ABSTRACT
Obstructive sleep apnea (OSA) and chronic obstructive pulmonary disease (COPD) coexist in 0.5–1% of the general population. Both OSA and COPD are associated with increased sympathetic nervous activity, and patients affected by both disorders have higher risk for increased morbidity and mortality as compared with patients with COPD or OSA alone.
We tested the hypothesis that patients with COPD and OSA (Overlap syndrome) have higher sympathetic and lower parasympathetic modulation of heart rate variability (HRV) in comparison with patients suffering from COPD or OSA alone.
HRV indices in the frequency domain were evaluated from daytime electrocardiographic recordings in 14 patients with both severe OSA (apnea–hypopnea index ≥ 30) and mild-to-moderate COPD and compared with those with OSA (n = 24) or COPD (n = 16) alone.
We found that, in the Overlap syndrome group, high-frequency (HF, 0.4–0.15 Hz) power was significantly lower (0.18 nu vs 0.34 nu in OSA and 0.44 nu in COPD patients, p < 0.01) and low-frequency (LF, 0.15–0.05 Hz) power was significantly greater (0.82 nu vs 0.66 nu in OSA and 0.57 nu in COPD patients, p < 0.01) compared with COPD and OSA groups. Patients with both OSA and COPD had higher LF/HF ratio as compared with patients in OSA and COPD groups (4.5 [5.9] vs 1.9 [2.6] and 1.3 [1.3], respectively, p < 0.01). For the Overlap syndrome group, there was a significant direct relationship between LF/HF ratio and residual volume (r2 = 0.62, p = 0.007).
These findings show that patients with both OSA and COPD have higher sympathetic modulation of heart rate compared with those with OSA or COPD alone. Furthermore, the findings provide a potential mechanism for the increased morbidity and mortality reported in patients suffering from both disorders, suggesting new therapeutic perspectives in Overlap syndrome.
Introduction
The prevalence of obstructive sleep apnea (OSA; defined as apnea–hypopnea index, AHI ≥ 5) is 22% in men and 17% in women (Citation1), while chronic obstructive pulmonary disease (COPD) has a prevalence of about 9%, according to a recent meta-analysis (Citation2). Together, the two disorders coexist in 0.5–1% of the general population (Citation3), and patients affected by Overlap syndrome have higher risk for increased morbidity and all-cause mortality compared with patients with COPD or OSA alone (Citation4).
There is accumulating evidence that both OSA and COPD have increased daytime sympathetic nervous activity. Compared with healthy subjects, COPD patients show increased heart rate at rest (Citation5), reduced heart rate variability (HRV) (Citation6), and higher levels of both blood norepinephrine and muscle sympathetic nerve activity (MSNA) ((Citation5, 7). In COPD, sustained hypoxemia, by increasing peripheral chemoreceptor activity, justifies augmented sympathetic autonomic discharges (Citation5, 8). In addition, oxidative stress, by stimulating metabo-receptors Citation9), and lung hyperinflation (Citation10–12), throughout decreasing baroreflex sensitivity, may contribute to increased sympathetic autonomic activity in COPD patients.
Neuro-humoral activation exposes COPD patients to increased morbidity and all-cause mortality, often related to cardiovascular diseases (Citation13). Thus, it has been proposed that β-blockers might have beneficial effects on survival in COPD patients (Citation14).
OSA patients exhibit a marked hyperactivity of sympathetic nervous system during wakefulness and sleep compared with subjects without sleep apnea (Citation15). This is due to (i) intermittent hypoxemia, leading to repeated stimulation of peripheral chemoreflex (Citation16), (ii) suspension of sympathetic outflow tonic inhibition by pulmonary stretch receptor activation ((Citation17, 18), and (iii) arousals from sleep, which often occur at the end of apnea, inducing an additional surge in sympathetic traffic Citation17). Similarly to COPD, sympathetic hyperactivity is related to increased morbidity and mortality due to cardiovascular events in OSA (Citation19).
HRV analysis is a valuable tool to investigate the sympathetic and parasympathetic components of the cardiac autonomic nervous system. It can be assessed in the time domain which evaluates time intervals between heartbeats or in the frequency domain (Citation20), from sequential R–R interval series, describing the magnitude of HRV as a function of frequency. The low-frequency (LF, 0.05–0.15 Hz) power is considered as an expression of both sympathetic and parasympathetic modulation (Citation21), while the high-frequency (HF, 0.15–0.5 Hz) power assesses the parasympathetic activity; the ratio of low frequency to high frequency (LF/HF) is an index of cardiac sympathovagal balance (Citation20).
The purpose of this study is to compare the HRV indices in a group of patients with COPD, a group of patients with OSA, and a group of patients suffering from Overlap syndrome. We hypothesize that patients with OSA+COPD would have higher HRV indices of sympathetic activity (LF and LF/HF) compared with patients with OSA or COPD alone, as a result of the summation of independent inputs stimulating sympathetic activity in subjects affected by both diseases.
Methods
Subjects
This investigation was performed at the Respiratory Medicine Department of Mellini Hospital, Chiari, Italy. Subjects suspected of having OSA were referred to our laboratories and were evaluated from June 2013 to June 2014 with all-night cardiorespiratory polygraphy (Somno Screen, SomnoMedics, Randersacker, Germany) at baseline. For all patients, the following data were recorded: age, sex, body mass index (BMI), neck and waist circumference, Epworth sleepiness scale, AHI, and average and minimum oxyhemoglobin saturation (SaO2%). Respiratory events were classified according to the American Academy of Sleep Medicine (AASM) criteria (Citation22). Arterial oxygen saturation was continuously measured by a finger-tip infrared pulsoximetry, and one electrocardiogram (ECG) derivation was also monitored. All subjects with OSA underwent a spirometry as part of routine clinical assessments. Patients with AHI >30 were included in the study and were divided on the basis of the spirometry results into two groups: OSA group and OSA+COPD group. COPD was defined, according to the American Thoracic Society criteria, by forced expiratory volume in 1st second/forced vital capacity (FEV1/FVC) ratio <70% after administration of 400 μg of nebulized albuterol (salbutamol) by metered-dose inhaler plus spacer (Citation23). COPD severity was classified following the Global Initiative on Obstructive Lung Disease criteria (Citation24) and subjects with mild-to-moderate COPD were recruited. Among them, a third group of COPD patients without OSA, excluded by cardiorespiratory polygraphy showing AHI < 5, was also selected.
All routine control examinations were performed within 3 weeks from ECG recordings. Only outpatients and COPD patients who were in stable conditions, free from acute exacerbations for at least 3 months before the study, were studied.
This study represents one aspect of a larger protocol that was approved by the Ethics Committee of Spedali Civili of Brescia, Italy (Citation25). Informed written consent was obtained from all subjects.
ECG recording
Subjects were studied supine in a temperature-controlled laboratory, 2–4 hours after food intake. Heart rate was derived from lead II recorded with a cardiorespiratory polygraphy (Somno Screen, Somnomedics, Randersacker, Germany). After 10 minutes of quiet rest, baseline signals were acquired during 10 minutes of spontaneous breathing.
Data analysis
The ECG signal was sampled at 512 Hz, digitized, and stored using customized software with a Labview software platform (National Instruments, Austin, TX) for subsequent analysis. Pulse (RR) interval series from each 10-minute ECG recording were analyzed in both time and frequency domains. Spectral analysis of HRV was quantified using fast Fourier transform analysis. Missing beats (<5% of total) were edited and replaced by linear interpolation. Spectra were calculated as the ensemble average of 256-beat sequences taken from a time series of 400–500 beats. The integrated power in the LF (0.05–0.15 Hz) and HF (0.15–0.50) ranges was calculated. The LF/HF ratio was calculated as estimates of the relative strength of cardiac sympathetic versus vagal heart rate modulation.
Statistics
For the primary analysis, data were analyzed by Kruskal–Wallis test for multiple group comparison. Secondary post hoc analysis was performed using Bonferroni–Dunn's test. Statistical significance was accepted if p < 0.05. Categorical variables were compared using chi-squared test. To assess the relative strength of factors that might influence the LF/HF ratio, a stepwise backward multiple regression analysis was performed with LF/HF ratio as the dependent variable and age, BMI, AHI, mean and minimum nocturnal SaO2, FEV1, residual volume (RV), KCO (Carbon Monoxide transfer coefficient), partial pressure of carbon dioxide (PaCO2), partial pressure of oxygen (PaO2), and medications as independent variables. Data are expressed as median [interquartile range]. Statistical analyses were performed using SPSS 19.00 (IBM, Armonk, NY) and GraphPad Prism 6.0 (McKiev Software, Boston, MA).
Results
Subjects
Fifty-four male subjects were progressively enrolled in the protocol: 16 had COPD, 24 had OSA, and 14 had OSA+COPD. Anthropometric data are shown in . COPD patients were older as compared with OSA patients and had lower BMI compared with OSA+COPD patients. Co-morbidities and medication distribution are summarized in . History of smoking and use of inhaled anticholinergics, β2-agonists, and corticosteroids were more frequent among the COPD and OSA+COPD group as compared with the OSA group. Other co-morbidities known to enhance sympathetic activity such as systemic hypertension, diabetes, ischemic cardiomyopathy were equally distributed among the groups.
Table 1. Anthropometric data, smoking habits, and sleepiness questionnaire score.
Table 2. Co-morbidities and medications of the patients analyzed.
Nocturnal respiratory polygraphy, spirometry, arterial blood gases
Results from cardiorespiratory polygraphy, spirometry, and arterial blood gas analysis are shown in . Patients with OSA and OSA+COPD had higher AHI, oxygen desaturation index (ODI), % analysis time with SaO2 < 90% (T90%), and lower mean and nadir nocturnal SaO2 than patients with COPD. On the other hand, COPD and OSA+COPD groups had lower FEV1/FVC and FEV1 than the OSA group. COPD group also had higher total lung capacity (TLC) as compared with the OSA+COPD group and lower KCO as compared with the OSA group. Arterial blood gases during wakefulness were not different among groups.
Table 3. Functional data from polygraphy, spirometry, and arterial blood gases.
Heart rate variability
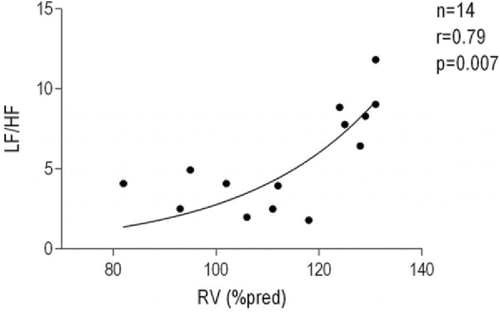
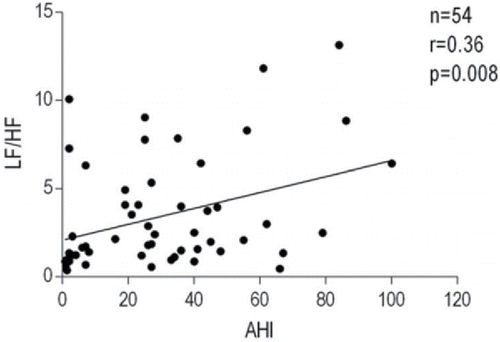
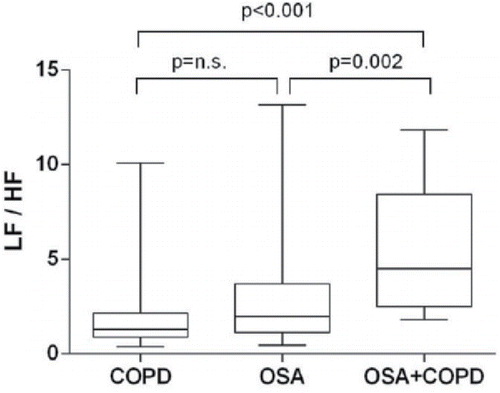
Heart rate and HRV indices during wakefulness are shown in . OSA+COPD group had an insignificant trend for increased heart rate at rest as compared with the OSA and COPD groups. There was no difference in total HRV power among the three groups. HF power was reduced in the OSA+COPD group as compared with the COPD group when expressed in msec2. When expressed in normalized units (HFnu and LFnu, the ratio between HF or LF over total power), HFnu was lower in the OSA+COPD group than in the OSA and COPD groups. On the other hand, LFnu was greater in the COPD+OSA group compared with either the COPD or OSA groups. Patients with OSA+COPD had significantly higher LF/HF ratio as compared with the OSA and COPD groups (). For OSA+COPD group, the univariate analysis revealed a significant direct relationship between LF/HF ratio and RV (r2 = 0.47, p = 0.007), and lowest nocturnal SaO2 (r2 = 0.30, p = 0.044); moreover, an inverse relationship between LF/HF ratio and awake PaCO2 (r2 = −0.32, p = 0.034) was found. The relationship between LF/HF ratio and RV (as % predicted) was best described by an exponential curve (r2 = 0.62, p = 0.007; ). Age, BMI, AHI, FEV1, TLC, mean nocturnal SaO2, PaO2, pH, HCO3−, and medications were not related to LF/HF ratio. The multivariate analysis showed only RV and PaCO2 as significant independent predictors of LF/HF ratio, which together accounted for 73% of its variance (r2 = 0.73, p = 0.001). In all patients, only AHI was related to LF/HF ratio (r2 = 0.13, p = 0.008) in both univariate and multivariate analyses (). No correlations were found between LF/HF ratio and other variables in the COPD group.
Table 4. Heart rate variability indices in time and frequency domain.
Figure 1. Patients with OSA and COPD have a higher index of sympathovagal balance (LF/HF ratio) of heart rate variability as compared with patients with a single disease. COPD, chronic obstructive pulmonary disease; OSA, obstructive sleep apnea; LF, low frequency; HF, high frequency.
Discussion
The main novel finding of this study was that patients with both OSA and COPD showed higher LF/HF ratio, lower HFnu, and higher LFnu as compared with patients with OSA or COPD alone. These results strongly suggest that, in patients with both disorders, there is a potentiation of independent abnormal influence of OSA and COPD on the autonomic nervous system, leading to an even greater predominance of sympathetic over parasympathetic activity in heart rate modulation.
Previous studies have shown a connection between COPD and sympathetic hyperactivity. Heindl et al. described for the first time the association between marked sympathetic activation, measured with MSNA, and chronic respiratory failure, underlying common basis for respiratory obstructive diseases and altered sympathovagal balance (Citation5). Whereas there are evidences showing relationships between low-grade systemic inflammation and sympathetic predominance in COPD patients (Citation26) and previous data showed that the level of sympathetic activation was enhanced by the presence of acute exacerbation (Citation27), some conflicting results exist in the HRV analysis data. In contrast to the negative results obtained by Camillo et al. in a group of 31 patients with moderate-to-severe COPD (Citation28), Mazzuco and coworkers found a positive relationship between FEV1 and HRV values in 16 moderate-to-severe COPD patients (Citation29). A study by Carvalho and coworkers reported that in COPD patients both LF and HF power are reduced, together with LF/HF ratio, indicating an overall loss of autonomic nervous system influence on HRV as compared with healthy controls (Citation30).
Less controversial data have been reported concerning OSA and sympathetic activation. Recently, Kim et al. demonstrated an unbalanced autonomic nervous system in OSA patients, showing increased LF power and LF/HF ratio, and their positive correlation with AHI (Citation31). To date, there is no striking evidence of modification of HF power in OSA patients as compared with controls (Citation32), even if Narkiewicz and coworkers showed that in 15 subjects with moderate-to-severe OSA (AHI > 20), HF power was significantly lower as compared with that of 16 controls (Citation33). Moreover, the normalized HF component of the RR interval correlated negatively with AHI. Finally, several works have shown that continuous positive airway pressure (CPAP) can ameliorate the HF power in OSA patients (Citation34–36).
So far, the sympathovagal modulation in patients with OSA and COPD together has not been explored; nevertheless, some analogies can be found in patients suffering from both heart failure and OSA. Spaak et al. found that patients with both heart failure and sleep apnea have a greater daytime MSNA as compared with those with heart failure alone (Citation37).
The reason for a greater sympathetic activation in patients with OSA and COPD could likely be a combination of factors specific for both disorders, like sustained or intermittent hypoxemia, oxidative stress, lung hyperinflation, and arousals from sleep. Moreover, these mechanisms also contribute to a loss of respiratory sinus arrhythmia, considering the reduction in HF power in patients with both diseases compared with those with OSA or COPD alone.
This study is the first to indicate how one possible mechanism underneath the increased mortality and morbidity in patients with Overlap syndrome as compared with patients with a single disease could be a greater level of sympathetic hyperactivity. Furthermore, our results confirm the significant positive correlation between AHI and degree of cardiac sympathetic activation in all patients included in the study: the higher the number of apnea–hypopnea episodes, the greater the value of LF/HF ratio.
We found the increased sympathetic autonomic surge to be positively correlated to RV. Significant correlation between RV and LF/HF ratio was already observed in the study of Mazzuco et al. (Citation29). Greater values of RV are expected to predispose COPD patients to pulmonary hyperinflation, which have been linked to impaired baroreflex sensitivity (Citation38) and, lastly, to an increased sympathetic autonomic outflow. Recently, in a cohort of 30 patients with Overlap syndrome, Kwon and coworkers (Citation39) showed that sleep efficiency negatively correlated with the inspiratory capacity to TLC ratio (IC/TLC%), a marker of lung hyperinflation, possibly leading to increased nocturnal work of breathing, which may disrupt sleep, causing arousals and raising the sympathetic tone.
The inverse relationship between PaCO2 and LF/HF ratio is more elusive at this time. Falls in PaCO2 might be due to higher hypoxia-related peripheral chemoreceptor stimulation (Citation40). Given the speculative nature of this hypothesis, the field clearly warrants further investigation.
This study presents several limitations. First, we did not measure HRV in a control group of healthy subjects matched for age and BMI to compare with our groups of patients; however, normal reference values can be derived from previous literature ((Citation8, 41–43). Moreover, the patients included were all men and not completely homogeneous; in particular, patients in the COPD group were older and thinner than those in the OSA group. Nonetheless, in multivariate analysis, including all patients or those with COPD alone, we did not find any correlation between LF/HF ratio and age or BMI and so it is unlikely that the differences in sympathetic or parasympathetic activity between COPD group and the other groups were driven by these parameters. Secondly, the significantly higher use of anticholinergics and β2-agonists in both COPD and Overlap syndrome groups as compared with the OSA group must be mentioned. Haarmann Citation44) and coworkers demonstrated that, in a cohort of 32 COPD patients, acute administration of salmeterol had no effects on sympathetic outflow measured with MSNA, whereas Wu recently showed that, in 70 moderate-to-severe stable COPD patients, there was a slight significant decrease in the HF and an increase in the LF component of HRV after 1 month of treatment with tiotropium (Citation45). Some patients in all groups were taking angiotensin-converting enzyme inhibitors (ACE-I) or angiotensin receptor blockers (ARBs) and β-blockers, whereas there were no significant differences among the groups in the use of these drugs. Some medications known to affect the autonomic balance and HRV variables such as β-blockers (Citation20) might have influenced our results. Despite this fact, the univariate and multivariate regression analyses did not show any significant correlation between medications and LF/HF in this groups of subjects. Third, the lack of blood pressure measurements did not allow us to calculate the baroreflex sensitivity in these groups of patients.
Fourth, while patients with COPD were treated, OSA patients were not. As LF/HF ratio was related to AHI in the whole group, it would have been interesting to measure HRV parameters after a period of CPAP treatment and measure the outcome in terms of AHI. Lastly, the small number of subjects and the design of the study did not allow us to draw firm conclusions regarding the relationship between RV, PaCO2, and LF/HF ratio in the Overlap syndrome group.
Conclusions
In patients affected by Overlap syndrome, the cardiac sympathetic autonomic activation is significantly higher than in patients with COPD or OSA alone. In fact, patients with both disorders had higher values of LF/HF ratio, higher LFnu, and lower HFnu, and this could play a pivotal role in increasing their morbidity and mortality risk. Mechanisms involved appear mainly related to the number of apnea and hypopnea during sleep, as well as to the increase in RV.
Declaration of interest
All authors declare that there are no conflicts of interest.
Funding
Dr Taranto-Montemurro is currently financially supported by the American Heart Association (15POST25480003).
References
- Franklin KA, Lindberg E. Obstructive sleep apnea is a common disorder in the population-a review on the epidemiology of sleep apnea. J Thorac Dis 2015; 7(8):1311–1322.
- Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Resp J 2006; 28:523–532.
- Ioachimesc OC, Teodorescu M. Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology 2013; 18(3):421–431.
- Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea, the overlap syndrome. Am J Resp Crit Care Med 2010; 182:325–331.
- Heindl S, Lehnert M, Criee CP, Hasenfuss G, Andreas S. Marked sympathetic activation in patients with chronic respiratory failure. Am J Resp Crit Care Med 2001; 164(4):597–601.
- Bedard ME, Marquis K, Poirier P, Provencher S. Reduced heart rate variability in patients with chronic obstructive pulmonary disease independent of anticholinergic or beta-agonist medications. COPD 2010; 7(6):391–397.
- Ashley C, Burton D, Sverrisdottir YB, Sander M, McKenzie DK, Macefield VG. Firing probability and mean firing rates of human muscle vasoconstrictor neurones are elevated during chronic asphyxia. J Physiol 2010; 588(Pt 4):701–712.
- Stein PK, Nelson P, Rottman JN, Howard D, Ward SM, Kleiger RE, et al. Heart rate variability reflects severity of COPD in PiZ alpha1-antitrypsin deficiency. Chest 1998; 113(2):327–333.
- MacNee W. Oxidants/antioxidants and COPD. Chest 2000; 117(5 Suppl 1):303S–317S.
- Burtscher M, Haider T, Domej W, Linser T, Gatterer H, Faulhaber M, et al. Intermittent hypoxia increases exercise tolerance in patients at risk for or with mild COPD. Resp Physiol Neurobiol 2009; 165(1):97–103.
- Raupach T, Bahr F, Herrmann P, Luethje L, Heusser K, Hasenfuss G, et al. Slow breathing reduces sympathoexcitation in COPD. Eur Resp J 2008; 32(2):387–392.
- Zulli R, Donati P, Nicosia F, De Vecchi M, Tantucci C, Romanelli G, et al. Increased QT dispersion: a negative prognostic finding in chronic obstructive pulmonary disease. Intern Emerg Med 2006; 1(4):279–286.
- Andreas S, Haarmann H, Klarner S, Hasenfuss G, Raupach T. Increased sympathetic nerve activity in COPD is associated with morbidity and mortality. Lung 2014; 192(2):235–241.
- Short PM, Lipworth SI, Elder DH, Schembri S, Lipworth BJ. Effect of beta blockers in treatment of chronic obstructive pulmonary disease: a retrospective cohort study. BMJ 2011; 342:d2549.
- Somers V, Dyken M, Clary M, Abboud F. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995; 96:1897–1904.
- Somers VK, Mark AL, Zavala DC, Abboud FM. Contrasting effects of hypoxia and hypercapnia on ventilation and sympathetic activity in humans. J Appl Physiol (1985) 1989; 67(5):2101–2106.
- Bradley TD, Tkacova R, Hall MJ, Ando S, Floras JS. Augmented sympathetic neural response to simulated obstructive apnoea in human heart failure. Clin Sci 2003; 104(3):231–238.
- Seals DR, Suwarno NO, Dempsey JA. Influence of lung volume on sympathetic nerve discharge in normal humans. Circ Res 1990; 67(1):130–141.
- Ge X, Han F, Huang Y, Zhang Y, Yang T, Bai C, et al. Is obstructive sleep apnea associated with cardiovascular and all-cause mortality? PLOS One 2013; 8(7).
- Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996; 93(5):1043–1065.
- Montano N, Ruscone T, Porta A, Lombardi F, Pagani M, Malliani A. Power spectrum analysis of heart rate variability to assess the changes in sympathovagal balance during graded orthostatic tilt. Circulation 1994; 90:1826–1831.
- Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med 2012; 8(5):597–619.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Resp Crit Care Med 2013; 187(4):347–365.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Resp Crit Care Med 2007; 176(6):532–555.
- Corda L, Novali M, Montemurro LT, La Piana GE, Redolfi S, Braghini A, et al. Predictors of nocturnal oxyhemoglobin desaturation in COPD. Resp Physiol Neurobiol 2011; 179(2–3):192–197.
- Chhabra SK, Gupta M, Ramaswamy S, Dash DJ, Bansal V, Deepak KK. Cardiac Sympathetic Dominance and Systemic Inflammation in COPD. COPD 2015; 12(5):552–559.
- Zamarrón C, Lado MJ, Teijeiro T, Morete E, Vila XA, Lamas PF. Heart rate variability in patients with severe chronic obstructive pulmonary disease in a home care program. Technol Health Care 2014; 22(1):91–98.
- Camillo CA, Pitta F, Possani HV, Barbosa MV, Marques DS, Cavalheri V, et al. Heart rate variability and disease characteristics in patients with COPD. Lung 2008; 186(6):393–401.
- Mazzuco A, Medeiros MW, Sperling MPR, Soares de Souza A, Alencar MCN, Arbex FF, et al. Relationship between linear and nonlinear dynamics of heart rate and impairment of lung function in COPD patients. Int J COPD 2015; 10:1651–1661.
- Carvalho TD, Pastre CM, De Godoy MF, Fereira C, O Pitta F, De Abreu LC, et al. Fractal correlation property of heart rate variability in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2011; 6(6):23–28.
- Kim YS, Kim SY, Park DY, Wu HW, Hwang GS, Kim HJ. Clinical implication of heart rate variability in obstructive sleep apnea syndrome patients. J Craniofac Surg 2015; 26(5):1592–1595.
- Flevari A, Vagiakis E, Zakynthinos S. Heart rate variability is augmented in patients with positional obstructive sleep apnea, but only supine LF/HF index correlates with its severity. Sleep Breath 2015; 19(1):359–367.
- Narkiewicz K, Montano N, Cogliati C, Van de Borne PJH, Dyken MED, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation 1998; 98(11):1071–1077.
- Shiina K, Tomiyama H, Takata Y, Yoshida M, Kato K, Saruhara H, et al. Effects of CPAP therapy on the sympathovagal balance and arterial stiffness in obstructive sleep apnea. Resp Med 2010; 106(6):911–916.
- Roche F, Court-Fortune I, Pichot V, Duverney D, Costes F, Emonot A, et al. Reduced cardiac sympathetic autonomic tone after long-term nasal continuous positive airway pressure in obstructive sleep apnoea syndrome. Clin Physiol 1999; 19(2):127–134.
- Palma JA, Iriarte J, Fernandez S, Alegre M, Valencia M, Artieda J, et al. Long-term continuous positive airway pressure therapy improves cardiac autonomic tone during sleep in patients with obstructive sleep apnea. Clin Autor Res 2015; 25(4):25–32.
- Spaak J, Egri ZJ, Kubo T, Yu E, Ando SI, Kaneko Y, et al. Muscle sympathetic nerve activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension 2005; 46:1327–1332.
- Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Resp Physiol Neurobiol 2002; 130:3–20.
- Kwon J, Wolfe L, Lu B, Kalhan R. Hyperinflation is associated with lower sleep efficiency in COPD with co-existent obstructive sleep apnea. COPD 2015; 6:441–445.
- Burton MD, Kazemi H. Neurotransmitters in central respiratory control. Respir Physiol 2000; 122(2–3):111–121.
- Balachandran JS, Bakker JP, Rahangdale S, Yim-Yeh S, Mietus JE, Goldberger AL, et al. Effect of mild, asymptomatic obstructive sleep apnea on daytime heart rate variability and impedance cardiography measurements. Am J Cardiol 2012; 109(1):140–145.
- Cheng ST, Wu YK, Yang MC, Huang CY, Huang HC, Chu WH, et al. Pulmonary rehabilitation improves heart rate variability at peak exercise, exercise capacity and health-related quality of life in chronic obstructive pulmonary disease. Heart Lung 43(3):249–255.
- Idiaquez J, Santos I, Santin J, Del Rio R, Iturriaga R. Neurobehavioral and autonomic alterations in adults with obstructive sleep apnea. Sleep Med 2014; 15(11):1319–1323.
- Haarmann H, Mohrlang C, Tschiesner U, Rubin DB, Bornemann T, Rüter K, et al. Inhaled β-agonist does not modify sympathetic activity in patients with COPD. BMC Pulm Med 2015; 15:46.
- Wu YK, Huang CY, Yang MC, Huang GL, Chen SY, Lan CC. Effect of tiotropium on heart rate variability in stable chronic obstructive pulmonary disease patients. J Aerosol Med Pulm Drug Deliv 2015; 28(2):100–105.