ABSTRACT
Mortality is one of the most important outcomes in patients with chronic obstructive pulmonary disease (COPD). Different predictors have been associated with mortality, including the patient's level of physical activity (PA). The objective of this work was to establish the relationship between changes in PA during a moderate-to-severe COPD exacerbation (eCOPD) and 1-year mortality after the index event. This was a prospective observational cohort study with recruitment of 2,484 patients with an eCOPD attending the emergency department (ED) of 16 participating hospitals. Variables recorded included clinical and sociodemographic data from medical records, dyspnea, health-related quality of life, and PA before the index eCOPD and 2 months after the hospital or ED discharge, as reported by the patient. In the multivariate analysis worsening changes in PA from baseline to 2 months after the ED index visit [odds ratio (ORs) from 2.78 to 6.31] was related to 1-year mortality, using the age-adjusted Charlson comorbidity index (OR: 1.22), and previous use of long-term domiciliary oxygen therapy or non-invasive mechanical ventilation at home (OR: 1.68). The same variables were also predictive in the validation sample. Areas under the receiver operating characteristic curve in the derivation and validation sample were 0.79 and 0.78, respectively. In conclusion, PA is the strongest predictor of dying in the following year, i.e., those with worsened PA from baseline to 2 months after an eCOPD or with very low PA levels have a higher risk.
Introduction
Mortality and chronic obstructive pulmonary disease (COPD) exacerbation (eCOPD) are two of the main outcomes in COPD. Different predictors have been associated with mortality depending on whether the endpoint is short or long term Citation(1). Exacerbation is not only an established predictor of mortality in COPD Citation(2) but is also considered an outcome Citation(3) with its predictors Citation(3) and consequences related to suffering exacerbations with different rate and severity, like decrement of pulmonary function Citation(4) and health-related quality of life (HRQoL) (Citation5,6).
Physical activity (PA) is a key issue in patients with COPD. Patients with COPD engage in less PA than healthy controls Citation(7), and PA is diminished in patients with COPD beginning with the mildest disease stages Citation(8). In patients whose clinical status is stable, physical inactivity has been shown to be detrimental to their prognosis (mortality and hospitalizations) (Citation9,10). In other words, PA is an instrument that can improve the quality and quantity of life by ameliorating different physiologic parameters.
An alternative approach to addressing the role of PA in patients with COPD is to evaluate the influence of PA on outcomes from a dynamic perspective, e.g., change in PA over time rather than from a static viewpoint. Studies that have taken this approach have demonstrated that changes in PA are related to changes in HRQoL Citation(11), hospitalizations Citation(12), and mortality Citation(13).
Information regarding the relationship between eCOPD and PA is available but scarce, and several questions remain to be answered. Moreover, more focus should be placed on evaluating how the change in PA during an eCOPD is related to several clinical outcomes. Thus, this study sought to determine the impact of mortality of failing on the ability to recover baseline PA levels after an eCOPD.
Methods
This was a prospective cohort study that included 16 hospitals belonging to the Spanish National Health Service. Patients with an eCOPD attending the EDs of the participant hospitals were informed of the goals of the study and invited to voluntarily participate and sign an informed consent. All information was kept confidential. The Institutional Review Boards of the participating hospitals and Galdakao-Usansolo Hospital Ethics Committee approved this project. Recruitment started in June 2008 and ended in September 2010. A description of the study protocol was published previously Citation(14).
Patients were eligible for the study if they presented to one of the participating EDs with symptoms consistent of an eCOPD. COPD was confirmed if the patient had a forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) quotient <70%. Exacerbation was defined as an event in the natural course of the disease characterized by a change in the patient's baseline dyspnea, cough, and/or sputum that was beyond normal day-to-day variations and may have warranted a change in regular medication Citation(15). For patients whose COPD was newly diagnosed in the ED to be included in the study, the diagnosis had to be confirmed by spirometry within 60 days of the index episode at a time when the patient was stable Citation(16). Patients were excluded from the study if, at the time they were observed in the ED, they had an eCOPD complicated by a comorbidity such as pneumonia, pneumothorax, pulmonary embolism, lung cancer, or left cardiac insufficiency. Other exclusion criteria included a diagnosis of asthma, extensive bronchiectasis, sequelae of tuberculosis, pleural thickening, or restrictive diseases. After patients were informed of the study protocol, those who did not wish to participate were also excluded.
Data collection
Data collected upon arrival in the ED included socioeconomic data, information about respiratory function (arterial blood gases, respiratory rate, and dyspnea), consciousness level measured by the Glasgow Coma scale (altered if <15) Citation(17), and presence of other pathologies recorded in the Charlson Comorbidity Index Citation(18). Additional data collected in the ED at the time a decision was made to admit or discharge the patient included the patient's symptoms, signs, and respiratory status at that moment.
For patients with known COPD, additional variables collected from medical records included baseline severity of COPD as measured by FEV1; hospital admissions during the previous 12 months for eCOPD; baseline therapy; and the presence of comorbidities such as diabetes, hypertension, ischemic heart disease and/or valve disease, cor pulmonale, hepatic disease, peptic ulcer disease, psychiatric disorders, rheumatic disease, history of stroke or deep-vein thrombosis, and other conditions needed to determine the Charlson Comorbidity Index. To estimate the age-adjusted Charlson comorbidity index for each decade after 40 years, one point is added up to 4 points (1 point for age group 41–50, 2 points for age group 51–60, 3 points for 61–70, and 4 points for 71 or older).
Patient-reported outcomes
Patients were asked questions about their PA, their general health, and their level of dyspnea before the index eCOPD and 2 months after the ED discharge. Patients discharged from the ED to home were also asked questions related to these variables (their PA, their general health, and their level of dyspnea), with reference to the index eCOPD before and 2 months after the index ED visit.
With regard to the PA level, we focused on walking. Participants were given six response options about their daily activity (walking) asking them to report their PA level just before the current exacerbation in a stable clinical condition: A.1: Could not leave the house; A.2: bedridden or in a chair; B: Could not leave the house, but could walk at home; C: Left the house, but could not walk more than 100 m; D: Could run errands and walk a few hundred meters, but not walking regularly, or work in the garden; and E: Walked regularly or could play sports. This PA scale was based on the health, activity, dyspnea, obstruction (HADO) score Citation(19) that has already been validated and used in previous studies. This scale showed a good correlation with the St. George's Respiratory Questionnaire activity sub-scale and with the EQ-5D Citation(20). For the purpose of our analysis, categories A1 and A2 were placed in the same category.
General health status was measured using the European Quality of Life-5-Dimensions (EuroQol-5D) questionnaire (Citation21,22). To measure the level of dyspnea at baseline and after 2 months, we used the Medical Research Council (MRC) breathlessness scale, which uses a 5-category scale that ranges from none (Grade 1) to almost complete incapacity (Grade 5) Citation(23). For the purpose of this study, the following three dyspnea categories were used: grade 1 and 2 (A), 3 (B), and 4 and 5 (C).
A manual was developed to guide data collection, and reviewers were trained to use it.
Definition of outcome measure
The main outcome variable was death at 1 year after the hospital admission or within 2 months after discharge to home from the ED and within 1 year of the index ED visit. Vital status was established by reviewing medical records and examining the hospital database and public death registries. Deaths were considered confirmed if the name, sex, and date of birth on the record matched those of the participant.
The secondary outcome was changes in the level of PA from baseline to 2 months after the ED visit.
Statistical analysis
The unit of analysis was a patient with eCOPD excluding those who died before 2 months in the participant's hospitals. The total sample was randomly divided in half into a derivation sample and a validation sample.
Descriptive analyses for both samples included the frequency and percentages for categorical variables and the mean and standard deviations for continuous variables. Chi square and Fisher's exact tests were used to test for statistical significance among proportions. For continuous variables, the Wilcoxon U-test was performed.
To identify risk factors associated with mortality at 1 year in patients with an exacerbation of COPD, we performed univariate analyses in the derivation sample using a univariate logistic regression. Variables that were statistically significant at the 0.20 level were entered in a multivariate logistic regression model.
We performed logistic regression models in the derivation sample to separately select the variables for prediction of death. Final predictive factors in the multivariate analysis were those with a significance level <0.05. Beta estimates, odds ratios (OR), and 95% confidence intervals (95% CI) were provided for the multivariate analysis. Final models were also adjusted by the variable hospital to see if the model changed. The predictive accuracy of the model was determined by calculating the area under the receiver operating characteristic curve (AUC). We validated the model in the validation sample by comparing AUC of both samples.
Although the final model considered predictors such as general health, level of dyspnea, or PA at baseline previous to the ED and after 2 months, we also built in the derivation sample an additional multivariate logistic regression model to just identify clinical predictors that were validated in the validation sample. Finally, to identify predictors of changes in PA from baseline to 2 months after the ED visit, general linear models were used.
All effects were considered significant at p < 0.05. All statistical analyses were performed using SAS for Windows statistical software, version 9.4 (SAS Institute, Inc., Carey, NC).
Results
From a total of 3,276 episodes of eCOPD, 198 (6%) were excluded because COPD was complicated by other major pathologies at the time of ED admission [cardiovascular conditions, 59 (29.8%); pneumonia, 55 (27.8%); cancer, 21 (10.6%); other respiratory problems, 13 (6.6%); or other conditions, 50 (25.2%)]. Fifty-six episodes of new-onset COPD were excluded when the COPD diagnosis was not confirmed by spirometry within 60 days of the index episode. Another 145 episodes were lost due to incomplete data without the possibility of retrieving the information needed for the study, and 390 episodes were excluded because the patient had more than one ED visit. Thus, the final baseline population included 2,487 patients. Of these, 1,537 (61.8%) were admitted to the hospital and 950 (38.2%) were discharged to home. Of those admitted, 192 patients were excluded because they died within 3 months from the ED index visit. Thus, a total of 2,295 patients were available at 1 year for the analyses (). Of them, 1,893 (82.5%) had full data on PA at follow-up. A comparison between responders and non-responders at 2 months showed that there were no differences in main clinical and sociodemographic variables among both groups except for the hospitalization rate up to 2 months of the ED index visit and the EuroQol score (Table E2) being worse those non-responders.
Figure 1. Flowchart of patients' recruitment and mortality during 1-year follow-up. eCOPD, chronic obstructive pulmonary disease exacerbation.
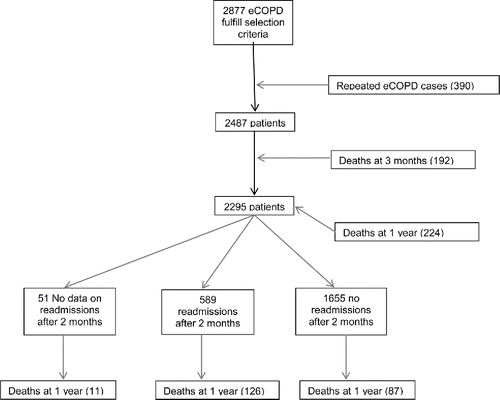
The total sample was randomly divided into a derivation and a validation cohort. No differences were found between both samples (). Variables that were related to mortality at 1 year in the univariate analysis are displayed in
Table 1. Descriptive statistics stratified by sample, derivation versus validation.
Table 2. Univariate analysis of predictors of 1-year mortality.
We attempted to determine which of the changes from baseline to 2 months in any of three parameters reported by the patient (general health, level of dyspnea, or PA) had more influence on mortality at 1 year. Of the three parameters, PA changes from baseline to 2 months were the best predictors of 1-year mortality. shows the relationship with mortality of the changes in PA from the ED arrival to 2 months after the ED index visit. Patients with lower levels of PA or who moved to the lowest levels at 2 months had the highest mortality rates. Conversely, patients at a higher level of PA or especially those who moved to the higher levels at 2 months had the lowest mortality rates at 1 year after the ED index visit. The same information is displayed by means of Kaplan-Meier curves (Figure E1).
Table 3. Mortality rates in 1 year on relation to physical activity at baseline and physical activity after 2 months.
The variables related to mortality at 1 year at p < 0.20 are presented in . Predictors of 1-year mortality in the multivariate analysis are displayed in . The only clinical predictors were age-adjusted Charlson comorbidity index and previous use of Long-Term Domiciliary Oxygen Therapy or Non-Invasive Mechanical Ventilation (LT-DOT or NIMV) at home. Importantly, changes in PA from baseline to 2 months were also related to 1-year mortality. More specifically, those patients who, at 2 months after the ED index visit and after adjusting by their baseline PA level and other significant variables described previously, “Could not leave the house or were bedridden or in a chair” had, in the derivation sample, an OR of 6.31 of dying at 1 year compared with those who “walked regularly or could play sports.” Participants who “could not leave the house, but could walk at home” had an OR of 6 and those who “left the house, but could not walk more than 100 m” had an OR of 2.78, all statistically significant. ORs in the validation sample for the same categories were 8.27, 4.24, and 3.13, respectively. AUC of this model in the derivation sample was 0.79 and 0.78 in the validation sample.
Table 4. Predictive factors of mortality in 1 year. Multivariate Analysis.
If we simply take into account the clinical parameters in the analysis, excluding patient-reported parameters, only the age-adjusted Charlson comorbidity index and previous use of LT-DOT or NIMV at home remain predictive of 1-year mortality and readmissions within the next 2 months after the ED index visit (). AUC for this model in the derivation and validation sample was 0.71.
Table 5. Clinical predictive factors of mortality in 1 year. Multivariate Analysis.
Significant predictors of changes in PA from baseline to 2 months after the ED visit were a lower basal FEV1%, previous use of LT-DOT or NIMV at home, the worse MRC basal dyspnea status prior to the exacerbation, and worse score in the age-adjusted Charlson Comorbidity Index (model 1). As an alternative to the last two variables, a worse general health status at baseline, as measured by the EuroQol-5D (model 2) and all adjusted by PA level at baseline, also predicted changes in PA (Table E2). Conversely, patients who had a readmission from the ED index visit within 2 months had a worsening in their PA level at 2 months (β estimate: −0.63; p-value: <0.0001).
Discussion
After a moderate-to-severe eCOPD, the change in the level of PA (baseline to 2 months after the ED attendance) had a meaningful impact in mortality at the 1-year follow-up. This impact was even greater than that of having a short-term readmission or a change in HRQoL within the same period of time after the index event.
The correlation between PA and mortality has been previously established. In a population study during a 20-year follow-up, Garcia-Aymerich and coworkers used self-reported PA levels to demonstrate that walking or biking less than 2 hours/week was associated with higher rates of all-cause and respiratory mortality Citation(10). Similarly, using accelerometers, García-Río and collaborators established a linear relationship between PA and mortality Citation(24). Moreover, Waschki and coworkers concluded that PA was the strongest predictor of mortality ahead of pulmonary function, body composition, exercise capacity, and HRQoL Citation(25).
The relationship between PA and mortality was further refined in a population study by Vaes et al. who showed that mortality was increased in patients with COPD who declined in their level of PA or changed to a low PA level from a baseline level that was moderately high Citation(13).
All these studies were carried out in stable patients. Our study adds to the literature by evaluating the association between a change in PA and 1-year mortality. In other words, we investigated how failure to recover the baseline level of PA after a moderate-to-severe eCOPD requiring evaluation at the ED or a hospital admission influences mortality in the following year.
On the other hand, eCOPD has been shown to impact on several outcomes but has also been linked to a decrease in the level of PA Citation(26). Our knowledge is very limited regarding the level of PA in patients with COPD during an admission resulting from an eCOPD Citation(26). One study reported that among patients evaluated in an ED for an eCOPD, the level of PA during the admission or 7 days after discharge from the ED in those patients who were not admitted was quite variable and increased after the discharge from the hospital or ED Citation(20).
The factors that influenced changes in the level of PA were related to the eCOPD itself (dyspnea at rest upon arrival to the ED, length of hospital stay, and use of anticholinergic or systemic corticosteroids to treat the eCOPD) and factors reflecting the patient's general clinical condition (age, HRQoL, living alone, and baseline FEV1%) Citation(20).
What these studies all confirmed is that the level of PA deteriorates during a moderate-to-severe eCOPD; however, little is known about the recovery period from the baseline level of PA after one eCOPD-related hospital admission or ED visit.
The median number of days that patients with COPD stayed at home during an exacerbation was 2.7 days/week in the post-eCOPD period (from day 1 to 35 after the beginning of the exacerbation), whereas during the baseline period, the median number of days was 2.1 days/week Citation(27). Therefore, it seems that after one COPD exacerbation, the PA recovery may be rapid. However, this article is focused on exacerbation in general with only 6.2% of the eCOPD resulting in hospitalization.
It seems that PA might increase during the 6 days following the discharge after one hospitalization and might change minimally or not at all for the following 6 weeks (Citation28,29). So again, there would be a relative quick recovery of PA at the beginning of the event; after that improvement, it would slow down. However, 1 month after one admission due to an exacerbation, patients with severe COPD had levels of PA lower than stable patients with COPD of similar characteristics Citation(26).
In our study, 2 months after the index episode, a significant number of patients (31%) had not reached their PA baseline level. It is even possible that patients with COPD with more frequent moderate-to-severe exacerbations were never able to recover their initial PA level. Little is known about the profile of patients or the type of eCOPD that may be prone to a slower recovery or an inability to recover.
Nevertheless, we demonstrated a relationship between a low level of recovery of the baseline PA level and a higher mortality rate during the following year.
From the point of view of mortality and hospitalizations for eCOPD, several factors have been noted as predictors of mortality in the long term after one hospitalization (age, FEV1, diabetes, ischemic heart disease, low body mass index, long-term oxygen therapy, and PaCO2 on admission) Citation(1). Our study adds one more predictor: failure to recover the baseline level of PA 2 months after the index event.
Several factors have been associated with the loss of PA during eCOPD, such as immobility, effects of the exacerbation itself, hypoxemia, increased CO2, and systemic inflammation. Furthermore, systemic corticosteroids used during the acute treatment lead to muscle weakness and further diminish the already reduced level of PA in patients with COPD.
Therefore, implementation strategies to bolster a quick recovery of the baseline PA level appear to be crucial. As has been pointed out in a recent official statement of the European Respiratory Society, several strategies, from behavioral changes to pharmacological therapy, could be potentially useful to improve PA but we need strategies focused in not losing PA too Citation(30). For example, pulmonary rehabilitation would likely be an effective therapy. In fact, early pulmonary rehabilitation has been included as a potential recommendation in the pulmonary rehabilitation guidelines Citation(31), based on studies that have reported a reduction in the readmission rate Citation(32).
However, some studies identified certain limitations in pulmonary rehabilitation as an intervention designed to provide a quick recovery after eCOPD, such as a low rate of uptake Citation(33) and a low, 9%, rate of compliance with the program Citation(34). In another study, early pulmonary rehabilitation intervention did not reduce the rate of readmission or improve the physical functioning recovery compared with a control group (Citation35,36).
The strengths of our study include the large size of the cohort from general outpatient clinics and the 1-year follow-up. We conducted statistical adjustments in an effort to prevent confounding factors from biasing the results.
Limitations of the study must also be noted. We used a questionnaire to establish the level of PA rather than using a direct measure such as an accelerometer or pedometer. Self-reports by study subjects may overestimate or underestimate PA.
Moreover, since baseline PA was reported retrospectively, a recall bias cannot be excluded. Directly assessing exercise capacity would have provided additional relevant data; however, we were not able to complete this type of assessment. We focused on walking as a measure of PA and did not include other activities. Further, as in any prospective study, 17.5% of patients lacked PA information at 2 months after the event. This could have biased the results because those patients were slightly more severely affected at baseline and in the follow-up. Although our patient population almost entirely comprised men (97%), it reflects the current distribution of COPD in Spain and does not imply any sampling bias.
In conclusion, we demonstrated that some patients failed to recover their baseline level of PA 2 months after an eCOPD. Furthermore, this lack of recovery is associated with an increased risk of any-cause mortality in the following year. Therefore, in patients with COPD following an eCOPD, interventions to revert PA levels to their previous baseline are mandatory.
Table_E2_1_.docx
Download MS Word (16.7 KB)Table_E2_1_.docx
Download MS Word (19 KB)Table_E2_1_.docx
Download MS Word (48.8 KB)Acknowledgment
The authors are grateful for the support of the 16 participating hospitals, as well as the ED physicians, other clinicians, and staff members of the various clinical, research, quality, and medical records units of these hospitals. They also gratefully acknowledge the patients who participated in the study.
Declaration of interest statement
We disclose any financial, consulting, and personal relationships with other people or organizations that could influence (bias) the author's work.
Funding
This work was supported in part by grants from the Fondo de Investigación Sanitaria (PI 06/1010, PI06/1017, PI06/714, PI06/0326, PI06/0664); Department of Health of the Basque Government (2012111008), Department of Education, Language Policy and Culture of the Basque Government (IT620-13); and the thematic networks - REDISSEC (Red de Investigación en Servicios de Salud en Enfermedades Crónicas) - of the Instituto de Salud Carlos III.
The IRYSS-COPD group included the following co-investigators: Dr. Jesús Martínez-Tapias (Hospital Virgen de las Nieves, Granada); Alba Ruiz (Hospital de Motril, Granada); Dr. Eduardo Briones (Unidad de Epidemiología. Distrito Sanitario Sevilla); Dr. Silvia Vidal (Unidad de Investigación, Hospital Costa del Sol, Marbella); Dr. Emilio Perea-Milla, Francisco Rivas (Servicio de Epidemiología, Hospital Costa del Sol, Málaga – REDISSEC); Dr. Maximino Redondo (Servicio de Laboratorio, Hospital Costa del Sol, Málaga-REDISSEC); Javier Rodríguez Ruiz (Responsable de Enfermería del Área de Urgencias, Hospital Costa del Sol, Málaga); Dra. Marisa Baré (Epidemiología y Evaluación, Corporació Sanitaria Parc Taulí-CSPT, Sabadell REDISSEC), Dr. Manel Lujan, Dra. Concepción Montón (Servicio de Neumología, CSPT/REDISSEC); Dra. Amalia Moreno, Dra. Josune Ormaza, Dr. Javier Pomares (Servicio de Neumología, CSPT); Dr. Juli Font (Medicina, Servicio de Urgencias; CSPT), Dra. Cristina Estirado, Dr. Joaquín Gea (Servicio de Neumología, Hospital del Mar, Barcelona); Dra. Elena Andradas (subdirectora de Promoción de la Salud y Epidemiología del Ministerio de Sanidad, Servicios Sociales e Igualdad); Dr. Juan Antonio Blasco (Unidad de Evaluación de Tecnologías Sanitarias, Agencia Laín Entralgo, Madrid); Dra. Nerea Fernández de Larrea (Subdirección General de Tecnología e Innovación Sanitarias. Consejería de Sanidad de la Comunidad de Madrid/REDISSEC); María del Puerto Cano Aguirre (Hospital de Torrejón, Madrid); Dra. Esther Pulido (Servicio de Urgencias, Hospital Galdakao-Usansolo, Bizkaia); Dr. Jose Luis Lobo (Servicio de Neumología, Hospital Txagorritxu, Araba); Dr. Mikel Sánchez (Servicio de Urgencias, Hospital Galdakao-Usansolo); Dr. Luis Alberto Ruiz (Servicio de Respiratorio, Hospital de Cruces, Bizkaia); Dra. Ane Miren Gastaminza (Hospital San Eloy, Bizkaia); Dr. Ramon Agüero (Servicio de Neumología, Hospital Marques de Valdecilla, Santander); Dr. Eva Tabernero and Carmen M. Haro (Hospital de Santa Marina); Dr. Gabriel Gutiérrez (Servicio de Urgencias, Hospital Cruces, Bizkaia); Dra. Belén Elizalde (Dirección Territorial de Gipuzkoa); Dr. Felipe Aizpuru (Unidad de Investigación, Hospital Txagorritxu, Álava/REDISSEC); Dra. Inmaculada Arostegui, Irantzu Barrio (Departamento de Matemática Aplicada, Estadística e Investigación Operativa, UPV/EHU-REDISSEC; Amaia Bilbao (Hospital Universitario Basurto/REDISSEC); Dr. Cristóbal Esteban (Servicio de Neumología, Hospital Galdakao-Usansolo, Bizkaia/REDISSEC); Dra. Nerea González, Susana Garcia, Iratxe Lafuente, Urko Aguirre; and Miren Orive, Edurne Arteta, and Dr. Jose M. Quintana (Unidad de Investigación, Hospital Galdakao-Usansolo, Bizkaia/REDISSEC).
References
- Singanayagam A, Schembri S, Chalmers JD. Predictors of mortality in hospitalized adults with acute exacerbation of chronic obstructive pulmonary disease. Ann Am Thoracic Soc 2013; 10:81–89.
- Soler-Cataluna JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60:925–931.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363:1128–1138.
- Donaldson GC, Seemungal TAR, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57:847–852.
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1998; 157:1418–1422.
- Esteban C, Quintana JM, Moraza J, Aburto M, Egurrola M, España PP, et al. Impact of hospitalisations for exacerbations of COPD on health-related quality of life. Respir Med 2009; 103:1201–1208.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171:972–977.
- Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J 2009; 33:262–272.
- Garcia-Aymerich J, Farrero E, Félez MA, Izquierdo J, Marrades RM, Antó JM. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 2003; 58:100–105.
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax 2006; 61:772–778.
- Esteban C, Quintana JM, Aburto M, Moraza J, Egurrola M, Perez-Izquierdo J, et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J 2010; 36:292–300.
- Esteban C, Arostegui I, Aburto M, Moraza J, Quintana JM, Aizpiri S, et al. Influence of changes in physical activity on frequency of hospitalization in chronic obstructive pulmonary disease. Respirology 2014; 19:330–338.
- Vaes AW, Garcia-Aymerich J, Marott JL, Benet M, Groenen MT, Schnohr P, et al. Changes in physical activity and all-cause mortality in COPD. Eur Respir J 2014; 44:1199–1209.
- Quintana JM, Esteban C, Barrio I, Garcia-Gutierrez S, Gonzalez N, Arostegui I, et al. The IRYSS-COPD appropriateness study: objectives, methodology, and description of the prospective cohort. BMC Health Serv Res 2011; 11:322.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007; 176:532–555.
- Gold PM. The 2007 GOLD Guidelines: a comprehensive care framework. Respir Care 2009; 54:1040–1049.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974; 2:81–84.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383.
- Esteban C, Quintana JM, Aburto M, Moraza J, Capelastegui A. A simple score for assessing stable chronic obstructive pulmonary disease. QJM 2006; 99:751–759.
- Esteban C, Quintana JM, Garcia S, Anton A, Gonzalez, Bare M, et al. Determinants of change in physical activity during moderate-to-severe COPD exacerbation. Int J Chron Obstruct Pulmon Dis 2016; 11:251–261.
- Brooks R. EuroQol: the current state of play. Health Policy 1996; 37:53–72.
- Badia X, Roset M, Montserrat S, Herdman M, Segura A. The Spanish version of EuroQol: a description and its applications. European Quality of Life scale. Med Clin (Barc ) 1999; 112(Suppl 1):79–85.
- Fletcher CM, Elmes PC, Fairbairn AS, Wood CH. The significance of respiratory symptoms and the diagnosis of chronic bronchitis in a working population. Br Med J 1959; 2:257–266.
- Garcia-Rio F, Rojo B, Casitas R, Lores V, Madero R, Romero D, et al. Prognostic value of the objective measurement of daily physical activity in patients with COPD. Chest 2012; 142:338–346.
- Waschki B, Kirsten A, Holz O, Müller KC, Meyer T, Watz H, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest 2011; 140:331–342.
- Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest 2006; 129:536–544.
- Donaldson GC, Wilkinson TM, Hurst JR, Perera WR, Wedzicha JA. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 171:446–452.
- Tsai LL, Alison JA, McKenzie DK, McKeough ZJ. Physical activity levels improve following discharge in people admitted to hospital with an acute exacerbation of chronic obstructive pulmonary disease. Chron Respir Dis 2016; 13:23–32.
- Hornikx M, Demeyer H, Camillo CA, Janssens W, Troosters T. The effects of a physical activity counseling program after an exacerbation in patients with Chronic Obstructive Pulmonary Disease: a randomized controlled pilot study. BMC Pulm Med 2015 Nov 4; 15:136.
- Watz H, Pitta F, Rochester CL, Garcia-Aymerich J, ZuWallack R, Troosters T, et al. An official European Respiratory Society statement on physical activity in COPD. Eur Respir J 2014; 44:1521–1537.
- Spruit MA, Singh SJ, Garvey C, ZuWallack Richard, Nici Linda, Carolyn Rochester, et al. on behalf of the ATS/ERS Task Force on Pulmonary Rehabilitation. An Official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188(8):e13–e64.
- Seymour JM, Moore L, Jolley CJ, Ward K, Creasey J, Steier JS, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax 2010; 65:423–428.
- Jones SE, Green SA, Clark AL, Dickson MJ, Nolan AM, Moloney C, et al. Pulmonary rehabilitation following hospitalisation for acute exacerbation of COPD: referrals, uptake and adherence. Thorax 2014; 69:181–182.
- Harrison SL, Robertson N, Graham CD, Williams J, Steiner MC, Morgan MD, et al. Can we identify patients with different illness schema following an acute exacerbation of COPD: a cluster analysis. Respir Med 2014; 108:319–328.
- Greening NJ, Williams JEA, Hussain SF, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ 2014; 349:g4315.
- Ko FW, Dai DL, Ngai J, Tung A, Ng S, Lai K, et al. Effect of early pulmonary rehabilitation on health care utilization and health status in patients hospitalized with acute exacerbations of COPD. Respirology 2011; 16:617–624.
