ABSTRACT
The use of oral and inhaled corticosteroids is associated with increases in the risk of infection, especially pneumonia. The risk of sepsis with corticosteroid treatment in patients with chronic obstructive pulmonary disease (COPD) has been little studied, however. We assessed whether the use of inhaled and oral corticosteroids in COPD is associated with an increase in the risk of sepsis. We carried out a retrospective cohort study using the administrative health databases of the province of Quebec, Canada, over the period 1990–2007. The cohort of patients with COPD included patients aged 55 years or older who used respiratory medications. A quasi-cohort analysis was used to estimate the rate ratio (RR) of sepsis in current users of inhaled corticosteroids and oral corticosteroids separately, after adjusting for differences in COPD disease severity and co-morbid conditions. The cohort included 163,514 patients treated for COPD, including 1,704 who were hospitalized for or died with sepsis during follow-up (incidence rate 1.94 per 1000 per year). The RR of sepsis associated with current use of inhaled corticosteroids was 0.98 (95%confidence interval [CI] 0.84–1.14). Current oral corticosteroid use was associated with a 66% increase in the risk of sepsis (RR 1.66; 95% CI: 1.35–2.05). The increase in risk remains for around 5 months after the oral corticosteroid exposure. Among patients treated for COPD, the risk of sepsis is not increased with inhaled corticosteroids, even at high doses, while the risk is increased with oral corticosteroids. This risk should be considered when treating exacerbations of COPD.
| Abbreviation list | ||
| CI | = | Confidence interval |
| ICD | = | International Classification of Diseases |
| RAMQ | = | Régie de l'assurance maladie du Québec or Quebec Health Insurance Board |
| RR | = | Rate ratio |
Introduction
Both inhaled and oral corticosteroids are used extensively in the treatment of chronic obstructive pulmonary disease (COPD): inhaled corticosteroids for maintenance therapy in approximately 70% of patients recruited for a recent clinical trial Citation(1), while oral corticosteroids are recommended in the treatment of acute exacerbations Citation(2). Corticosteroids have multiple effects on the immune system with the potential to increase susceptibility to infection Citation(3). Corticosteroids have been associated with infectious complications, including pneumonia and tuberculosis Citation(4–6). Oral corticosteroids have been found to increase the risk of postoperative sepsis among patients undergoing surgery for inflammatory bowel disease Citation(7). Sepsis is increasing in frequency, especially severe sepsis defined on the basis of associated organ dysfunction Citation(8,9).The study of sepsis in COPD is especially pertinent given that pneumonia is a common precursor of sepsis and is more frequent in COPD patients Citation(10) with a further increase in users of inhaled corticosteroids Citation(11). We therefore carried out an observational study examining the risk of sepsis in subjects with respiratory disease treated with inhaled and oral corticosteroids between 1990 and 2007.
Methods
Data source
The current study is based on the databases of the Régie de l'assurance maladie du Québec (RAMQ), the health insurance program of the province of Québec, Canada. The databases contain information on demographics and medical services rendered for all residents of the province and are linked to death records. Au: Please advise if the edits made to the sentence “The prescription drugs database includes …” are correct. The prescription drugs database includes outpatient prescription medications dispensed to all people aged 65 years or older, social welfare recipients and, all other residents who opted to join the provincial drug plan since 1996. This study was approved by the Research Ethic Committee of the Jewish General Hospital (Protocol #10–126).
Study design
The source population for this study consisted of subjects dispensed at least one prescription between 1990 and 2005 for any of the following respiratory medications: any form of β agonist, theophylline, ipratropium or tiotropium bromide, and inhaled corticosteroid. To form a cohort of patients with COPD, we identified all subjects, aged 55 years or older, with three or more prescriptions for these medications (except inhaled corticosteroids) in any 1 year and on at least two different dates. To ensure a new-user cohort, we included only those with no respiratory medications during the 2 years before the first of these three prescriptions. Subjects who before cohort entry had a mention of asthma, either as primary or secondary diagnosis during a hospitalization, or using nedocromil, ketotifen, cromolyn or antileukotrienes were excluded. All subjects in the cohort were followed from cohort entry, taken as the date of the third prescription, until the first hospitalization for sepsis, death, end of RAMQ drug coverage, or 31 March 2007. In a sensitivity analysis, we restricted the cohort to subjects who also had a diagnostic code for COPD. Because of the large size of the cohort, the time-varying nature of corticosteroid use and the large number of covariates, we used a nested case-control design with a quasi-cohort approach to the analysis to estimate the association between corticosteroid exposures and the risk of sepsis.
Sepsis cases and controls
Cases of sepsis were defined as a hospitalization for or death from sepsis. The first hospitalization with an admission with a primary diagnosis of sepsis was identified in the RAMQ hospitalization database (International Classification of Diseases (ICD)-9 codes 995.92, 785.52, 038, ICD-10 codes A40, A41) during cohort follow-up. A subject was considered to have died of sepsis if sepsis was listed as the initial cause of death on the death certificate whether death occurred in or out of hospital. The date of admission or death was called the index date.
For the nested case-control design, we identified, for each case, 10 controls matched on the case's age within 1 year and cohort entry month were selected at random from all subjects without the outcome of interest on the case's index date. For the few cases with no eligible controls, the age and calendar time matching criteria were relaxed to within 5 years and the same year, respectively. When less than 10 potential controls were available for a case, all members of the risk set were included as controls for that case.
Inhaled and oral corticosteroid exposure
Prescriptions for inhaled corticosteroids, alone or in a combination inhaler, dispensed between cohort entry and the index date were identified. These include inhaled beclomethasone, fluticasone, budesonide, triamcinolone and flunisolide. All doses of inhaled corticosteroids were converted to fluticasone-equivalent doses on the basis of relative topical potency and what experts consider to be comparable doses according to the United States Citation(12) and Canadian asthma consensus statements Citation(13). Dose equivalences used are beclomethasone 100µg, budesonide 80µg, triamcinolone 200µg, fluticasone 50µg and flunisolide 200µg. The converted doses were categorized as high (fluticasone 1000µg/day or more), moderate (500–999µg/day) and low (less than 500µg/day).
All prescriptions for oral corticosteroids dispensed between cohort entry and the index date were identified.
Data analysis
A quasi-cohort analysis, based on the nested case-control approach within the cohort, was performed Citation(14). Subjects were considered current inhaled corticosteroid or oral corticosteroid users if their index date was preceded by a prescription for inhaled corticosteroid (or oral corticosteroid) by 60 days or less. Non-use, defined as the index date preceded by no such prescription during the entire prior year, was taken as the reference category. Subjects who were not current users but whose index date was preceded by a prescription dispensed between 61 and 365 days previously were classified as discontinued users. Quasi-rates of sepsis were estimated using the sampling fraction to multiply the event rate Citation(14). Rate ratios (RRs) of sepsis associated with current use of inhaled and oral corticosteroids were estimated directly from the odds ratios computed by conditional logistic regression, as the two are equivalent in this nested case-control design. The RRs, with 95% confidence intervals (CIs), were adjusted for age, sex, severity of respiratory disease, and other conditions associated with a risk of sepsis. Severity of respiratory disease was measured by the number of prescriptions for other respiratory drugs and oral corticosteroids, both number of prescriptions in the prior year and occurrence of a prescription in the prior month, antibiotics and the occurrence of a hospitalization with a primary diagnosis of COPD measured in the year prior to the index date. Co-morbidity included cardiac disease defined by a prescription for cardiotropes, antihypertensives, diuretics or vasodilators; diabetes defined by insulin or use of oral hypoglycemic agents; central nervous system drugs included benzodiazepines, major tranquilizers, anticonvulsants and drugs for parkinsonism; osteoporosis drugs were calcium, vitamin D and bisphosphonates; antirheumatic drugs were gold salts, methotrexate, azathioprine, hydroxychloroquine and chloroquine. Non-steroidal anti-inflammatory drugs, antidepressive agents and narcotics were considered as separate categories. For the RRs with oral corticosteroids, the same adjustments were made except for oral corticosteroid use since this was the exposure of interest, but adjusting for use of inhaled corticosteroids.
The risk of sepsis according to the time from the last prescription to the index date was estimated using a cubic splines model fit by conditional logistic regression. For inhaled corticosteroids, current users were also classified as users of fluticasone, budesonide or other inhaled corticosteroid (beclomethasone, flunisolide or triamcinolone). Dose response was assessed using the fluticasone-equivalent daily dose of current users, classified as high, moderate and low. All analyses were carried out using SAS software (version 9.4).
Results
The cohort included 163,514 patients who generated 879,183 person-years of follow-up, during which 1,704 were hospitalized with a primary diagnosis of sepsis or death with sepsis listed as the initial cause of death, for an incidence rate of 1.94 cases of sepsis per 1000 per year (95% CI: 1.85–2.03). provides characteristics of subjects according to the current use of inhaled corticosteroids. Current users of inhaled corticosteroids were more frequently hospitalized for COPD and pneumonia in the prior year and had been dispensed more respiratory drugs and more proton pump inhibitors than non-users or those who had discontinued use.
Table 1. Quasi-cohort characteristics based on inhaled corticosteroid exposure.
The adjusted RR of sepsis with current use of inhaled corticosteroids (RR 0.98 95% CI 0.84–1.14) excludes a significant excess risk (). There is also no increased risk observed at the highest doses equivalent to 1000µg or more of fluticasone (RR 1.06 95% CI 0.89–1.27), or according to the type of inhaled corticosteroid, whether fluticasone (RR 0.98 CI 0.82–1.16) or budesonide (RR 0.95 95% CI 0.72–1.25). In a sensitivity analysis, restricting the cohort to subjects who had a diagnostic code for COPD, there were 1,482 cases of sepsis, and the adjusted RR of sepsis with current use of inhaled corticosteroids was 1.02 95% CI 0.87–1.18.
Table 2. Quasi-rates, crude and adjusted rate ratios of sepsis associated with exposure to inhaled corticosteroids.
provides subject characteristics according to use of oral corticosteroids. Approximately 94% of the prescriptions in the year prior to the event were for prednisone, with an average duration of 17.7 days and a median of 14 days. As expected, current users of oral corticosteroids had more severe respiratory disease as evidenced by a COPD hospitalization in the last year and use of respiratory medications. Various co-morbidities were also more common.
Table 3. Quasi-cohort characteristics based on oral corticosteroid exposure.
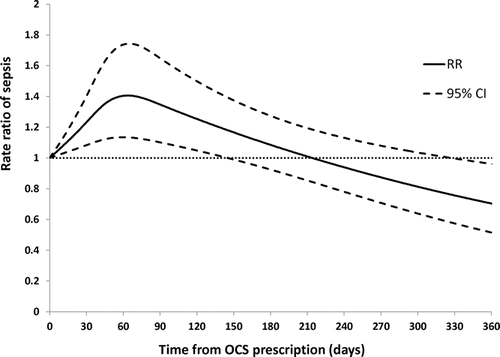
shows that current use of oral corticosteroids is associated with a 66% increase in the risk of sepsis (RR 1.66 95% CI 1.35–2.05). The risk is still present 2–6 months after exposure (RR 1.39 95% CI 1.12–1.72). suggests that the increase in risk is no longer statistically significant at 150 days after exposure, where the lower limit of the confidence interval crosses 1. We repeated the analysis of the association between use of oral corticosteroids and sepsis excluding subjects hospitalized for pneumonia or COPD in the past year since we do not capture medication use in hospital and also to see whether the association persisted in those with less severe disease. The increase in risk was the same, RR 1.61 95% CI 1.30–2.01. In the sensitivity analysis, restricting the cohort to subjects who had a diagnostic code for COPD, current exposure to oral corticosteroids was similarly associated with an increased risk of sepsis, RR 1.64 95% CI 1.32–2.03.
Table 4. Quasi-rates, crude and adjusted rate ratios of sepsis associated with exposure to oral corticosteroids.
Discussion
In our population-based cohort of over 160,000 patients with presumed COPD followed for an average 5.4 years, we found an incidence rate of sepsis of 1.94 per 1,000 persons per year. This rate is somewhat higher than the rates obtained from administrative data during a similar time period in the United States, which increased from 0.67 cases/1,000 in 1993 to 1.32 cases/1,000 in 2003 Citation(8) for the population as a whole. This is not surprising since COPD patients are at an increased risk of pneumonia Citation(10) and therefore probably of sepsis.
Current use of an inhaled corticosteroid defined by a prescription dispensed within 60 days of the sepsis event was neither associated with an increased risk of sepsis even at high doses equivalent to a daily dose of fluticasone 1000 mcg or more, nor was there any difference in risk between fluticasone and budesonide, the two most commonly prescribed inhaled corticosteroids. This differs from previous studies of pneumonia where the risk appeared greater with fluticasone as compared to budesonide Citation(15–17).
A 66% increase in the risk of sepsis was observed with current use of oral corticosteroids with an increase in risk persisting for approximately 150 days or 5 months. Despite the fact that use of oral corticosteroids for the treatment of acute exacerbations of COPD is considered standard therapy Citation(2), there is surprisingly little information on the safety of oral corticosteroids used in an outpatient setting. In a recent meta-analysis of treatment of acute exacerbations of COPD with systemic corticosteroids, only 2 of 20 studies were carried out in the outpatient setting Citation(18). Overall, hyperglycemia was the only commonly observed adverse effect found in excess with systemic corticosteroid therapy. We could find no studies examining the risk of sepsis with oral corticosteroids in COPD.
The duration of the outpatient prescriptions for oral corticosteroid was long with a median of 14 days. This is longer than current recommendations Citation(2), and one would hope that shorter durations of treatment would be associated with less risk of sepsis.
A limitation of the present study is the inaccuracy of diagnostic codes for sepsis. Gaeski Citation(19) reported a greater than threefold variability in the annual incidence of sepsis depending on the coding algorithm used. In addition, the coding for sepsis in our data appears much less detailed than the coding in U.S. databases. For example, in U.S. databases, ICD-9 codes for severe sepsis/sepsis with organ dysfunction (995.92) and septic shock (785.52), which have been increasingly used in the United States Citation(20), do not appear in our database; all our sepsis cases are based on the ICD-9 code for septicemia (038) or the equivalent ICD-10 codes (A40, A41). Thus, coding in the Quebec databases appears more like that found in the U.K., where 99% of ICD-10 codes for sepsis in adults were A40 or A41 Citation(21). Overall, administrative codes for sepsis have low sensitivity but high specificity Citation(22) such that we are certainly underestimating the rate of sepsis. There is no reason, however, to suspect that this would differ for users of corticosteroids.
A further limitation is our definition of COPD based on initiation of use of respiratory medication after the age of 55 years without prior evidence of asthma. While we have successfully used this definition to study the risk of severe pneumonia in relation to use of inhaled corticosteroids in this same population Citation(15), we cannot be sure that subjects do not have asthma. This may be less relevant today given the increasing recognition of overlap syndromes Citation(23). We also cannot be sure that subjects actually have COPD as defined by spirometry. We carried out a sensitivity analysis, restricting the cohort to subjects who also had a diagnostic code for COPD, and the results were almost identical. These diagnostic codes for COPD have been validated in another Canadian database very similar to ours Citation(24). Furthermore, there is the possibility that, despite our extensive adjustments for severity of disease, there is residual confounding such that more severe respiratory disease in those receiving oral corticosteroids accounts for the increase in risk for sepsis. We are also not able to dissociate the risk of sepsis related to the occurrence of an exacerbation from that of its treatment with oral corticosteroids. The present study reflects therapy for presumed airway disease as it was applied in the last two decades, and one might hope that the use of shorter courses of oral corticosteroids Citation(25) might limit the occurrence of sepsis in the COPD population.
Conclusion
We could not find evidence of an increase in the risk of sepsis with inhaled corticosteroids, even at high dose, while current use of oral corticosteroids, presumably for the treatment of acute exacerbations of COPD, was associated with a 66% increase in sepsis, leading to hospitalization or death.
Declaration of interest
Dr. Suissa has received funding for research, advisory board meetings or as speaker from AstraZeneca, Boehringer Ingelheim, Merck, and Novartis. Drs. Ernst, Brassard and Ms. Coulombe do not have any conflicts of interest to disclose.
Acknowledgments
The author Pierre Ernst is guarantor of the content of the manuscript. All authors contributed to the design of the study, carried out or verified the analyses and contributed to the drafting of the manuscript. The funding source had no input into the design or interpretation of the study. The authors would like to thank Melissa Dahan for help in preparing the manuscript.
Funding
This research was funded in part by a grant from the Canadian Institutes of Health Research (CIHR) and the Canadian Foundation for Innovation (CFI). Dr. Suissa is the recipient of the James McGill Professorship award.
References
- Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med 2014; 371(14):1285–1294.
- Criner GJ, Bourbeau J, Diekemper RL, Ouellette DR, Goodridge D, Hernandez P, et al. Executive summary: prevention of acute exacerbation of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest 2015; 147(4):883–893.
- Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med 1976; 84(3):304–315.
- Singh S, Amin AV, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease: a meta-analysis. Arch Intern Med 2009; 169(3):219–229.
- Wolfe F, Caplan L, Michaud K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia. Arthritis Rheum 2006; 54(2):628–634.
- Toruner M, Loftus EV Jr., Harmsen WS, Zinsmeister AR, Orenstein R, Sandborn WJ, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008; 134(4):929–936.
- Aberra FN, Lewis JD, Hass D, Rombeau JL, Osborne B, Lichtenstein GR. Corticosteroids and immunomodulators: postoperative infectious complication risk in inflammatory bowel disease patients. Gastroenterology 2003; 125(2):320–327.
- Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med 2007; 35(5):1244–1250.
- Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med 2012; 40(3):754–761.
- Almirall J, Bolibar I, Balanzo X, Gonzalez CA. Risk factors for community-acquired pneumonia in adults: a population-based case-control study. Eur Respir J 1999; 13(2):349–355.
- Ernst P, Gonzalez AV, Brassard P, Suissa S. Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med 2007; 176(2):162–166.
- National Asthma Education Program NHLBI. Practical guide for the diagnosis and management of asthma. 1997.
- Boulet LP, Becker A, Berube D, Beveridge R, Ernst P. Canadian Asthma Consensus Report, 1999. Canadian Asthma Consensus Group. Canadian Med Assoc J 1999; 161(11 Suppl):S1–61.
- Suissa S. The Quasi-cohort approach in pharmacoepidemiology: upgrading the nested case-control. Epidemiology 2015; 26(2):242–246.
- Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 2013; 68(11):1029–1036.
- Crim C, Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J 2009; 34(3):641–647.
- O'Byrne PM, Pedersen S, Carlsson LG, Radner F, Thoren A, Peterson S, et al. Risks of Pneumonia in Asthmatic Patients Taking Inhaled Corticosteroids. Am J Respir Crit Care Med 2011; 183:589–595.
- Walters JA, Tan DJ, White CJ, Gibson PG, Wood-Baker R, Walters EH. Systemic corticosteroids for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 9:CD001288.
- Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med 2013; 41(5):1167–1174.
- Rhee C, Murphy MV, Li L, Platt R, Klompas M. Comparison of trends in sepsis incidence and coding using administrative claims versus objective clinical data. Clin Infect Dis 2015; 60(1):88–95.
- McPherson D, Griffiths C, Williams M, Baker A, Klodawski E, Jacobson B, et al. Sepsis-associated mortality in England: an analysis of multiple cause of death data from 2001 to 2010. BMJ Open 2013; 3(8).
- Jolley RJ, Sawka KJ, Yergens DW, Quan H, Jette N, Doig CJ. Validity of administrative data in recording sepsis: a systematic review. Crit Care 2015; 19(1):139.
- Postma DS, van den Berge M. The different faces of the asthma-COPD overlap syndrome. Eur Respir J 2015; 46(3):587–590.
- Gershon AS, Wang C, Guan J, Vasilevska-Ristovska J, Cicutto L, To T. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD 2009; 6(5):388–394.
- Walters JA, Tan DJ, White CJ, Wood-Baker R. Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 12:CD006897.