1 during the run-in period on oral prednisolone. The annual rate of decline in post-bronchodilator FEV1 was −54 mL (95% CI −40 to −69 mL) in the budesonide group and −56 mL (95% CI −40 to −72 mL) in the placebo group. The difference in time trend between budesonide and placebo was not statistically significant (p = 0.89) (). Similarly, there was no statistically significant difference (p = 0.30) between the annual decline in DLCO in the budesonide group −0.38 mmol/kPa/min (95% CI −0.31 to −0.46) and in the placebo group −0.32 mmol/kPa/min (95% CI −0.24 to −0.41) (). The annual change in CT density parameters and LFT parameters in both groups with the corresponding p-values are summarised in .
Table 3 Annual change in CT lung density parameters and lung function parameters in budesonide and placebo groups
Figure 3 Biannual decline in FEV1 (A) and annual decline in DLCO (B) as average of the treatment groups. The continuous line represents the budesonide group and the stippled line the placebo group. The bars represent the standard error of the mean. The figures below graph A refer to the number of patients who underwent 2, 3, 4, 5, 6, 7, 8 and 9 spirometries in the budesonide and the placebo groups, respectively. The figures below graph B refer to the number of patients who underwent 2, 3, 4 and 5 measurements of diffusion capacity in the budesonide and the placebo groups, respectively.
Exacerbations
The total number of patients, who experienced exacerbations throughout the study in the budesonide group was 69 (54%) and in the placebo group 62 (49%) (p = 0.45). Patients on budesonide had a total of 265 exacerbations during the study, whereas patients on placebo had 198 exacerbations, corresponding to 0.8 and 0.7 exacerbations/patient/year respectively. The difference was not statistically significant (p = 0.15).
DISCUSSION
Smoking cessation is the principal line of management in COPD, because it is the only available intervention that decelerates the progression of the disease. Nevertheless, only a minority of patients are capable of quitting smoking regardless the type of intervention used (Citation[29]). This fact is also evident from our study, in which only 14% of participants quit smoking, despite direct counselling and smoking cessation courses with the use of nicotine replacement therapy. Therefore, primarily asthma medications, including ICS, have been used in COPD. Indeed, ICS are widely prescribed for COPD because of their anti-inflammatory properties by inference from their indisputable effectiveness in asthma.
The nature of inflammation in COPD is quite complex involving macrophages, neutrophils, CD8+ T-lymphocytes and mast cells (Citation[30]). In addition, there is smooth muscle proliferation and goblet cell hyperplasia (Citation[31]). Recently, it has been shown that inflammation increases in intensity with increasing severity of airway obstruction (Citation[32]). In other words, the intensity of inflammation is maintained even when a substantial amount of lung tissue is damaged by emphysema. The variable effects of ICS on airway inflammation may reflect the heterogeneity of the disease and may explain some of the positive effects on exacerbations and health-related quality of life (Citation[3], Citation[11], Citation[14], Citation[15]), and the increased exacerbation rates associated with withdrawal of ICS(Citation[33], Citation[34]). ICS in combination with long-acting β2-agonists have been shown to improve lung function, reduce the frequency of exacerbations and improve health status in patients with severe COPD (FEV1 < 50% predicted)(Citation[1]).
The natural history of emphysema in smokers is not fully understood, because current knowledge is mainly based on cross-sectional pathological studies or longitudinal physiological studies. CT lung density offers an opportunity to monitor emphysema non-invasively, since longitudinal pathological validation is unfeasible, and quantitative CT certainly adds to our understanding of various aspects of the pathophysiology of emphysema. As yet longitudinal data on CT lung density in smoker's emphysema are still scarce. Several CT density parameters are used to assess emphysema, particularly PD15 and RA-910 (also called emphysema index) (17–22,25–27,35).
We have earlier shown that PD15 is superior to RA-910 in monitoring longitudinal changes in emphysema due to two reasons: (Citation[1]) PD15 can detect changes in lung density in patients with mild disease, and (Citation[2]) the method of volume adjustment for PD15 is more robust than that for RA-910 (Citation[36], Citation[26]). For these reasons, PD15 was the pre-specified primary endpoint in the statistical analysis plan of this study. Therefore, the statistically significant difference in RA-910, which was one of the secondary endpoints, between the budesonide and the placebo arms should be taken with a caveat. This significant difference would at best, support the non-significant trend observed with PD15. the p-value of this trend (0.09) either suggests that budesonide does not influence the fall in lung density in smokers with COPD, or represent a type 2 error indicating that the study was underpowered.
The latter might be the case as both PD15 and RA-910 suggest a slower fall in lung density in patients on budesonide than those on placebo. Our data provide no explanation for this difference. Smoking cessation reduces the intensity of inflammation in COPD with positive impact on symptoms and lung function (Citation[37]). This influence was eliminated in the current study by censoring of data from patients, who stopped smoking from the reported date of smoking cessation. The observed non-significant trend cannot be explained by differences in smoking habits, because patients in both treatment groups were heavy smokers with a mean smoking history of 56 pack-years at entry and were exposed to the same burden of smoking throughout the study ().
Budesonide did not influence the accelerated decline in lung function, probably due to the relative insensitivity and variability of LFT in emphysema (Citation[38]). In the current study, the annual decline in FEV1 was similar to the reported decline in other studies (Citation[3], Citation[5], Citation[6]), and neither a clinically nor statistically significant difference was observed between the two groups. Also the rate of decline in the diffusion capacity (DLCO) was accelerated in both groups of patients compared to healthy non-smokers (Citation[24]).
The difference in the rate of the decline between the treatment groups was not statistically significant (p = 0.30). It is tempting in this regard to consider CT lung density a more direct measure of disease severity than measures of lung function. The difference in PD15 between the budesonide and the placebo group was 0.7 g/L/yr (95% CI −0.1 to 1.5). This difference can be approximately calculated to an average loss of 5 g lung tissue (or 0.7%) per year. When the lack of effective pharmacotherapy of emphysema and the chronicity of the disease is taken into consideration, a difference of this magnitude in the rate of decline in lung density must be considered clinically important. Nevertheless, further studies using CT densitometry as the endpoint of interventional studies is needed before a recommendation can be made in this regard.
This study has a number of methodological limitations, most importantly the change of the scanner midway in the study. The change of CT scanner became necessary during the course of the study due to ethical considerations. During the study, new knowledge about the optimal scanning parameters for lung densitometry led us to modify the tube current in order to considerably reduce the radiation dose, a major ethical issue, when repeated scans are required. Besides the reconstruction algorithm was changed from high spatial resolution (hard) to low spatial resolution (soft). Soft algorithm is recommended in lung densitometry studies because of better contrast resolution (Citation[39]).
It is well known that change of scanner and/or reconstruction algorithm has a significant influence on the estimation of the degree of emphysema (Citation[39], Citation[40]); therefore, we included the type of scanner and thus the scanner setting as an explanatory variable in the regression model. This factor added undoubtedly to the level of uncertainty (i.e., the variance) of the results and could be the cause of the lack of statistical significance for PD15. The change of scanner can be considered a limitation of the current study and should be avoided as much as possible during the course of a study; nevertheless, it is an expression of a real-life situation imposed by the rapid progression of CT technology and the continuous search of radiological departments for updating their aging scanners.
Information about smoking cessation was solely based on the patients' reports and was not confirmed by a blood or urine cotinine test. The definition of exacerbation in the protocol was based solely on symptoms and not related to treatment as the most common definition is today. No patient-reported outcomes were collected. The study was initiated before the results of the ISOLDE study were published. These results drew attention to the importance of those “soft” outcome variables in monitoring the effect of treatment on COPD (Citation[3]). This must be an important aspect of any future longitudinal CT study in emphysema. Other targets for future research are the longitudinal regional changes in CT lung density, the influence of smoking cessation on CT lung density and the therapeutic effect of combined ICS and long-acting β2-agonist in emphysema.
In conclusion, long-term budesonide inhalation shows a non-significant trend towards reducing the progression of emphysema as determined by the CT-derived 15thpercentile lung density from annual CT scans in current smokers with moderate to severe COPD.
The study was funded by AstraZeneca, Denmark. The authors would like to acknowledge the financial support of The Alpha-1 Foundation, Miami, USA. The Pulmo-CMS software is supported by the European Union (grant nr. RNDV.07773), and SBS, AD and BS are members of the SPREAD group.
REFERENCES
- Celli B R, MacNee W. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004; 23(6)932–946
- Saetta M, Kim W D, Izquierdo J L, Ghezzo H, Cosio M G. Extent of centrilobular and panacinar emphysema in smokers' lungs: pathological and mechanical implications. Eur Respir J 1994; 7(4)664–671
- Burge P S, Calverley P M, Jones P W, Spencer S, Anderson J A, Maslen T K. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ 2000; 320(7245)1297–1303
- Pauwels R A, Lofdahl C G, Laitinen L A, Schouten J P, Postma D S, Pride N B, et al. Long-term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue smoking. European Respiratory Society Study on Chronic Obstructive Pulmonary Disease. N Engl J Med 1999; 340(25)1948–1953
- The Lung Health Study Research Group. Effect of inhaled triamcinolone on the decline in pulmonary function in chronic obstructive pulmonary disease. N Engl J Med 2000; 343(26)1902–1909
- Vestbo J, Sorensen T, Lange P, Brix A, Torre P, Viskum K. Long-term effect of inhaled budesonide in mild and moderate chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 1999; 353(9167)1819–1823
- Confalonieri M, Mainardi E, Della P R, Bernorio S, Gandola L, Beghe B, et al. Inhaled corticosteroids reduce neutrophilic bronchial inflammation in patients with chronic obstructive pulmonary disease. Thorax 1998; 53(7)583–585
- Gizycki M J, Hattotuwa K L, Barnes N, Jeffery P K. Effects of fluticasone propionate on inflammatory cells in COPD: an ultrastructural examination of endobronchial biopsy tissue. Thorax 2002; 57(9)799–803
- Llewellyn-Jones C G, Harris T A, Stockley R A. Effect of fluticasone propionate on sputum of patients with chronic bronchitis and emphysema. Am J Respir Crit Care Med 1996; 153(2)616–621
- Keatings V M, Jatakanon A, Worsdell Y M, Barnes P J. Effects of inhaled and oral glucocorticoids on inflammatory indices in asthma and COPD. Am J Respir Crit Care Med 1997; 155(2)542–548
- Paggiaro P L, Dahle R, Bakran I, Frith L, Hollingworth K, Efthimiou J. Multicentre randomised placebo-controlled trial of inhaled fluticasone propionate in patients with chronic obstructive pulmonary disease. International COPD Study Group. Lancet 1998; 351(9105)773–780
- Spencer S, Calverley P M, Sherwood B P, Jones P W. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001; 163(1)122–128
- Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2003; 361(9356)449–456
- Calverley P M, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J 2003; 22(6)912–919
- Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J 2003; 21(1)74–81
- Thurlbeck W M. Chronic Airflow Obstruction in Lung Disease, 1st ed. W. B. Saunders Company. 1976
- Flenley D C. Diagnosis and follow-up of emphysema. Eur Respir J Suppl 1990; 9: 5s–8s
- Gould G A, MacNee W, McLean A, Warren P M, Redpath A, Best J J, et al. CT measurements of lung density in life can quantitate distal airspace enlargement–an essential defining feature of human emphysema. Am Rev Respir Dis 1988; 137(2)380–392
- Muller N L, Staples C A, Miller R R, Abboud R T. “Density mask”. An objective method to quantitate emphysema using computed tomography. Chest 1988; 94(4)782–787
- Gevenois P A, De Maertelaer V, De Vuyst P, Zanen J, Yernault J C. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1995; 152(2)653–657
- Dirksen A, Dijkman J H, Madsen F, Stoel B, Hutchison D C, Ulrik C S, et al. A randomized clinical trial of alpha(1)-antitrypsin augmentation therapy. Am J Respir Crit Care Med 1999; 160: 1468–1472, (5 Pt 1)
- Soejima K, Yamaguchi K, Kohda E, Takeshita K, Ito Y, Mastubara H, et al. Longitudinal follow-up study of smoking-induced lung density changes by high-resolution computed tomography. Am J Respir Crit Care Med 2000; 161: 1264–1273, (4 Pt 1)
- Quanjer P H, Tammeling G J, Cotes J E, Pedersen O F, Peslin R, Yernault J C. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16: 5–40
- Cotes J E, Chinn D J, Quanjer P H, Roca J, Yernault J C. Standardization of the measurement of transfer factor (diffusing capacity). Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl 1993; 16: 41–52
- Stoel B C, Stolk J. Optimization and standardization of lung densitometry in the assessment of pulmonary emphysema. Invest Radiol 2004; 39(11)681–688
- Shaker S B, Dirksen A, Laursen L C, Skovgaard L T, Holstein-Rathlou N H. Volume adjustment of lung density by computed tomography scans in patients with emphysema. Acta Radiol 2004; 45(4)417–423
- Stolk J, Dirksen A, van der Lugt A A, Hutsebaut J, Mathieu J, de Ree J, et al. Repeatability of lung density measurements with low-dose computed tomography in subjects with alpha-1-antitrypsin deficiency-associated emphysema. Invest Radiol 2001; 36(11)648–651
- Fischer B M, Mortensen J, Dirksen A, Eigtved A, Hojgaard L. Positron emission tomography of incidentally detected small pulmonary nodules. Nucl Med Commun 2004; 25(1)3–9
- Wagena E J, van der Meer R M, Ostelo R J, Jacobs J E, van Schayck C P. The efficacy of smoking cessation strategies in people with chronic obstructive pulmonary disease: results from a systematic review. Respir Med 2004; 98(9)805–815
- Jeffery P K. Structural and inflammatory changes in COPD: a comparison with asthma. Thorax 1998; 53(2)129–136
- Saetta M, Turato G, Baraldo S, Zanin A, Braccioni F, Mapp C E, et al. Goblet cell hyperplasia and epithelial inflammation in peripheral airways of smokers with both symptoms of chronic bronchitis and chronic airflow limitation. Am J Respir Crit Care Med 2000; 161: 1016–1021, (3 Pt 1)
- Hogg J C, Chu F, Utokaparch S, Woods R, Elliott W M, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(26)2645–2653
- Jarad N A, Wedzicha J A, Burge P S, Calverley P M. An observational study of inhaled corticosteroid withdrawal in stable chronic obstructive pulmonary disease. ISOLDE Study Group. Respir Med 1999; 93(3)161–166
- van d er, Valk J, Monninkhof E, van der P J, Zielhuis G, van Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease: the COPE study. Am J Respir Crit Care Med 2002; 166(10)1358–1363
- Park K J, Bergin C J, Clausen J L. Quantitation of emphysema with three-dimensional CT densitometry: comparison with two-dimensional analysis, visual emphysema scores, and pulmonary function test results. Radiology 1999; 211(2)541–547
- Dirksen A, Friis M, Olesen K P, Skovgaard L T, Sorensen K. Progress of emphysema in severe alpha 1-antitrypsin deficiency as assessed by annual CT. Acta Radiol 1997; 38: 826–832
- Willemse B WM, Postma D S, Timens W, ten Hacken N H. The impact of smoking cessation on respiratory symptoms, lung function, airway hyperresponsiveness and inflammation. Eur Respir J 2004; 23: 464–476
- Gurney J W, Jones K K, Robbins R A, Gossman G L, Nelson K J, Daughton D, et al. Regional distribution of emphysema: correlation of high-resolution CT with pulmonary function tests in unselected smokers. Radiology 1992; 183(2)457–463
- Kemerink G J, Kruize H H, Lamers R J, van Engelshoven J M. Density resolution in quantitative computed tomography of foam and lung. Med Phys 1996; 23(10)1697–1708
- Shaker S B, Dirksen A, Laursen L C, Maltbaek N, Christensen L, Sander U, et al. Short-term reproducibility of computed tomography-based lung density measurements in alpha-1 antitrypsin deficiency and smokers with emphysema. Acta Radiol 2004; 45(4)424–430