ABSTRACT
In patients with stable COPD, proadrenomedullin (MR-proADM) has been shown to be a good predictor for mortality. This study aims to provide an external validation of earlier observed cut-off values used by Zuur-Telgen et al. and Stolz.et al. in COPD patients in stable state and at hospitalization for an acute exacerbation of COPD (AECOPD). From the COMIC cohort study we included 545 COPD patients with a blood sample obtained in stable state (n = 490) and/or at hospitalization for an AECOPD (n = 101). Time to death was compared between patients with MR-proADM cut-off scores 0.71 and 0.75 nmol/L for stable state or 0.79 and 0.84 nmol/l for AECOPD. The predictive value of MR-proADM for survival was represented by the C statistic. Risk ratios were corrected for sex, age, BMI, presence of heart failure, and GOLD stage. Patients above the cut-off of 0.75 nmol/l had a 2-fold higher risk of dying than patient below this cut-off (95% CI: 1.20–3.41). The cut-off of 0.71 nmol/l showed only a borderline significantly higher risk of 1.67 (95% CI: 0.98–2.85). The corrected odds ratios for one-year mortality were 3.15 (95% CI 1.15–8.64) and 3.70 (95% CI 1.18–11.6) in patients with MR-proADM levels above versus below the cut-off of respectively 0.75 and 0.71 nmol/l measured in stable state. MR-proADM levels in samples at hospitalization for an AECOPD were not predictive for mortality in this validation cohort. MR-proADM in stable state is a powerful predictor for mortality.
KEYWORDS:
Introduction
Chronic obstructive pulmonary disease (COPD) will be the third-leading cause for mortality worldwide by 2020 Citation(1). The prediction of mortality is relevant to identify patients in whom adjustment of care may be appropriate. Airflow limitation (Citation2, 3), low body mass index (BMI) Citation(4), breathlessness Citation(5), exercise capacity (Citation6, 7), and the frequency and severity of exacerbations Citation(8) can predict prognosis of COPD. The explained variance in mortality by these parameters, however, is very low. Their integration into multidimensional indices, such as the BODE index Citation(9) and ADO score Citation(10) predicts survival better than the individual variables.
An easily accessible marker with a close relation to survival in COPD would constitute an important step forward. Recent publications show promising results for proadrenomedullin regarding prediction of survival (Citation11–14)
Adrenomedullin (ADM) has immune-modulating, meta-bolic, and vascular actions. It can behave both as a hormone and as a cytokine, and can simultaneously control pulmonary blood flow, leukocyte migration, and electrolyte balance (Citation15–17). The ADM-sensitive receptors are ubiquitously present in the body and receptors for ADM and ADM itself have been established in the lung in high concentrations Citation(18). ADM is rapidly cleared from the circulation and therefore difficult to measure Citation(19). The more stable precursor of ADM, midrange-proadrenomedullin (MR-proADM), closely reflects the level of active ADM Citation(20)
Recently, we reported that MR-proADM measured in stable state showed to be a strong predictor for mortality in COPD patients when the median MR-proADM value (0.71 nmol/l) was used as cut-off value (corrected Hazard Ratio (cHR) 2.98 (95% CI 1.51–5.90) Citation(12). Stolz et al showed the optimal cut-off value of MR-proADM in stable should be 0.75 nmol/l in stable state reflecting 68.1% sensitivity and 70.7% specificity Citation(13).
In our population, the MR-proADM value at hospitalization for an acute exacerbation of COPD (AECOPD) showed a median of 0.79 nmol/l and when this was used as a cut-off value a non-significant cHR of 1.58 (95%CI 0.80–3.14) was seen. Stolz et al reported a median of 0.84 nmol/l in a population assessed at hospitalization for an AECOPD. When this median was taken as a cut-off the cHR was a little higher (cHR 1.82 (95% CI 0.93–3.55) but still non-significant. Before MR-proADM, either assessed in stable state or during AECOPD, can be used as a risk stratification marker, these cut-off values should be externally validated (Citation21, 22).
The aim of our study was to investigate whether the previous cut-off point of 0.71 nmol/l remains a good predictor for mortality when used in a new, but similar population. Also the cut-off value in stable state (0.75 nmol/l) determined by Stolz et al. Citation(13) will be tested in the new validation sample of population. Furthermore, the cut-off values of MR-proADM when assessed at hospitalization for AECOPD in our previous study (0.79 nmol/l) and the cut-off value determined by Stolz et al. (0.84 nmol/l) Citation(11) will be validated.
Materials and methods
Setting and study population
This study was part of the COMIC study, a single-center cohort study on the immune status of COPD patients as a determinant for survival. From December 2005 till April 2010, 795 patients were included with a follow-up period of three years. The COMIC study was approved by medical ethical committee Twente, at Enschede (P05-49). All patients provided written informed consent.
Patients had to meet the following criteria: Citation(1) a clinical diagnosis of COPD according to the GOLD standard Citation(23); Citation(2) current or former smoker; Citation(3) age ≥40 years; Citation(4) no medical condition compromising survival within the follow-up period or serious psychiatric morbidity; Citation(5) absence of any other active lung disease (e.g. sarcoidosis); Citation(6) no maintenance therapy with antibiotics; and Citation(7) ability to speak Dutch. Patients were enrolled when hospitalized for an AECOPD or when visiting the outpatient clinic in stable state. To be included in the AECOPD group, patients had to be hospitalized for an AECOPD and be able to produce an adequate sputum sample at the day of hospitalization Citation(24). An AECOPD was defined as an acute negative change from the baseline, reported by the patient, in dyspnea and/ or sputum volume and/or color of sputum (yellowish or greenish sputum) and/or cough, which may warrant additional treatment of prednisolone with or without antibiotics by a physician in a patient with underlying COPD. To be included in the stable state group patients had to meet the following criteria; no use of antibiotic and/or prednisolone 4 weeks prior to enrolment and no exacerbation less than 4 weeks before study entry. Also data on common co-morbidities like hypertension, myocardial infarction, and heart failure were obtained as they are probably related to MR-proADM due to its vascular activity and related to survival (Citation25–28). Patients were asked to complete a questionnaire about their dyspnea in stable state (mMRC)(29). All patients were treated according the current COPD standard. Eighty percent of the patients used inhaled corticosteroids during follow-up Citation(30).
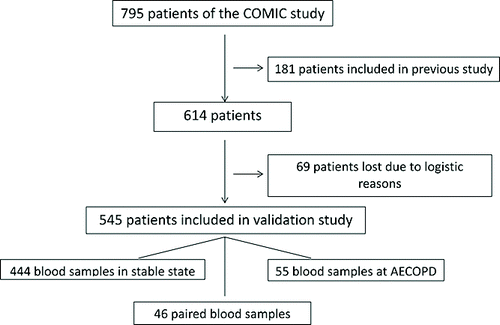
The initial report(12) concerned 181 patients of the 795 patients included in the COMIC study. These patients had paired blood samples (stable state and at hospitalization for AECOPD). In this validation study, we obtained 490 blood samples in stable state and 101 blood samples at hospitalization for an AECOPD from 545 of the remaining 614 patients (). In these samples levels of MR-proADM were determined. Forty-six patients were able to give a paired sample. These patients were not included in the previous study because at the time of analysis they did not have this paired sample.
Outcomes
The primary outcome parameter was survival, based on all-cause mortality. Date of death was verified from the municipal administration.
Measurements of MR-proADM
MR-proADM levels were measured in EDTA-plasma (ethylenediaminetetraacetic acid anticoagulant) with an automated sandwich immunoassay using a time-resolved amplified cryptate emission technology (TRACE) Citation(31) (KRYPTOR®; Thermo Scientific Biomarkers, [formerly B.R.A.H.M.S. AG] Hennigsdorf, Germany). The KRYPTOR MR-proADM assay has a measuring range of 0.05–100 nmol/l and a functional sensitivity of 0.25 nmol/l. The reference interval of MR-proADM in a healthy subset is 0.23–0.64 nmol/l (median 0.41 nmol/l) (Citation13, 20).
Statistical analysis
Continuous variables are expressed as mean (± standard deviation (SD)), and categorical variables as counts (percentages). Differences in baseline characteristics between survivors and non-survivors in stable state and during AECOPD were analyzed by T-test for continuous variables or Chi square tests for categorical variables. Differences in MR-proADM levels between survivors and non-survivors and between stable state and hospitalization for an AECOPD were analyzed by T-test. Patients were classified as having high or low levels of MR-proADM based on the cutoff level of 0.71 nmol/l in stable state and 0.79 nmol/l at hospitalizations for an AECOPD. As a secondary analysis, the test characteristics of the cut-off levels of the MR-proADM by Stolz et al. Citation(13), in stable state 0.75 nmol/l and at hospitalization for an AECOPD 0.84 nmol/l, were assessed. Time from stable state or first hospitalization for AECOPD to death was analyzed by Kaplan-Meier survival curves with log rank tests. Hazard ratios (HRs) were determined by Cox regression analysis. All analyses were corrected for age, sex, BMI, GOLD stage, heart failure in a multivariate Cox regression analysis, and the C statistic was calculated Citation(32). One–and two-year survival after stable state or hospitalization for an AECOPD were analyzed by logistic regression analyses and corrected for the same parameters. The sensitivity, specificity, and positive and negative predictive value were calculated for one–and two-year survival, and Receiver Operating Curves (ROC) were plotted. All tests were two-sided and a p-value of 0.05 was considered statistically significant. The data were analyzed using SPSS, version 20 (SPSS Inc. Chicago IL).
Results
Baseline characteristics
The baseline characteristics of the patients with a sample in stable state (490) and those with a blood sample taken at hospitalization for an AECOPD (101) are presented in . Forty-six patients had a paired sample. The median follow-up after inclusion at stable state and at hospitalization for AECOPD was 33 and 24 months, respectively. Mortality was 26% and 54%, respectively.
Table 1. Baseline characteristics.
MR-proADM levels in survivors and non-survivors
MR-proADM levels were significantly higher in non-survivors compared to survivors when measured in stable state. There was no significant difference in MR-proADM levels between survivors and non-survivors at hospitalization for AECOPD ().
Table 2. MR-proADM results.
Stable state
MR-proADM in stable state
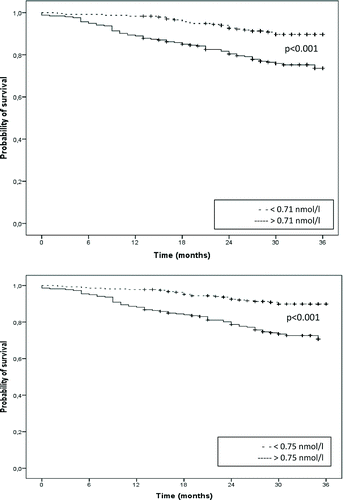
Survival time was borderline significantly worse in patients with high stable-state MR-proADM levels (>0.71 nmol/L) than in those with low levels with a corrected HR of 1.67 (95% CI 0.98–2.85) and a C statistic of 0.76 ( and ). The 1–and 2-year corrected Odds Ratios (cORs) for mortality of patients with high versus low MR-proADM levels were, respectively, 3.70 (95% CI 1.18–11.6) and 1.94 (95% CI 0.99–3.79) ().
Table 3. Survival analysis based on different cut-off values of MR-proADM as well in stable state as in AECOPD.
Table 4. One–and two-year mortality risk based on different cut-off values of MR-proADM as well in stable state as in AECOPD.
When we used the median MR-proADM level of Stolz et al. (0.75 nmol/L)(Stolz ERJ 2013), survival time was significantly worse in patients with high versus low MR-proADM levels with a cHR of 2.02 (95% CI 1.20–3.41) and a C statistic of 0.77 ( and ). The 1–and 2-year cORs for mortality of patients with high versus low MR-proADM levels were 3.15 (95% CI 1.15–8.64) and 2.23 (95% CI 1.15–4.32), respectively ().
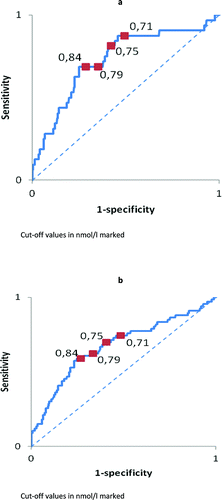
shows cut-off points of different MR-proADM levels for one–and two-year survival. When higher cut-off values than the median of our population were used, the sensitivity decreased, while the specificity increased. shows the ROC curves of the one–and two-year survival and the same pattern was observed.
Table 5. Sensitivity and specificity of MR-proADM for 1-year survival (a priori 6.5%) and 2-year survival (a priori 13.5%).
Hospitalization for AECOPD
MR-proADM at hospitalization for AECOPD
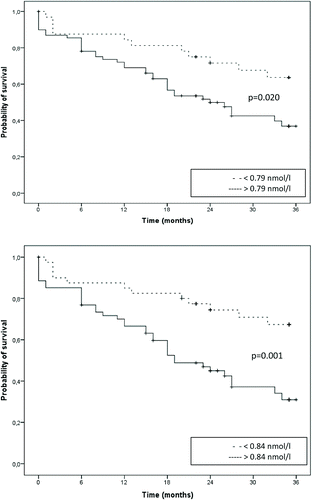
In patients with high MR-proADM levels (above median of 0.79 nmol/L) at hospitalization for AECOPD, survival was not significantly worse than in those with low levels, with a cHR of 1.51 (95% CI 0.69–3.33) and a C statistic of 0.73 ( and ). The 1–and 2-year cORs for mortality of patients with high versus low MR-proADM levels were, respectively, 1.77 (95% CI 0.45–6.92) and 2.28 (95% CI 0.68–7.60) ().
When we used the median MR-proADM level of Stolz et al. (0.84 nmol/L)(34), survival time was not significantly worse in patients with high versus low MR-proADM levels with a cHR of 1.91(95% CI 0.87–4.20) and a C statistic of 0.74 ( and ). The 1–and 2-year corrected ORs for mortality of patients with high versus low MR-proADM levels were 1.76 (95% CI 0.49–6.40) and 2.66 (95% CI 0.82–8.64), respectively ().
Discussion
This validation study confirms that high MR-proADM levels, measured in patients with COPD in stable state, are associated with a higher mortality risk. When corrected for potential confounding variables, this concerns a 1.7 times higher risk with a cut-off value of 0.71 nmol/l and a 2.0-fold risk with a cut-off value of 0.75 nmol/l. The 1.9–and 2.2-fold increased risk of 2-year mortality associated with MR-proADM levels measured in stable state, with a cut-off value of 0.71 and 0.75 nmol/l respectively is clinically relevant, because the absolute mortality risk in these COPD patients is high with 13% not surviving two years. At one year it was even increased: 3.7-fold and 3.2-fold, respectively. The C statistic for both cut-off values, 0.76 and 0.77, is slightly higher than the BODE index (0.74) Citation(9). This result is in line with our original analysis Citation(12).
We confirm the observation that high MR-proADM levels at hospitalization for AECOPD were related to a shorter survival, but this observation was non-significant after correction for age, sex, GOLD stage, heart failure and BMI. It could be that there are more competitive causes of death around the time of an AECOPD and therefore MR-proADM is less predictive than in stable state.
It is important to realize that from the population of the COMIC study some patients entered the cohort at an admission to the hospital, while others entered the cohort in stable state in a teaching hospital outpatient clinic. These patients will have more severe COPD than the population seen by general practitioners. Also within our cohort there is a difference in overall mortality rates between patients who provided a blood sample in stable state versus those who provided a sample at a hospitalization for an AECOPD, with, respectively, 26% and 54%. However, within these two distinct groups, those with the higher levels of MR-proADM again had the higher risk of dying. The median value of MR-proADM in the AECOPD group of patients was higher than in our previous group. This can be explained by the higher mortality rate in this group. In the previous study the patients had to be able to give also a blood sample in stable state, this will possibly be a group of patients with less severe COPD.
The current validation data suggest that a higher cut-off value (0.75 nmol/l), especially when measured in a stable period of the disease, is a better predictor for mortality than the 0.71 nmol/l we had previously found. As shown in and , increasing the cut-off value results in worse sensitivity and thus worse negative predictive value, while it increases specificity, and thus provides better positive predictive values. The choice of the cut-off point therefore depends on the intention of use. For example, when MR-proADM is used to select patients that may benefit from a more intensive treatment based on the high mortality risk, a higher sensitivity would be preferred. Although also some patients with a lower mortality risk will receive this intensive treatment, there will be no undertreatment. But when MR-proADM would be used to select patients who are considered for palliative care, we would like to have a cut-off with a higher specificity since we do not want to start palliative care in patients with low mortality risk.
Figure 4. (a) ROC curve at stable state for one-year survival. Cut-off values in nmol/l marked. (b) ROC curve at stable state for two-year survival. Cut-off values in nmol/l marked.
In this study, we also applied the cut-off value as observed in the PROMISE-COPD study by Stolz et al. Citation(13). It is surprising that their cut-off performed better in our validation cohort than our own derived cut-off value from our training data set, because the mortality rate in the COMIC study is substantially higher than the mortality rate in the PROMISE-COPD study. Overall mortality during follow-up was 20.3% (N = 261), of which respectively 205 (30.6%) in the COMIC and 56 (9.1%) in the PROMISE-COPD study Citation(33). The fact that the higher cut-off level in a low mortality population, nevertheless, is a good predictor for mortality in a high mortality population might mean that this cut-off value of 0.75 nmol/l might be used for a large range of patients with COPD. Perhaps even higher cut-off levels, e.g. using the 75th percentile instead of the median, might also be a useful cut-off value. This should be investigated in a larger cohort because enough statistical power is needed when using the 75th percentile instead of the median. Additionally, as always, the optimal cut-off depends on the intended use.
With this study, the prognostic value of MR-proADM has been firmly established and further research may lead to useful clinical applications. Schuetz et al. Citation(14) have proposed several areas for further studies including longitudinal measurement of MR-proADM, and risk stratification in COPD patients based on MR-proADM levels alone or added to existing score systems levels like it has been done by Albrich et al. Citation(34) in patients with low respiratory tract infections to shorten length of stay.
Since it has been shown that by adding MR-proADM to existing risk prediction systems (Citation33, 35) the accuracy of mortality prediction increases, it would be interesting to investigate whether or not adding other biomarkers to these models or to MR-proADM alone improves mortality prediction even more. Also adding MR-proADM to the GOLD standard classification may be valuable.
Funding
The authors thank the Research Department of Thermo scientific biomarkers [formerly B.R.A.H.M.S. AG], Hennigsdorf, Germany for supplying the MR-proADM kits, financial support for the determination of the biomarkers and the use of the KRYPTOR®. An unrestricted research grant of Glaxo Smith Kline was obtained for the COMIC cohort. Thermofisher supported this study by providing the Kryptor and the MR-proADM kits.
Authors' contributions
M.C. Zuur-Telgen: contributed to the study design, laboratory work, statistical analysis, and writing of the manuscript. Dr. M.G.J. Brusse-Keizer: contributed to the study design, statistical analysis, and writing of the manuscript. Dr. P.D.L.P.M. van der Valk: contributed to the study design and writing of the manuscript. Prof. dr. van der Palen: contributed to the study design, statistical analysis, and writing of the manuscript. Prof. dr. H.A.M. Kerstjens: contributed to the study design and writing of the manuscript.
Declaration of interest
The authors report no conflicts of interest.
Notation of prior abstract publication/presentation
The abstract is accepted for poster presentation at the ERS 2014 in Munich, Germany.
References
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997; 349(9063):1436–1442.
- Sapey E, Stockley RA. COPD exacerbations. 2: aetiology. Thorax 2006; 61(3):250–258.
- Anthonisen NR, Wright EC, Hodgkin JE. Prognosis in chronic obstructive pulmonary disease. Am Rev Respir Dis 1986; 133(1):14–20.
- Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr 2005; 82(1):53–59.
- Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002; 121(5):1434–1440.
- Cote CG, Casanova C, Marin JM, Lopez MV, Pinto-Plata V, de Oca MM, et al. Validation and comparison of reference equations for the 6-min walk distance test. Eur Respir J 2008; 31(3):571–578.
- Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T. Analysis of the factors related to mortality in chronic obstructive pulmonary disease: role of exercise capacity and health status. Am J Respir Crit Care Med 2003; 167(4):544–549.
- Soler-Cataluna JJ, Martinez-Garcia MA, Roman SP, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60(11):925–931.
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de OM, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(10):1005–1012.
- Puhan MA, Garcia-Aymerich J, Frey M, ter RG, Anto JM, Agusti AG, et al. Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet 2009; 374(9691):704–711.
- Stolz D, Christ-Crain M, Morgenthaler NG, Miedinger D, Leuppi J, Muller C, et al. Plasma pro-adrenomedullin but not plasma pro-endothelin predicts survival in exacerbations of COPD. Chest 2008; 134(2):263–272.
- Zuur-Telgen MC, Brusse-Keizer MG, Vandervalk PD, van der Palen J, Kerstjens HA, Hendrix MG. Stable state MR-proadrenomedullin level is a strong predictor for mortality in COPD patients. Chest 2013.
- Stolz D, Kostikas K, Blasi F, Boersma W, Milenkovic B, Lacoma A, et al. Adrenomedullin refines mortality prediction by the BODE index in COPD: the “BODE-A” index. Eur Respir J 2014; 43(2):397–408.
- Schuetz P, Marlowe RJ, Mueller B. The prognostic blood biomarker proadrenomedullin for outcome prediction in patients with chronic obstructive pulmonary disease (COPD): a qualitative clinical review. Clin Chem Lab Med 2015; 53(4):521–539.
- Linscheid P, Seboek D, Zulewski H, Keller U, Muller B. Autocrine/paracrine role of inflammation-mediated calcitonin gene-related peptide and adrenomedullin expression in human adipose tissue. Endocrinology 2005; 146(6):2699–2708.
- Yoshibayashi M, Kamiya T, Kitamura K, Saito Y, Kangawa K, Nishikimi T, et al. Plasma levels of adrenomedullin in primary and secondary pulmonary hypertension in patients <20 years of age. Am J Cardiol 1997; 79(11):1556–1558.
- Cheung BM, Hwang IS, Li CY, WS O, Tsang KW, Leung RY, et al. Increased adrenomedullin expression in lungs in endotoxaemia. J Endocrinol 2004; 181(2):339–345.
- Ichiki Y, Kitamura K, Kangawa K, Kawamoto M, Matsuo H, Eto T. Distribution and characterization of immunoreactive adrenomedullin in human tissue and plasma. FEBS Lett 1994; 338(1):6–10.
- Struck J, Tao C, Morgenthaler NG, Bergmann A. Identification of an Adrenomedullin precursor fragment in plasma of sepsis patients. Peptides 2004; 25(8):1369–1372.
- Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem 2005; 51(10):1823–1829.
- Zuur-Telgen MC, Brusse-Keizer MG, van der Palen J, Vandervalk PD, Kerstjens HA, Hendrix MG. Response. Chest 2014; 146(2):e65–e66.
- Khorfan R. Usefulness of midrange-proadrenomedullin as a predictor of mortality in patients with COPD. Chest 2014; 146(2):e65.
- Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2011. Available from: http://www.goldcopd.org/. 2011.
- Telgen MC, Brusse-Keizer MG, van der Valk P, van der Palen J, Kerstjens HA, Hendrix MG. Impact on clinical decision making of quality control standards applied to sputum analysis in COPD. Respir Med 2011; 105(3):371–376.
- Jougasaki M, Burnett JC, Jr. Adrenomedullin: potential in physiology and pathophysiology. Life Sci 2000; 66(10):855–872.
- vonHaehling S., Filippatos GS, Papassotiriou J, Cicoira M, Jankowska EA, Doehner W, et al. Mid-regional pro-adrenomedullin as a novel predictor of mortality in patients with chronic heart failure. Eur J Heart Fail 2010; 12(5):484–491.
- Behnes M, Papassotiriou J, Walter T, Fiedler E, Sauer T, Lang S, et al. Long-term prognostic value of mid-regional pro-adrenomedullin and C-terminal pro-endothelin-1 in patients with acute myocardial infarction. Clin Chem Lab Med 2008; 46(2):204–211.
- Khan SQ, O'Brien RJ, Struck J, Quinn P, Morgenthaler N, Squire I, et al. Prognostic value of midregional pro-adrenomedullin in patients with acute myocardial infarction: the LAMP (Leicester Acute Myocardial Infarction Peptide) study. J Am Coll Cardiol 2007; 49(14):1525–1532.
- Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93(3):580–586.
- Koehorst-Ter HK, Kort S, van der Palen J, van Beurden WJ, Movig KL, van d V, et al. Quality of life and adherence to inhaled corticosteroids and tiotropium in COPD are related. Int J Chron Obstruct Pulmon Dis 2016; 11:1679–1688.
- Bello S, Lasierra AB, Minchole E, Fandos S, Ruiz MA, Vera E, et al. Prognostic power of proadrenomedullin in community-acquired pneumonia is independent of aetiology. Eur Respir J 2012; 39(5):1144–1155.
- Nam B-H, D'Agostino R the area under the ROC curve. In Huber-Carol C, Balakrishnan N, Nikulin MS, Mesbah M, eds. Goodness-of-fit tests and model validity. Boston: Birkhäuser, 2002; 273–277.
- Brusse-Keizer M, Zuur-Telgen M, van der Palen J, VanderValk P, Kerstjens H, Boersma W, et al. Adrenomedullin optimises mortality prediction in COPD patients. Respir Med 2015; 109(6):734–742.
- Albrich WC, Ruegger K, Dusemund F, Schuetz P, Arici B, Litke A, et al. Biomarker-enhanced triage in respiratory infections: a proof-of-concept feasibility trial. Eur Respir J 2013; 42(4):1064–1075.
- Grolimund E, Kutz A, Marlowe RJ, Vogeli A, Alan M, Christ-Crain M, et al. Long-term prognosis in COPD exacerbation: role of biomarkers, clinical variables and exacerbation type. COPD 2015; 12(3):295–305.