ABSTRACT
Inhaled corticosteroid (ICS) use in chronic obstructive pulmonary disease (COPD) patients is associated with a reduction of exacerbations and a potential risk of pneumonia. The objective was to determine if ICS use, with or without long-acting β2-agonist, increases pneumonia risk in COPD patients. A cohort study was performed using linked hospital and drug prescription databases in the Lazio region. Patients (45+) discharged with COPD in 2006–2009 were enrolled and followed from cohort entry until first admission for pneumonia, death or study end, 31 December, 2012. A nested case-control approach was used to estimate the rate ratio (RR) associated with current or past use of ICS adjusted for age, gender, number of exacerbations in the previous year and co-morbidities. Current users were defined as patients with their last ICS prescribed in the 60 days prior to the event. Past users were those with the last prescription between 61 and 365 days before the event. Current use was classified into three levels (high, medium, low) according to the medication possession ratio. Among the cohort of 19288 patients, 3141 had an event of pneumonia (incidence rate for current use 87/1000py, past use 32/1000py). After adjustment, patients with current use were 2.29 (95% confidence interval [CI]: 1.99–2.63) times more likely to be hospitalised for pneumonia with respect to no use; for past use RR was 1.23 (95% CI: 1.07–1.42). For older patients (80+), the rate was higher than that for younger patients. ICS use was associated with an excess risk of pneumonia. The effect was greatest for higher doses and in the very elderly.
Background
Inhaled corticosteroids (ICSs) are widely used in patients with asthma and chronic obstructive pulmonary disease (COPD) Citation(1,2). Their role in treatment of asthma is well established, whereas their usefulness in COPD is less clear, as it is characterised by a type of airway inflammation which is partly resistant to treatment with corticosteroids.
In COPD management, recent guidelines recommend adding ICS to long-acting β2-agonists (LABAs) or long-acting muscarinic antagonists (LAMAs) for patients with severe disease and/or frequent exacerbations Citation(3). It has been shown that ICS use reduces the overall frequency of exacerbations Citation(4–6) but the effects on airway inflammation are still controversial. The most important/frequent adverse drug reactions related to ICS are oropharyngeal candidiasis, dysphonia and increased risk of cataracts Citation(7–9).
Several studies have given evidence for an increased risk of pneumonia in COPD patients and particularly in patients receiving ICS Citation(6,10). However, there is the need for studies to determine subgroups of COPD patients particularly susceptible to harmful effects of ICS. As regard mortality after pneumonia and ICS use, the available evidence is controversial Citation(3,6,10–13).
The present study took advantage of the database established for the conduction of a large Italian multicenter study aimed to investigate the comparative effectiveness and safety of inhaled drugs in COPD, based on a cohort of more than sixty-thousand COPD patients, enrolled from electronic healthcare records (“the OUTPUL study”) Citation(14–16). As a part of this project here we present an analysis of the subgroup of patients residing in Lazio. The main objective of the present study was to measure whether ICS treatment, with or without LABA, is associated with an increased risk of community-acquired pneumonia among patients discharged after a COPD exacerbation. We tested the hypothesis that elderly people are more vulnerable to the risk of pneumonia when exposed to ICS. Finally, we measured the association of ICS and 30-day mortality among hospitalised COPD patients with pneumonia.
Methods
Data sources
Different health information systems were used to study the population of the Lazio region (over 5,700,000 residents). The Hospital Information System (HIS) routinely collects data from all regional hospitals, including patient demographic data, admission referral source, discharge status, discharge diagnoses, procedures and the regional code of the facility. Data relating to emergency room visits are available in the Healthcare Emergency Information System (HEIS), which comprises information from all regional hospitals. The Outpatient Specialist Service Information System (OSSIS) collects data from ambulatory services, visits and laboratory and diagnostic procedures. The Drug Claims Registry (PHARM) comprises individual records for each medical prescription that is dispensed from public and private pharmacies, and the date of dispensing. The registry is limited to drugs dispensed to outpatients, and reimbursed by the healthcare system. Drugs are identified by the national drug register code, which is related to the Anatomical Therapeutic Chemical (ATC) classification system. Mortality endpoints were retrieved from the Regional Mortality Information System (MIS), which uses the International Classification of Disease, Ninth Revision (ICD-9-CM codes) to code the cause of death. Record linkage procedures on the basis of a unique anonymous patient identifier allowed all the information to be integrated with the patient's socio-demographic characteristics, hospitalisation and date of death.
Study population
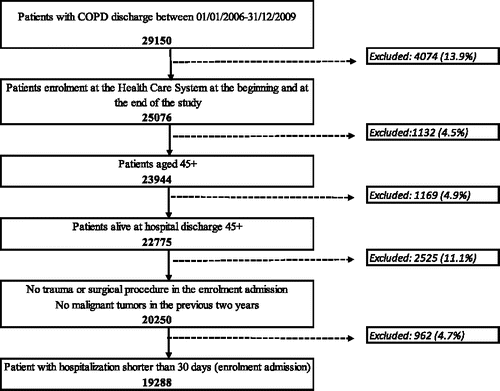
We conducted a nested case-control study in a prospective cohort of patients, resident in the Lazio region, discharged with COPD (moderate to severe cases) during the years 2006–2009. COPD patients were identified from HIS, according to the following inclusion criteria: main diagnosis of COPD or, alternatively, main diagnosis of COPD-related causes along with a secondary diagnosis of COPD (for details see Appendix 1). Discharge from the first hospitalisation which met selection criteria during the enrolment period was considered as the date of cohort entry. Exclusion criteria were: not being alive at discharge, secondary diagnosis of major trauma or major surgeries, cancer diagnoses in the 2 years preceding the index admission, hospitalisation longer than 30 days (95th percentile), more than 50% of follow-up spent in in-patient regimen, or not being registered in the regional healthcare system throughout the entire study period. We excluded patients with a primary or secondary diagnosis of asthma in the year before the index admission. Moreover we excluded those who were treated with oral corticosteroids within 90 days prior to the date of enrolment. All patients in the cohort were followed from cohort entry (date of enrolment) until first hospitalisation for pneumonia, death, maximum of 5 years of follow-up or end of the study (31 December, 2012) whichever came first.
Case and control definition
Following a validated algorithm Citation(17–19), pneumonia events were classified into three subtypes according to the timing and setting of onset: Healthcare-associated pneumonia (HCAP), Nosocomial pneumonia (NP) and Community-acquired pneumonia (CAP). HCAP category includes patients with pneumonia who were exposed to the healthcare system in terms of dialysis or chemotherapy in the last 30 days before the index episode of care, or patients who had an admission to an acute-care hospital for at least 2 days or had surgery within the past 180 days. NP refers to infections acquired during hospitalisation or alternatively, patients with hospitalisations for 2 or more days in the 10 days before admission for pneumonia. The third category, CAP, considered any other incident episode not included in the definition of NP or HCAP and defined on the basis of pneumonia codes (main or secondary diagnosis) registered in the first hospital admission or emergency department admission during follow-up. We limited the study to CAP cases only. The pneumonia event was defined as ‘index date’. (for details see Appendix 1).
Controls were chosen from the COPD cohort, selecting four COPD patients who did not experience the outcome at the date of the related case at index date, matching for 5-year age classes and length of individual follow-up. Thus, a control could have been identified as a case later in the follow-up.
Exposure
ICS comprised beclometasone, budesonide, flunisolide, betamethasone, fluticasone, triamcinolone, mometasone, ciclesonide, fluticasone furoate, salbutamol and sodium cromoglicate (ATC codes: R03BA; R03AK04 for associations with corticosteroids). ICS was dispensed alone or in combination with a LABA (ATC: R03AC12, R03AC13, R03AC18, R03AK06, R03AK07). We considered all prescriptions of study drugs between date of enrolment and index date. We defined ‘current users' as those patients with an ICS prescription within 60 days prior to the pneumonia event. In case the last prescription was dispensed between 60 and 365 days prior to the index date, the patient was defined as a ‘past user’. All patients without prescriptions of ICS in the year prior to the pneumonia event were defined as ‘no users'. For current users we defined three levels of doses according to their Medication Possession Ratio (MPR) that is the total number of treatment days divided by the follow-up time, which was classified as high (≥75%), medium (50–74%) and low (<50%).
Covariates
Covariates were retrieved at baseline, using information referring to the 365 days before the index date. Specifically, patients were characterised with respect to sex, age (controlled by design), co-morbidities (ischaemic heart disease, congestive heart failure, cerebrovascular disease, dementia, peripheral vascular disease, diabetes, anxiety, neurological disease, psychiatric disease, chronic kidney diseases, liver disease, hypertension and respiratory failure) and use of respiratory medications: adrenergics in combination with corticosteroids or other drugs (excluding anticholinergics), tiotropium, anticholinergics, LABA, xanthines, antibacterials for systemic use and glucocorticoids.
COPD severity was approximated by the number of COPD exacerbations in the year preceding the index date. As a secondary definition of severity, we classified severity into three levels (mild, moderate and severe) on the basis of drug prescriptions starting from enrolment until the first pneumonia episode. Categorisation into mild, moderate and severe COPD is done breaking down the individual follow-up period into 6-month intervals. Therefore early events are not associated with a shorter observation time. Mild patients were those not treated at all during the observation period, moderate patients were those with regular COPD treatment, and severe patients had also oxygen or nebulised therapy prescriptions Citation(20). Details on ICD-9-CM and ATC codes are reported in Appendix1.
Statistical analysis
Hospitalisation rates for pneumonia were calculated. Adjusted rate ratios (RRs) of pneumonia for past, current and no users were estimated with 95% confidence intervals (CIs) by conditional logistic regression. For current users, analyses were conducted according to three levels of doses. After testing a potential interaction between ICS and age classes, the analysis was stratified by age (45–64, 65–79, ≥80). Statistical significance was set at p < 0.05 for main effects and p < 0.10 for interactions. As a secondary objective, we investigated mortality for all causes after pneumonia. In this case we restricted the analyses to pneumonia cases. Adjusted odds ratios (ORs) of 30-day mortality separately for current, past and no users were estimated with 95% CIs through logistic regression.
Several sensitivity analyses were performed. In the first analysis we defined ‘current users' as patients with ICS use in the past 90 days, rather than 60 days as in the main analysis. In the second analysis, pneumonia cases were identified through hospital diagnoses only (HIS), whereas emergency room visits (HEIS) were not taken into account. In the third analysis, we refined the definition of severity using also drug prescription data. Finally, we identified patients if they were users of ICS only or ICS and LABA within 60 days from index date.
Results
This study included 19288 patients with COPD (). At enrolment admission, 45% were men and aged 75.5 (±9.9) years. In the main analysis maximum follow-up was 5 years. Mean duration of follow-up was 1.3 (±1.3) years. During follow-up 3141 patients were hospitalised for pneumonia (cases), corresponding to a pneumonia rate of 4.7 per 100 person-years.
shows the demographic and clinical characteristics of pneumonia cases and their controls. There were more men among controls and they had a lower number of hospitalisations for COPD in the year before index date. According to the secondary definition of severity almost half of the cases were classified as mild, others were equally distributed between ‘moderate’ and ‘severe’. Controls were mainly mild patients (75%). Cases had more co-morbidities than controls, in particular, with respect to respiratory failure (11.1% vs 7.5%) and hypertension (11.0% vs 8.1%). A similar difference was observed for previous use of respiratory medications, with 46% of cases with prescriptions for tiotropium compared to 36% of the control patients.
Table 1. Characteristics of pneumonia cases and their matched controls among COPD patients.
shows crude and adjusted rates for pneumonia cases associated with current and past use of ICS. After adjustment, current use was associated with a more than twofold pneumonia risk (RR = 2.29, 95%CI 1.99–2.63) with respect to no use. Past use was associated to a risk increase of 23% (RR = 1.23, 95%CI 1.07–1.42). In current users, a dose-related trend was tested, with high doses being associated to an almost twofold risk.
Table 2. Crude and adjusted rate ratios of pneumonia associated with past and current use of ICS among patients with COPD, and doses for current use.
The role of age as a potential effect modifier was investigated and stratified results are reported in . Risk rates increased by age group, both for current and for past use.
Table 3. Crude and adjusted rate ratios of pneumonia, by age, associated with past and current use of ICS among patients with COPD, and doses for current use.
All-cause mortality within 30 days of being discharged from hospital for pneumonia is reported in . A total of 487 deaths among the 3141 pneumonia patients were found (15.5%). Both current and past users had a lower risk of death than non-users, even if results were not statistically significant. Pneumonia mortality is equal to 4% of total mortality Citation(1).
Table 4. Crude and adjusted OR of 30-day mortality in hospitalised COPD patients with pneumonia associated with past and current use of ICS, and doses for current use.
In the first sensitivity analysis (Table S1-Supplementary material), we calculated crude and adjusted RRs of pneumonia, extending the definition of ‘current use’ from 60 to 90 days before the index date. RRs turned out to be a little lower but results were robust, except for past use, for which results lost statistical significance. The dose-related trend was significant (p < 0.05). In the second sensitivity analysis (Table S2-Supplementary material), we restricted pneumonia cases to those identified by HIS. After adjustment, current use of ICS was very similar to the main analysis (RR = 2.24, 95%CI 1.88–2.68). The flawing of the association between past use and pneumonia risk observed in the first sensitivity analysis was even stronger here and no association was detected (RR = 0.86, 95%CI 0.71–1.04). The results remained stable when applying a more complex severity definition (Table S3-Supplementary material). When trying to distinguish users of ICS plus LABA from those using ICS alone, we found only 5% using both and do not report results here.
Discussion
Inhaled corticosteroids reduce the frequency of exacerbations especially when combined with an inhaled LABA Citation(21–24). The present study shows that ICS treatment for current users is associated with a more than twofold risk of pneumonia in comparison to non-users. Also past exposure increases risk by 23% in comparison to no use. There is a dose-response relationship, with the highest ICS doses associated to the highest risk increase. Moreover, risk is higher in old ages. Mortality risk in hospitalised COPD with pneumonia is lower for users of ICS with respect to non-users. The association between ICS use and pneumonia was previously reported by several studies and our results confirm the findings of other observational studies which gave evidence of an excess risk of pneumonia in ICS users Citation(2,7–13,20,25–29).
The risk of pneumonia associated with ICS use was evidenced the first time in the large prospective Towards a Revolution in COPD Health (TORCH) study. This study compared the efficacy of fluticasone propionate, salmeterol or their combination (salmeterol/fluticasone 50/500 µg twice daily) versus placebo in COPD patients over a 3-year period. Patients receiving ICS, alone or in a combination with LABA, had a higher rate of pneumonia (19.6%) than the placebo group (12.3%) Citation(5).
In particular, Suissa reported an excess risk of 69% of serious pneumonia Citation(4). Joo found that patients with current use of ICS had an excess risk of 38% Citation(21). Ernst reported an adjusted RR of 1.70 for patients hospitalised for pneumonia, and the RR was greatest with the highest doses (RR = 2.25) Citation(8). The Inspire study comparing salmeterol plus fluticasone with tiotropium reported double the rate of pneumonia with salmeterol/fluticasone than with trotopium Citation(10). Kardos reported more pneumonia events in the ICS/LABA group compared to LABA alone. A large observational study in Canada found that elderly patients with COPD with a current ICS prescription were 70% more likely to be hospitalised with a primary diagnosis of pneumonia Citation(29). In a recent Cochrane meta-analysis on 43 studies, 26 with fluticasone and 17 with budesonide, both medications were found to increase risk of pneumonia Citation(22).
Recent studies have reported a stronger association between pneumonia for higher doses of ICS, suggesting a dose-response relationship Citation(29–32). Mullerova studied the association between ICS and CAP in 40414 COPD patients and showed an incidence rate of this subtype of pneumonia of 22.4 per 1000 person-years, underlining that age over 65 years was an important risk factor for incidence of CAP Citation(7,33).
The mechanism of the association of ICS therapy with pneumonia remains unclear. One potential explanation is that ICS therapy may increase pneumonia risk in patients with COPD by increasing local airway immunosuppression. This could diminish the ability of the innate immune system to defend against primary bacterial infections or post viral superinfection Citation(12). Consistent with this hypothesis, our results demonstrated that higher ICS doses are associated with increased pneumonia risk in a dose-dependent manner.
Also for mortality, our results are in line with previous studies Citation(34). In a cohort study, Malo de Molina reported a lower 30- and 90-day mortality for COPD patients hospitalised with pneumonia, using ICS Citation(3). Chen found an independent association with a decreased risk of short-term mortality after hospitalisation for pneumonia Citation(6). In the Cochrane meta-analysis mentioned above, no significant difference in overall mortality rates was observed between either fluticasone or budesonide Citation(22). One explanation maybe that ICS can increase risk of pneumonia, but decrease risk of mortality since they decrease the level of inflammation of pneumonia and, consequently, severity Citation(11).
There are several strengths in this study. We controlled estimates for COPD severity, accounting for the number of COPD-related hospitalisations in the year prior to the index date. Furthermore, we controlled for ICS doses, both considering that higher ICS doses might be a proxy of COPD severity and controlling for a potential relationship between ICS load and pneumonia risk. Moreover, in a distinct sensitivity analysis we constructed a measure based on COPD medication. Results were similar in the main and the sensitivity analyses. Second, this large observational study included patients who would not have been included in clinical trials. Among these patients also pneumonia risk is expected to be higher. Consequently, if ICS use is associated with pneumonia, this association might be mitigated in clinical trials. Third, the study population refers to medium-severe COPD patients, identified by hospital discharge records, which increases internal validity. Validity of information regarding severity was previously proven Citation(35,36). Fourth, we used a validated algorithm to identify CAP. Fifth, we controlled for potential bias of exposure misclassification by varying the exposure window of current user (60 or 90 days). Lastly, given the large dataset, we could analyse findings according to different age categories and could show higher risk in old age.
Limitations need to be discussed. In general, observational studies based on administrative data lack information relating to confounding factors such as clinical data, functional status and smoking history. The principal limitation is that our data did not comprise X-ray results for pneumonia definition. The identification of pneumonia in patients with COPD is imperfect and prone to misclassification. Clinical signs and symptoms associated with pneumonia are similar to COPD exacerbation. Moreover, diagnosis is very sensitive to the quality of ICD-9-CM coding. Second, we cannot exclude residual confounding although we controlled for respiratory drugs other than ICS in the year prior to the index date, and by prescriptions of oral corticosteroids in combination with LABA and antibiotics. Third, in our study we did not distinct between budesonide and fluticasone. The majority of studies reported such distinction that seems to show an excess of pneumonia among fluticasone users. Fourth, COPD severity was not defined by lung function, but on the basis of surrogate markers, including use of inhaler medications, exacerbation treatments and hospital admissions Citation(37,38). All these proxies have not been validated against the standard measure defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD), such as the Forced expiratory volume (FEV), and therefore their accuracy in establishing COPD severity is unknown.
In conclusion, current and past use of ICS, with or without LABA, is associated with an increased risk of CAP, especially for current use and for patients treated with high doses. No association was detected between ICS use and mortality in COPD patients with pneumonia. Although not novel, our study adds to the knowledge on the topic using a population-based study design which is not commonly used. Our study highlights the importance for clinicians to balance the beneficial effects of ICS therapy with the evidence of harmful effects in terms of pneumonia, especially among very old patients.
icop_a_1254172_sm5536.docx
Download MS Word (31.1 KB)Acknowledgments
The authors are very grateful to Roberta Macci and Sandra Magliolo for their support in finding the cited articles.
Declaration of interest
The author reports no conflicts of interest in this work.
Funding
The project was partially funded by the Ministry of Health - Agenzia Italiana del Farmaco (AIFA); Prot. FARM8ZBT93; and the Italian Medicines Agency, grant number FARM87BT93.
Authors' contributions
NA and SC conceived the idea of the study and together with RP were responsible for the design of the study. VB, LB and SC were responsible for the acquisition of the data. SC was responsible for undertaking the data analysis and produced the tables and graphs. NA, UK, LB, VB and MDM provided input into the data analysis. UK, RP, MD, UK, GF and MDM contributed to the interpretation of the results. The manuscript was drafted by SC, NA and UK and then shared with all authors for critical revision.
References
- Suissa S. Statistical treatment of exacerbations in therapeutic trials of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 173(8):842–846.
- Calverley P, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. for the TORCH investigators. Statistical treatment of exacerbations in therapeutic trials of chronic obstructive pulmonary disease. N Engl J Med 2007; 356:775–789.
- Malo de Molina R, Mortensen EM, Restrepo MI, Copeland LA, Pugh MJ, Anzueto A. Inhaled corticosteroid use is associated with lower mortality for patients with COPD and hospitalized with pneumonia. Eur Respir J 2010; 36(4):751–757.
- Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 2013; 68:1029–1036.
- Crim C, Calverley P.M.A, Anderson J.A, Celli B, Ferguson G.T, Jenkins C, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J 2009; 34:641–647.
- Chen D, Restrepo M.I, Fine M.J, Pugh M.J.V, Anzueto A, Metersky M.L, et al. Observational study of inhaled corticosteroids on outcomes for COPD patients with pneumonia. Am J Respir Crit Care Med 2011; 184:312–316.
- Sibila O, Anzueto A, Restrepo M.I. The paradoxical effect on pneumonia of chronic inhaled corticosteroids. Clin Pulm Med 2013; 20(1):6–10.
- Ernst P, Gonzales A.V, Brassard P, Suissa S. Inhaled Corticosteroid Use in Chronic Obstructive Pulmonary Disease and the Risk of Hospitalization for Pneumonia. AM J Respir Crit Care Med 2007; 176:162–166.
- Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med 2010; 123:1001–1006.
- Wedzicha J, Calverley P, Seemungal T, Hagan G, Ansari Z, Stockley R, for the INSPIRE Investigators. The Prevention of Chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med 2008; 177:19–26.
- Iannella H, Luna C, Waterer G. Inhaled corticosteroids and the increased risk of pneumonia: what's new? A 2015 updated review. Ther Adv Respir Dis 2016; 10(3):235–255. pii: 1753465816630208.
- Drummond MB, Dasenbrook EC, Marshall WP, Murphy DJ, Fann E. Inhaled corticosteroids in patients with stable chronic obstructive pulmonary disease. A systematic review and meta-analysis. JAMA 2008; 300(20):2407–2416.
- Janson C, Larsson K, Stallberg B, Stratelis G, Goike H, Jorgensen L, et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting β2 agonist: observational matched cohort study (PATHOS). BMJ 2013; 346:f3306.
- Di Martino M, Agabiti N, Bauleo L, Kirchmayer U, Cascini S, Pistelli R, et al. Use patterns of long-acting bronchodilators in routine COPD care: the OUTPUL study. COPD 2014; 11(4):414–423.
- Kirchmayer U, Cascini S, Agabiti N, Di Martino M, Bauleo L, Formoso G, et al. One-year mortality associated with COPD treatment: a comparison of tiotropium and long-acting beta2-agonists in three Italian regions: results from the OUTPUL study. Pharmacoepidemiol Drug Saf 2016; 25(5):578–589. doi: 10.1002/pds.3961.
- Di Martino M, Agabiti N, Cascini S, Kirchmayer U, Bauleo L, Fusco D, et al. OUTPULStudy Group. The effect on total mortality of adding inhaled corticosteroids to long-acting bronchodilators for COPD: A real practice analysis in Italy. COPD 2016; 13(3):293–302. doi: 10.3109/15412555.2015.1044861.
- Cascini S, Agabiti N, Antonelli Incalzi R, Pinnarelli L, Mayer F, Arcà M, et al. Pneumonia burden in elderly patients: a classification algorithm using administrative data. BMC Infect Dis 2013; 13:559.
- Venditti M, Falcone M, Corrao S, Licata G, Serra P, the Study Group of the Italian Society Of Internal Medicine. Outcomes of patients hospitalized with community-acquired, health care-associated, and hospital-acquired pneumonia. Ann Intern Med 2009; 150:19–26.
- Giorgi Rossi P, Agabiti N, Faustini A, Ancona C, Tancioni V, Forastiere F, et al. The burden of hospitalised pneumonia in Lazio, Italy. 1997–1999. Int J Tuberc Lung Dis 2004; 8(5):528–536.
- Müllerova H, Chigbo C, Hagan GW, Woodhead MA, Miravitlles M, Davis KJ, et al. The natural history of community acquired pneumonia in COPD patients:a population database analysis. Respir Med 2012; 106(8):1124–1133. doi:10.1016/j.rmed.2012.04.008.
- Joo MJ, Au DH, Fitzgibbon ML, Lee TA. Inhaled corticosteroids and risk of pneumonia in newly diagnosed COPD. Respir Med 2010; 104:246–252.
- Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014; 10(3):CD010115. doi: 10.1002/14651858.CD010115.pub2.
- Singanayagam A, Chalmers JD, Hill AT. Inhaled corticosteroids and risk of pneumonia: evidence for and against the proposed association. QJM 2010; 103(6):379–385. doi: 10.1093/qjmed/hcq023.
- Festic E, Scanlon PD. Incident pneumonia and mortality in patients with chronic obstructive pulmonary disease. A double effect of inhaled corticosteroids? Am J Respir Crit Care Med 2015; 191(2):141–148. doi: 10.1164/rccm.201409-1654PP.
- Marzoratti L, Iannella HA, Waterer GW. Inhaled corticosteroids and the increased risk of pneumonia. Ther Adv Respir Dis 2013; 7(4):225–234. doi: 10.1177/1753465813480550.
- Mapel D, Schum M, Yood M, Brown J, Miller D, Davis K. Pneumonia among COPD patients using inhaled corticosteroids and long-acting bronchodilators. Primary Care Respir J 2010; 19(2):109–117.
- Singh S, Amin VA, Loke YK. Long-term use of inhaled corticosteroids and the risk of pneumonia in chronic obstructive pulmonary disease. Arch Intern Med 2009; 169(3):219–229.
- Restrepo M.I, Mortensen E.A, Anzueto A. Are COPD patients with pneumonia who are taking inhaled corticosteroids at higher risk of dying? Eur Respir J 2011; 38:1–3.
- Kardos P, Wencker M, Glaab T, Vogelmeier C. Impact of Salmeterol/Fluticasone propionate versus salmeterol on exacerbations in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007; 175:144–149.
- Di Santostefano R, Sampson T, Le HV, Hinds D, Davis KJ, Bakerly ND. Risk of pneumonia with inhaled corticosteroid versus long-acting bronchodilator regimens in chronic obstructive pulmonary disease: A new-user cohort study. PLoS ONE 2014; 9(5):e97149. doi:10.1371/journal.pone.0097149. eCollection 2014.
- Yawn BP, Li Yunfeng, Tian H, Zhang J, Arcona S, Kahler KH. Inhaled corticosteroid use in patients with chronic obstructive pulmonary disease and the risk of pneumonia: a retrospective claims data analysis. Int J COPD 2013; 8:295–304.
- Anzueto A, Ferguson GT, Feldman G, Chinsky K, Seibert A, Emmett A, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD 2009; 6(5):320–329.
- Mullerova H, Chigbo C, Hagan GW, Woodhead MA, Miravitlles M, Kourtney JD, Wedzicha JA. The natural history of community-acquired pneumonia in COPD patients: A population database Analysis. Respir Med 2012; 106:1124–1133.
- Liapikou A, Toumbis M, Torres A. Managing the safety of inhaled corticosteroids in COP and the risk of pneumonia. Exp Opin Drug Saf 2015; 14(8):1237–1247.
- Fano V, D'Ovidio M, del Zio K. The role of the quality of hospital discharge records on the comparative evaluation of outcomes: the example of chronic obstructive pulmonary disease (COPD). Epidemiol Prev 2012; 36(3–4):172–179.
- Bauleo L, Agabiti N, Kirchmayer U, Piras G, Cascini S, Di Martino M, et al. Accuracy of COPD diagnosis reported in hospital discharge registry:an epidemiological study in Rome. 38th Congress of Italian Epidemiological Association, Naples, 5–7 November 2014.
- Finney L, Berry M, Singanayagam A, Elkin SL, Johnston SL, Mallia P. Inhaled corticosteroids and pneumonia in chronic obstructive pulmonary disease. Lancet Respir Med 2014; 2(11):919–932. doi: 10.1016/S2213-2600(14)70169-9.
- Lee MC, Lee CH, Chien SC, Chang JH, She HL, Wang JY, et al. Inhaled Corticosteroids Increase the Risk of Pneumonia in Patients With Chronic Obstructive Pulmonary Disease: A Nationwide Cohort Study. Medicine (Baltimore) 2015; 94(42):e1723. doi: 10.1097/MD.0000000000001723.