ABSTRACT
Chronic obstructive pulmonary disease (COPD) is a prevalent condition in adults aged ≥40 years characterized by progressive airflow limitation associated with chronic inflammatory response to noxious particles in the airways and lungs. Smoking, genetics, air pollution, nutrition and other factors may influence COPD development. Most hospitalizations and deaths for COPD are caused by its acute exacerbations, which greatly affect the health and quality of life of COPD patients and pose a high burden on health services. The aims of this project were to identify trends, geographic patterns and risk factors for COPD exacerbations, as revealed by hospitalizations and deaths, in the Basque Country, Spain, over a period of 12 years (2000–2011). Hospitalization and mortality rates for COPD were 262 and 18 per 100,000 population, respectively, with clusters around the biggest cities. Hospital mortality was 7.4%. Most hospitalized patients were male (77.4%) and accounted for 72.1% of hospital mortality. Hospitalizations decreased during the study period, except for 50–64 year-old women, peaking significantly. Using a multivariate modeling approach it was shown that hospitalizations were positively correlated with increased atmospheric concentrations of NO2, CO, PM10, and SO2, and increased influenza incidence, but were negatively associated with increased temperatures and atmospheric O3 concentration. COPD exacerbations decreased in the Basque Country during 2000–2011, but not among 50–64-year-old women, reflecting the high smoking prevalence among Spanish women during the 1970–1990s. The main metropolitan areas were those with the highest risk for COPD exacerbations, calling attention to the role of heavy car traffic. Influenza virus, cold temperatures, and increased atmospheric NO2, CO, PM10, and SO2 (but decreased O3) concentrations were identified as potential contributors to the burden of COPD exacerbations in the community. These findings are important for both the understanding of the disease process and in providing potential targets for COPD-reducing initiatives and new avenues for research.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is characterized by persistent airflow limitation that is usually progressive and associated with a chronic inflammatory response to noxious particles or gases in the airways and lungs Citation(1). Although smoking is the main risk factor, genetic factors (e.g. a1-antitrypsin deficiency), air pollution, occupational exposure to airborne pollutants, early-life environmental factors, socioeconomic status, and nutrition may also influence the development of COPD (Citation1–2).
In Europe, the prevalence of COPD is 5–10% among adults aged ≥40 years Citation(2) and 10% in Spain according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) criteria Citation(3). In 2011, the standardized rates of hospitalization and death for COPD were respectively 200 and 18 per 100,000 population in Europe, and 257 and 18 in Spain Citation(2).
Most hospitalizations and deaths for COPD are caused by its acute exacerbations, which are defined as the sustained worsening of the patient's condition from the stable state and beyond normal day-to-day variation, presenting with an acute onset and needing for a change in medication Citation(4). COPD exacerbations greatly affect the health and quality of life of COPD patients and pose a high burden on health services. Common etiologic factors of COPD exacerbations include infections Citation(5), air pollution (Citation6–5), withdrawal of maintenance treatment with inhaled corticosteroids Citation(7), or sudden changes in ambient temperature Citation(8). The relative importance of these factors, however, may vary over geographic locations Citation(9).
The aim of this study was to identify trends, geographic patterns and risk factors for COPD exacerbations, as revealed by hospitalizations and deaths, in the Basque Country, Spain, over a period of 12 years (2000–2011).
Materials and methods
Study area
The Basque Country is an autonomous community of Northern Spain, situated at the Eastern edge of the Cantabrian Coast. It has an area of 7,234km2. It is divided in three administrative provinces: Gipuzkoa, Bizkaia, and Araba, whose capitals have a population of approximately 186 (Donostia), 352 (Bilbao) and 239 (Gasteiz) thousand inhabitants, respectively. The total population in the Basque Country is about 2.1 million people and has remained quite stable throughout the study period: from 2000 to 2011 Citation(10).
Data collection
COPD hospitalizations were identified by first-listed discharge diagnoses as defined by the International Classification of Diseases, 9th revision (ICD-9), codes 490–492, 494 and 496. Respiratory causes of hospitalization were defined by the ICD-9 codes 460–519. Data regarding COPD hospitalizations for the study period were obtained from the Certificates of Hospital Discharge of the Spanish National Health System Register (CMBD).
COPD mortality was defined according to the ICD-10 codes J40-J44 and J47, and the data were obtained for the study period from the Mortality Register of the Basque Country Government Citation(11). Hospital mortality rates for the study period were determined based on patients who died during their hospitalization for COPD. Also this information was obtained from the CMBD.
Data on atmospheric concentrations of air pollutants were obtained for the study period from the Air Quality Surveillance Network of the Department of Environment of the Government of the Basque Country. We used data from 12 urban monitoring stations in Bilbao, Donostia, and Gasteiz. We used weekly averages for nitrogen dioxide (NO2 in μg/m3), carbon monoxide (CO in mg/m3), sulfur dioxide (SO2 in μg/m3) and particulate matter (PM2.5 and PM10 in μg/m3). In the case of tropospheric ozone, O3 (μg/m3), weekly mean and maximum 8-hour average concentrations were calculated. We only included the data from stations with at least 75% of valid measurements for each week.
Data on temperature (°C) and relative humidity (%) for the study period were obtained from the Spanish Meteorology Agency (AEMET). The weather stations were located in Igeldo (Donostia), Bilbao Airport (Bilbao), and Foronda-Txokiza (Gasteiz). We obtained the minimum, maximum and daily mean temperatures and the relative humidity measured at 00, 07, 13, and 18 hours (Current local time in UTC). We then calculated the mean weekly temperature and relative humidity.
Data on influenza consisted of the weekly incidence rate per 100,000 population of influenza-like illness as derived from the Basque Influenza Surveillance Network for the study period (hereinafter called Influenza). This network manages weekly epidemiological and virological surveillance for the entire Basque Country, with data covering 3% of the population.
Data analysis
Descriptive analysis
We described the age and sex distribution, as well as the trends, of the rates of COPD hospitalization, mortality, and hospital mortality in the Basque Country during the study period. The European Standard Population was used for direct age standardization of the rates. Age- and sex-specific rates were calculated for the age groups <50, 50–64, 65–74, and >74 years. Analyses were performed on a yearly basis and by 4-year periods.
For seasonal comparison, we defined the warm period (spring–summer) as the one spanning from week 15 to week 40, and the cold period (autumn–winter) from week 41 to week 14, of each year.
Trend analysis
A linear regression model was used to analyze temporal trends in COPD hospitalization, mortality and hospital mortality during the study period. The slope and significance of these trends were calculated by age group and sex.
Spatial distribution
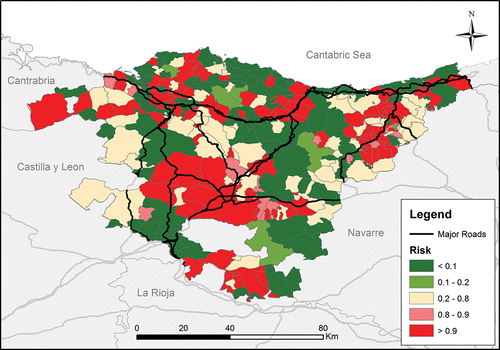
To examine the spatial distribution and potential clustering of COPD hospitalizations (as the hospitalization dataset was the largest and was therefore used for spatial analysis), we calculated the standardized incidence ratio (SIR) for each municipality of the Basque Country. Sex- and age-specific rates were used for SIR estimations. A smoothed SIR map was then created using the hierarchical Bayes method proposed by Besag et al. Citation(12), which effectively reduces the over-variability and over-dispersion in the estimates Citation(13). The posterior distribution of the relative risk was approximated by the INLA algorithm by defining a prior normal distribution of the random terms and a normal distribution for the constant. Model goodness-of-fit was assessed using the Deviance Information Criterion (DIC). Analysis was performed using INLA package in R Citation(14). Municipalities were considered to have a high risk for COPD exacerbations if at least 80% of the posterior mean of the smoothed SIR values were >1 Citation(15).
Multivariate analysis
Associations of exogenous factors, i.e. atmospheric concentrations of NO2, NO, SO2, O3, CO, PM2.5, PM10, temperature (minimum, mean, and maximum values) and relative humidity, with the weekly number of COPD hospitalizations were examined for the three capital cities (Donostia, Bilbao, and Gasteiz) using a time-series Poisson generalized linear model (GLM) with a log link function and a first-order autoregressive term Citation(16) developed using a standardized protocol Citation(17). The corresponding city populations served as offset variable in the model. To account for clustering of observations at the city level, the analysis was stratified by city. Independent variables were first tested univariately and those showing a p < 0.10 for the association with the outcome were included in a multivariate model built in backward stepwise fashion (p < 0.05). The model also included a linear trend and the significant lagged terms of the covariates tested for.
To avoid multicollinearity, Spearman's pairwise correlation coefficients between all the exogenous factors were calculated prior to multivariable analysis. If a high correlation between the factors was detected (), the one contributing the less to the model fit, as revealed by the Akaike Information Criterion (AIC), was excluded. However, because all the pollutants were found to be highly correlated with each other, in order to assess them as independent variables in the multivariate model while avoiding multicollinearity, a principal component analysis (PCA) was used to transform the original five correlated pollutant variables into three uncorrelated principal components (PCs) as performed elsewhere (Citation18–19). Variables were standardized by city prior to PCA. Statistical analysis was performed using STATA 11.
Results
Hospitalization and mortality rates per 100,000 population, and the percentage of hospital mortality, are summarized in . Overall, there was a decreasing temporal trend for all indicators of COPD exacerbations, also when looking at the sex- and age-specific rates. The only exception concerned the group of 50–64-year-old women, who had experienced an increase in hospitalizations and mortality during the study period (). Female cases were always older, on average, than the male ones.
Table 1. Hospitalizations, mortality and hospital mortality.
Table 2. Hospitalization and mortality trends.
Hospitalization
Overall, there were 3,244,066 all-cause hospitalizations during the study period, and among these, the ICD-9 codes 490–492, 494 or 496 were the primary diagnosis for 67,403 hospitalizations. These COPD cases represented 2.1% of all hospitalizations, and 19.9% of all hospitalizations for respiratory causes (ICD-9 codes 460–519). Men accounted for 77.5% of all COPD hospitalizations. The mean age (±SD) was 74.1±11.5, with the highest number of hospitalizations occurring at 76 years of age.
Looking at the age distribution, 3.3% of hospitalizations concerned the <50-year-olds, 12.8% the 50–64-year-olds, 28% the 65–74-year-olds, and 55.9% the >74-year-olds. The standardized mean hospitalization rate for the entire study period was 268.1 per 100,000 habitants: 286.8 for men and 58.7 for women. There were 3.4 times more hospitalizations in men than in women, and 2.8 times more hospitalizations in the >74-year-olds. Hospitalization rates showed a decreasing trend in all age groups except in women aged 50–64 years (from 57.1 to 89.5 per 100,000 population).
Looking at seasonality, the largest numbers of hospitalizations were recorded in the months of January (14.5%), December (10.3%), and February (10.1%), and the lowest in August (4.9%). The mean number of hospitalizations per day (15.3) went from a maximum of 27.1 in January to 10.4 and 9.2 in July and August, respectively.
The spatial distribution of the hospitalization incidence is depicted in . Areas with the highest risk of experiencing hospitalizations due to COPD exacerbations clustered around the cities of Bilbao, Gasteiz, and Goierri region (Gipuzkoa).
Mortality
Overall, 235,406 people died in the Basque Country during the study period, and for 9,541 (4.1%) fatalities COPD was the main cause (ICD-10: J40-44, 47), meaning that COPD accounted for 38.1% of deaths due to respiratory causes (ICD-10: J00-J99). The standardized mean mortality rate of the entire study period was 18.4 per 100,000 population: 37.4 for men and 7.7 for women. Mortality rates showed a decreasing trend in all age groups except in women aged 50–64 years (from 3.1 to 4.7 per 100,000 population ).
Looking at the age distribution, 0.5% of mortalities concerned the <50-year-olds, 4.4% the 50–64-year-olds, 14.5% the 65–74-year-olds, and 80.6% the >74-year-olds. Regarding seasonality, the largest numbers of mortalities were recorded in the months of January (13.8%) and February (10.4%), and the lowest in September (6.2%). The mean number of mortalities per day was 2.6, with a maximum of 12 in the month of January.
Regarding the spatial distribution of COPD mortality, this was clustered around the main cities of Bilbao, Donostia, and Gasteiz.
Hospital mortality
The mean hospital mortality was 7.4% in the whole study period. Hospital mortality decreased mainly in recent years, both in men and women. Looking at the age distribution, hospital mortality was 2.6% in 50–64 year-olds, 5.5% in 65–74-year-olds, and 9.9% in >74-year-olds. The largest number of hospital deaths occurred between December and March (51.2%).
Effects of environmental factors
Weekly and daily mean values of pollutants met the regulations on air quality, established by the Spanish Royal Decree 102/2011 of 28 January, based on limit values for the protection of human health of Directive 2008/50/EC of the European Parliament Citation(20) and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe.
Only PM10 and NO2 exceeded the limit in Bilbao. In Donostia and Gasteiz, only PM10 exceeded the limit, but these limit excesses always lasted less than 35 days/year as established by European regulations (Directive 2008/50/CE) (Supplementary Table 1). shows the correlation coefficients for the mean concentrations of the gaseous pollutants and meteorological indicators. CO was strongly positively correlated with NO2 (r = 0.77) and SO2 (r = 0.75), moderately positively correlated with PM10 (r = 0.56) and PM2,5 (r = 0.53) and inversely correlated with O3 (r = −0.60). NO2 was moderately correlated with PM10 (r = 0.55), PM2,5 (r = 0.55), and SO2 (r = 0.66). O3 was inversely correlated with all other variables. Temperature was also inversely correlated with pollutants except O3. Relative humidity showed low or no correlation with the pollutants. Except for particulate matters and relative humidity, the other environmental factors varied seasonally. CO, NO2, and SO2 concentrations and influenza rates were significantly higher in the cold season, whereas O3 concentration and temperature were higher in the warm season (). Regarding hospitalization, mortality, and hospital mortality in cold season are always higher than in warm season ().
Table 3. Correlation between variables.
Table 4. Comparison between Cold and Warm season.
Seasonal influenza epidemics occurred between November and February. The maximum and minimum standardized incidence rates were <100 cases (2000–01) and 832 cases (2001–02) per 100,000 population. The most prevalent influenza A virus strains were H3N2 and H1N1, depending on the influenza season. The pandemic AH1N1(pdm)09 strain caused a peak in week 43 of the year 2009, corresponding to a incidence rate of 546 per 100,000 population.
The PCA allowed for the reduction of the pollutant variables from 5 to 3, which explain together 91% of the original variance: the relationships between each principal component and the original pollutant variables are given in . The first PC (PC1) is strongly positively correlated with five of the original variables, but negatively associated with O3. This PC can therefore be considered as a proxy for high concentrations of NO2, CO, P10, and SO2, but low O3 concentrations. Conversely, the second PC (PC2) is positively correlated with O3 and can therefore be considered as a proxy of high O3 concentrations.
Table 5. Relationships between each principal component (Comp) and their original pollutants variables: assumed variance in % (AsVar), significant pair-wise correlation coefficient (r) (P < 0.05) and overall proportions of explained variance.
The final multivariable Poisson GLM revealed that the environmental factors significantly associated with the weekly occurrence of COPD hospitalizations were the mean temperature, influenza rate, and PC1 (). Specifically, the weekly number of COPD hospitalizations increased significantly with increasing influenza rates in the same week, and with increasing concentrations of NO2, CO, PM10, and SO2 (but decreasing O3 concentrations) in the previous week. Conversely, the weekly number of COPD hospitalizations decreased with concurrently increasing mean temperature.
Table 6. Output of the multivariable Poisson regression model predicting COPD cases with environmental factors.
The over-dispersion test revealed that the data were not over-dispersed and that a Poisson model was appropriate.
Discussion
This study aimed to describe the epidemiology of COPD exacerbations in the Basque Country during 2000–2011. The standardized COPD hospitalization rates we found were similar to the Spanish average (256) and higher than the European one (200) Citation(2). As an estimated 73% of deaths for COPD in high-income countries are attributable to smoking, the trends in COPD hospitalizations and mortality observed here can be explained by the parallel decrease in smoking in the population Citation(21). However, while COPD hospitalization and mortality decreased in both men and women, an opposite trend was observed in women aged 50–64 years. This is in agreement with previous studies showing that in the last decades, mortality rates have decreased in men, but have increased or remained stable in women (Citation22–24). Moreover, the increasing trend in COPD mortality found in 50–64-year-old women might well be a reflection of the “20-year delay” reported in the literature Citation(25) between the increase in smoking prevalence and the one in mortality, which in Spanish women occurred during the 1970s and 1990s, respectively Citation(26). This follows the various stages of the global tobacco epidemic Citation(27). The decrease in COPD mortality among men and the expected stabilization of that in women might be able to level out the sex-related differences in COPD mortality in the years to come.
The proportion of fatal hospitalizations found here was similar to those reported elsewhere (Citation28–30) and so was the decreasing trend observed during the study period Citation(22). We also found a positive association between COPD hospitalizations and influenza incidence and a negative association with temperature, with the highest number of COPD hospitalizations being observed in the cold months (December–February), reflecting the potentially detrimental effects of high-incidence concomitant respiratory infections and cold temperatures in people with underlying COPD (Citation31–33).
Time-series and case-crossover studies focused on single air pollutants have consistently provided evidence for associations between air pollutants and hospital admissions for COPD (Citation34–36). However, in the real world, the human body is exposed to multiple air pollutants together; thus, air pollution epidemiology has recently begun to shift toward examining multi-pollutant exposures as a way to better understand the combined health effects of several air pollutants at once. Yet, the joint assessment of the health effects of numerous and potentially inter-correlated air pollutants may be statistically unfeasible using conventional multivariable regression modeling without the use of a dimension-reduction technique like PCA, which has proved useful in assessing the association between multiple pollutants and health parameters in previous studies (Citation37–39). Here, the PCA revealed that PC1 (explaining over 62% of the original variance in the data) could be used to represent the effect of the linear combination of 5 pollutants (NO2, CO, O3, PM10, and SO2), with NO2 contributing the most. The positive association between COPD hospitalizations and increasing levels of PC1 in the previous week we found confirms previous findings based on single-pollutant studies regarding NO2, SO2, CO, and PM10 (Citation34,Citation40–41). However, in the specific case of O3, the association is still largely unclear, as it has been reported to be both positively (Citation9,Citation33,Citation42), especially in the warm season Citation(35), and negatively (Citation43–44) associated with COPD.
The effect of air pollutant concentrations on COPD hospitalizations was examined at different lags in time. Lagged (or delayed) effects of air pollutants may be epidemiologically significant both in the long- or short-term, depending mainly on the cause of COPD hospitalization, e.g. immediate in cases of pneumonia and more prolonged in COPD patients with respiratory infections Citation(41). A report of the Monitoring Atmospheric Composition and Climate (MACC) project in the UK concluded that the influence of air quality on COPD hospital admissions is indeed greatest at a lag of ∼1 week Citation(45). Also in our study, the only significant effect on COPD hospitalizations was observed with a 1-week lag and it is believed that the relevance of this period is that it allows for alert of both patients and healthcare providers Citation(45). Yet, it may also be argued that this lag period is the time during which the condition of COPD patients will deteriorate to the point of requiring hospital admission.
A few other studies have used Geographic Information Systems (GIS) to explore the spatial distribution of COPD cases (Citation46–47), as GIS may help identify areas where the population is more prone to experience COPD or its exacerbations. Once a specific cluster is identified, further epidemiological investigations are required to understand the specific risk factors shaping these clusters. In the Basque Country, COPD hospitalizations appeared to cluster mainly around the metropolitan areas of Bilbao, Gasteiz, and Goierri, which are indeed characterized by the presence of highways and heavy car traffic ().
Besides the potential for ecological fallacy typical of ecological studies like the present one, this study has some other limitations related to the identification of COPD episodes via registers, as it relied entirely on the quality of coding of the patients' medical condition on hospital discharge and death certificates. Therefore, the identification of episodes, despite being carried out by trained medical coders, has a number of difficulties. Specifically, variation in the selection of the primary diagnosis, clinical acumen and thoroughness in the recording of the diagnoses, as well as the coding criteria adopted by the different hospitals, might have undermined, to some extent, the quality of the data used. On the other hand, this study covered a large region and period of time, resulting in a long series of observations both in space and time, all being obtained from well-established registers in the Basque Country.
In conclusions, COPD exacerbations leading to hospitalization or death have decreased in the Basque Country during 2000–2011. However, there were some significant sex-related differences, with 50–64-year-old women showing increased COPD hospitalizations and deaths, possibly reflecting the high smoking prevalence among Spanish women between the 1970s and 1990s. Moreover, the main metropolitan areas of the Basque Country were those with the highest risk for COPD exacerbations. The gradual decline in the Basque Country's traditional industrial activities and associated pollutant emissions since the 1970–1980s in favor of the development of the service sector and new tech economy further points to car traffic as the key determinant of COPD exacerbations. Significant positive associations between COPD hospitalizations and increased influenza incidence, cold ambient temperatures, and increased NO2, CO, PM10, and SO2 (but decreased O3) concentrations were also found, further confirming their potential as significant environmental triggers contributing to the burden of COPD exacerbations in the community. It is therefore clear that public health authorities should lead the implementation of measures to minimize the detrimental effects of factors like atmospheric pollution and seasonal influenza on COPD exacerbations, as the control of these factors is largely beyond the possibilities of individual patients and their clinicians. Rather, the control of these factors relies essentially on government-sponsored interventions aimed to reduce car pollution (e.g. limit car use in densely populated areas, potentiate and promote public transportation, incentivize purchase of low-emission vehicles, etc.) and to limit seasonal flu epidemics by further promoting influenza vaccine uptake and other preventive measures (e.g. hand washing, stay home when sick, etc.), as well as continuing running anti-smoking campaigns. Our findings are therefore important for both the understanding of the disease process and in providing evidence-based targets for COPD-reducing initiatives and new avenues for epidemiological research.
Supplementary Table 1
Download MS Word (24.7 KB)References
- Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014; www.goldcopd.org. Accessed 15 January 2015.
- European respiratory Society (ERS). European Lung White Book. http://www.erswhitebook.org/chapters/ Accessed 15 January 2015.
- Soriano, JB, Miratvilles, M, Borderías, L, Duran-Tauleria, E, GArcia Rio, F, Martinez, J, et al. Diferencias geográficas en la prevalencia de EPOC en España: relación con hábito tabáquico, tasas de mortalidad y otros determinantes. Arch Bronconeumol 2010; 46(10):552–530.
- Rodriguez-Roisin, R. Toward a consensus definition for COPD Exacerbations. Chest 2000; 117: 398–401 DOI:10.1378/chest.117.5_suppl_2.398S.
- Wedzicha, JA, Seemungal, TAR. COPD exacerbations: defining their cause and prevention. Lancet 2007; 370:786–796.
- Anderson, HR, Spix, C, Medina, S, Schouten, JP, Castellsague, J, Rossi, G, et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the Aphea project. Eur Respir 1997; 10: 1064–1071.
- Wouters, EFM, Postma, DS, Fokkens, B, Hop, WC, Prins, J, Kuipers, AF, et al. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax 2005; 60:480–487. doi: 10.1136/thx.2004.034280.
- Donaldson, GC, Seemungal, T, Jeffries, DJ, Wedzicha, JA. Effect of temperature on lung function and symptoms in chronic obstructive pulmonary disease. Eur Respir J 1999; 13:844–849.
- Ko, FWS, Tam, W, Wong, TW, Chan, DPS, Tung, AH, Lai, CKW, et al. Temporal relationship between air pollutants and hospital admissions for chronic obstructive pulmonary disease in Hong Kong. Thorax 2007; 62(9):780–785.
- EUSTAT. Basque statistics office. Poblacion estimada de la C.A. de Euskadi 1975–2010. Available at http://www.eustat.es. Accessed 15 January 2015.
- Departamento de Salud. Gobierno Vasco. Mortalidad en la Comunidad Autónoma del País Vasco 2011. http://www.osakidetza.euskadi.eus/contenidos/informacion/estado_salud/es_5463/adjuntos/informe%20mortalidad%202011.pdf Accessed 15 January 2015.
- Besag, J, York, J, Mollié A. Bayesian image restoration, with two applications in spatial statistics. Ann Inst Statist Math 1990; 43:1–59.
- Lawson, AB, Browne, WJ, Vidal-Rodeiro, CL. Disease Mapping with WingBUGS and MLwiN. Hoboken, NJ: John Wilwy & Sons, Ltd. 2003.
- Rue, H, Martino, S, Chopin, N. Approximate Bayesian inference for latent Gaussian models using integrated nested Laplace approximations (with discussion). J R STAT SOC, Series B 2009; 71(2):319–392.
- Richardson, S, Thomson, A, Best, N, Elliott, P. Interpreting posterior relative risk estimates in disease-mapping studies. Environ Health Perspect 2004; 112(9):1016–1025.
- Breiman, L, Friedman, JH. Estimating Optimal Transformations for Multiple Regression and Correlation. J Am Statist Assoc 1985; 80:580–598.
- Katsouyanni, K, Schwartz, J, Spix, C, Touloumi, G, Zmirou, D, Zanobetti, A, et al. Short term effects of air pollution on health: a European approach using epidemiologic time series data: the APHEA protocol. J Epidemiol Community Health 1996; 50(1):S12–S18.
- Riley, A, Banks, L, Fintzi, J, Gould, TH, Hartin, K, Schaal, L, et al. Multi-pollutant mobile platform measurements of air pollutants adjacent to a major roadway. Atmos Environ 2014; 98:492–499.
- Messer, LC, Jagai, JS, Rappazzo, KM, Lobdell, D. Construction of an environmental quality index for public health research. Environ Health 2014; 13:39.
- Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Accessed 15 January 2015.
- Maninno, DM, Buist, AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet 2007; 370:765–773.
- Brown, DW, Croft, JB, Greenlund, KJ. Trends in Hospitalization with Chronic Obstructive Pulmonary Disease—United States, 1990–2005. COPD 2010, 7:59–62.
- Akinbami, LJ, Liu, X. Chronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998–2009. NCHS 2011:63.
- Ricroft, CE, Heyes, A, Lanza, L, Becker, K. Epidemiology of chronic obstructive pulmonary disease: a literature review. Int J Chron Obstruct Pulmon Dis 2012; 7:457–494.
- Adair, T, Hoy, D, Dettrik, Z, Lopez, AD. 100 years of mortality due to chronic obstructive pulmonary disease in Australia: the role of tobacco consumption. Int J Tubercul Lung Dis 2012; 16(12):1699–1705.
- Fernández, E, Schiaffino, A, García, M, Saltó E, Villalbí JR, Borras, JM. Prevalencia del consumo de tabaco en España entre 1945 y 1995. Reconstrucción a partir de las Encuestas Nacionales de Salud. Med Clin (Barc) 2003; 120(1):14–16.
- Lopez, AD, Collishaw, NE, Piha, T. A descriptive model of the cigarette epidemic in developed countries. Tob control 1994; 3:242–247.
- San Roman Terán, CM, Guijarro Merino, R, Gómez Huelgas, R, Montero Ribas, L. Epidemiologia hospitalaria de la EPOC en España. Rev Clin Esp 2007; 207 Supl 1:3–7.
- Connors, AF, Dawson, NV, Thomas, C, Harrell, FEJ, Desbiens, N, Fulkerson, WJ, et al. Outcomes following acute exacerbation of severe chronic obstructive pulmonary disease. Am J Respir Care Med 1996; 154:939–967.
- Groenewegen, KH, Schols, AMWJ, Wouters, EFM. Mortality and Mortality-Related Factors After Hospitalization for Acute Exacerbation of COPD. Chest 2003; 124:459–467.
- Arbex, MA, de Souza Conceição, GM, Cendon, SP, Arbex, FF, Lopes, AC, Moysés, EP, et al. Urban air pollution and chronic obstructive pulmonary disease-related emergency department visits. J Epidemiol Community Health 2009; 63:777–783. doi:10.1136/jech.2008.078360.
- Jenkins, CR, Celli, B, Anderson, JA, Ferguson, GT, Jones, PW, Vestbo, JC, et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J 2012; 39:38–45. doi: 10.1183/09031936.00194610.
- Qiu, H, Yu, ITS, Wang, XR, Tian, LW, Tse, LA, Wong, TW. Season and humidity dependence of the effects of air pollution on COPD hospitalizations in Hong Kong. Atmos Environ 2013; 76:17–80.
- Yang, Q, Chen, Y, Krewski, D, Burnett, RT, Shi, Y, McGrail, KM. Effect of short-term exposure to low levels of gaseous pollutants on chronic obstructive pulmonary disease hospitalisations. Environ Res 2005; 99(1):99–105.
- Medina-Ramón, M, Zanobetti, A, Schwartz, J. The Effect of Ozone and PM10 on Hospital Admissions for Pneumonia and Chronic Obstructive Pulmonary Disease: A National Multicity Study. Am J Epidemiol 2006; 163:579–588.
- Yang, CY, Chen, CJ. Air Pollution and Hospital Admissions for Chronic Obstructive Pulmonary Disease in a Subtropical City: Taipei, Taiwan. J Toxicol Env Health, Part A 2007; 70:1214–1219.
- Pires, JCM, Sousa, SIV, Pereira, MC, Alvim-Ferraz, MCM, Martins, FG. Management of air quality monitoring using principal component and cluster analysis - Part II: CO, NO2 and O-3. Atmos Enrivon 2008; 42(6):1261–1274.
- Taspinar, F. Improving artificial neural network model predictions of daily average PM10 concentrations by applying principle component analysis and implementing seasonal models. J Air Waste Manage 2015; 65(7):800–809.
- Vellingiri, K, Kim, KH, Jeon, JY, Brown, RJC, Jung, MC. Changes in NOx and O-3 concentrations over a decade at a central urban area of Seoul, Korea. Atmos Environ 2015; 112:116–125.
- Santus, P, Russo, A, Madonini, E, Allegra, L, Blasi, F, Centanni, S, et al. How air pollution influences clinical management of respiratory diseases. A case-crossover study in Milan. Resp Res 2012; 13:95.
- Faustini, A, Stafoggia, M, Colais, P, Berti, G, Bisanti, L, Cadum, E, et al. Air pollution and multiple acute respiratory outcomes. Eur Respir J 2013; 42(2):304–313.
- Ghozikali, MG, Mosaferi, M, Safari, GH, Jaafari, J. Effect of exposure to O-3, NO2, and SO2 on chronic obstructive pulmonary disease hospitalizations in Tabriz, Iran. Environ Sci Pollut Res 2015; 22(4):2817–2823.
- Linn, WS, Szlachcic, Y, Gong H Jr, Kinney, PL, Berhane, KT. Air Pollution and Daily Hospital Admissions in Metropolitan Los Angeles. Environ Health Perspect 2000; 108(5):427–434.
- Atkinson, RW, Carey, IM, Kent, AJ, van Staa, TP, Anderson, HR, Cook, DG. Long-term exposure to outdoor air pollution and the incidence of chronic obstructive pulmonary disease in a national English cohort. Occup Environ Med 2015; 72(1):42–48. doi: 10.1136/oemed-2014-102266. Epub 2014 August 20.
- Sarran, C, Agnew, P, Davis, L. The Influence of Air Quality on COPD Hospital Admissions. A report for MACC 2010.
- Wang, WQ, Ying, YY, Wu, QY, Zhang, HP, Ma, DD, Xiao, W. A GIS-based spatial correlation analysis for ambient air pollution and AECOPD hospitalizations in Jinan, China. Resp Med 2015; 109(3):372–378.
- Crighton, EJ, Ragetlie, R, Luo, J, To, T, Gershon, A. A spatial analysis of COPD prevalence, incidence, mortality and health service use in Ontario. Health reports 2015; 26(3):10–18.