ABSTRACT
Symptoms, clinical course, functional and biological data during an exacerbation of chronic obstructive pulmonary disease (EXCOPD) have been investigated, but data on physiological changes of respiratory mechanics during a severe exacerbation with respiratory acidosis requiring noninvasive mechanical ventilation (NIMV) are scant. The aim of this study was to evaluate changes of respiratory mechanics in COPD patients comparing data observed during EXCOPD with those observed during stable state in the recovery phase. In 18 COPD patients having severe EXCOPD requiring NIMV for global respiratory failure, we measured respiratory mechanics during both EXCOPD (T0) and once the patients achieved a stable state (T1). The diaphragm and inspiratory muscles effort was significantly increased under relapse, as well as the pressure-time product of the diaphragm and the inspiratory muscle (PTPdi and PTPes). The resistive loads to breathe (i.e., PEEPi,dyn, compliance and inspiratory resistances) were also markedly increased, while the maximal pressures generated by the diaphragm and the inspiratory muscles, together with forced expired volumes were decreased. All these indices statistically improved but with a great intrasubject variability in stable condition. Moreover, tension-time index (TTdi) significantly improved from the EXCOPD state to the condition of clinical stability (0.156 ± 0.04 at T0 vs. 0.082 ± 0.02 at T1 p < 0.001). During an EXCOPD, the load/capacity of the respiratory pump is impaired, and although the patients exhibit a rapid shallow breathing pattern, this does not necessarily correlate with a TTdi ≥ 0.15. These changes are reverted once they recover from the EXCOPD, despite a large variability between patients.
Introduction
Acute hypercapnic respiratory failure can occur when the respiratory muscles cannot cope with the inspiratory workload Citation(1). An imbalance between the load and the capacity of the respiratory system has been shown to be the cause of diaphragmatic fatigue in long-term ventilator-dependent patients or in those who fail a trial of weaning from mechanical ventilation (MV) Citation(2–5), even if this has not been ever directly demonstrated using “gold standard” methods such as stimulation of phrenic nerve.
Indirect evidence indicates that an imbalance between the force and the capacity of the respiratory system may also be the cause of acute respiratory failure when the patients are requiring invasive or NIMV to sustain their breathe, but this has never been demonstrated in “real life” experimental settings Citation(5).
For example, Purro et al. Citation(6) found that an increased load and respiratory muscle weakness were present in the early phases of an EXCOPD, together with a rapid shallow breathing pattern. However, no comparison could be made with the data obtained in the same patient, far from the phase of instability.
Therefore, the aim of the study was to verify, for the first time, whether the load/capacity balance during a severe acute exacerbation of COPD requiring noninvasive ventilation could be reversed in the “recovery” period.
For this reason, in this selected group of patients, we performed a comparison of pulmonary mechanics, respiratory muscle strength and effort both during an episode of exacerbation and some weeks later, when the same patients recovered from it.
Methods
Patients
This was a prospective physiological study carried out over a 36-month period on consecutive COPD patients having Acute Hypercapnic Respiratory Failure (AHRF) due to EXCOPD and admitted to 2 respiratory units (the Rehabilitation Units at the Institute of Pavia, Salvatore Maugeri Foundation and Respiratory and Critical Care Unit of Sant'Orsola Malpighi Hospital, Bologna) and requiring NIMV according to the following criteria: pH <7.35; PaCO2 >45 mm Hg; respiratory rate >25 breaths/min and signs of impending respiratory fatigue (rib cage-abdominal paradox and use of accessory respiratory muscles). All patients gave their informed consent to the study that was approved by the IRCCS Salvatore Maugeri Foundation Ethics Committee with the number 276 CEC. All patients had a known and established history of COPD lasting for at least 5 years and previously diagnosed according to the GOLD criteria Citation(7). Patients with cancer, congestive chronic heart failure (left ventricular ejection fraction < 40%), morbid obesity (body mass index > 35) or with previous domiciliary NIMV were excluded. EXCOPD was defined according to the Anthonisen criteria Citation(8), and all patients included in the study received maximal medical treatment plus oxygen and NIMV until the achievement of clinical stabilization, which was defined as the ability to maintain normal blood gas without NIMV, a resting respiratory rate <24 breaths/min and absence of use of accessory respiratory muscles or rib cage-abdominal paradox. During the rest of hospital stay, patients underwent a program of clinical stabilization and rehabilitation, including physical exercise, nutritional assessment, optimization of medical therapy and educational programs.
Protocol
Respiratory mechanics were assessed twice: the first evaluation (T0) was done within 24 hours of the hospital admission and few hours after the starting of NIMV, when clinical condition allowed the recordings during a brief trial (15 minutes) of spontaneous breathing. The second evaluation (T1) was performed at least 5 weeks after the onset of the acute respiratory failure. A period of 5 weeks was considered sufficient to reach a phase of clinical stability, when the patients returned to, or very close to, the respiratory functional status prior the EXCOPD, with full regular chronic inhalation treatment, including the previous long-term oxygen therapy and without the use of systemic steroids. If the patients were still hospitalised for a rehabilitation period, they performed the second evaluation before the discharge, otherwise, they were asked to come back for the second evaluation as outpatient.
All data were recorded at the patient's bedside, and 15-minute measurements were performed in one single session. Pulmonary function tests were performed at T0, as soon as the patient was able to perform the manoeuvre, and at T1.
Measurements
Respiratory function
Forced expiratory volume in the first second (FEV1) and forced vital capacity (FVC) were measured with a portable spirometer (PONY class 1 type B, Cosmed, Rome, Italy) during the interval-free of NIMV. The best values (expressed in ml) of three consecutive measurements were chosen for analysis.
Blood gas analysis
Blood gas analysis was performed on a blood sample drawn from the radial artery and processed with an ABL 4 blood gas analyzer (Radiometer Medical ApS, Denmark).
Respiratory mechanics
The patients were studied in the semi-supine position and during spontaneous breathing. For the experimental procedure of this study, flow (V') and pressure at the airway opening (Pao) were measured by means of a heated pneumotachograph and a differential pressure transducer (Honeywell, Freeport, IL, USA ± 300 cmH20). The pneumotachograph was inserted between the mouthpiece and a connector with a side port for Pao measurement. A nose-clip to avoid air leaks was applied during the manoeuvre to test the breathing pattern. Volume (VT) was obtained by numerical integration of the flow signal. Changes in oesophageal pressure (Ppl) and gastric pressure (Pga) were estimated by means of latex, 100 mm balloon-catheter (Microtek, Zutphen, The Netherlands) with the catheter connected to a differential pressure transducer Citation(9). The proper position of the oesophageal balloon-catheter was verified using the occlusion test Citation(10). The relative contribution of the rib cage and expiratory muscles to tidal breathing was assessed as the ratio of swings in Pga to swings in Pes (ΔPga/ΔPes). ΔPes was measured from the beginning of effort to its nadir. ΔPga was measured from the beginning of effort (also identified from the Pes tracing) to its maximum excursion Citation(11). Activation of expiratory muscles during expiration (Pga,exp) was assessed using the expiratory Pga rise measured as peak expiratory Pga from the lowest value reached after inspiration Citation(12). Flow (V') and airways pressure opening (Pao) were measured using a pneumotachograph connected to the mouth with a rigid mouthpiece and a pressure transducer placed at the mouth of the patient, respectively, Tidal volume (VT), respiratory frequency (f), and minute ventilation (V'E), were computed from the flow signal. Total cycle duration (TTOT), inspiratory time (TI), expiratory time (TE), mean inspiratory flow (VT/TI) and duty cycle (TI/TTOT) were calculated from the flow signal, as average values of 5-minute periods of continuous recording of flow and volume. Intrinsic dynamic positive end-expiratory pressure (PEEPi,dyn) was measured as the negative deflection in Pdi swing from the onset of inspiratory effort to the onset of inspiratory flow, as described by Appendini et al. Citation(4). Changes in the magnitude of the effort of the inspiratory muscles were estimated from changes in Pes swing, while the diaphragmatic effort was calculated using the Pdi trace.
The pressure time integrals of the diaphragm and the other inspiratory muscles were calculated per breath (PTPdi/b and PTPes/b, respectively) and per minute (PTPdi/min and PTPes/min) as the area under the curve from the beginning of effort to the end of the inspiratory flow Citation(4).
Maximal transdiaphragmatic pressure (Pdi max) was calculated by means of the maximal inspiratory effort generated with a maximal sniff manoeuvre during a verbal encouragement to breathe with the maximal strength Citation(13). The best of the three efforts was considered for the data analysis.
The load/capacity ratio of the diaphragm was calculated from the ratio between Pdi and Pdimax.
Transpulmonary pressure was used to calculate pulmonary resistance at mid inspiration (Rawe) according to Neergaard–Wirtz elastic subtraction technique Citation(14).
The reciprocal values of elastance were used as dynamic compliance (Cldyn). The tension-time index of the diaphragm (TTdi) was computed using Pdimax according to the method of Bellemare and Grassino: TTdi = Pdi/Pdi,max x Ti/Ttot. Citation(2). Mean inspiratory Pdi was also expressed as a fraction of Pdimax. A value of TTdi > 0.15 is expression of an imbalance between pressure output of the respiratory muscles and load sustained by the muscles themselves, causing the patient to be no longer able to maintain the imposed breathing pattern.
All the signals recorded during the measurement of respiratory mechanics were digitised by means of an analogue-to-digital converter with a 12-bit resolution (Data Translation 2801/A) and fed into a Pentium processor personal computer at a sampling frequency of 100 Hz.
Statistical analysis
Results are expressed as mean ± 1 standard deviation (SD). Differences between acute respiratory failure and stable phase were evaluated by the Student's paired t-test. A p-value <0.05 was considered as statistically significant. We created a new binary variable (“task failure exacerbator”) defined as 1 = patient with a TTI >0.15 during exacerbation; 0 = patient with a TTI ≤ 0.15 during exacerbation. Then, by stepwise logistic regression, we conducted an exploratory analysis to detect if baseline predictive variables were able to predict “task failure exacerbator.”
Results
A total of 18 patients were enrolled, and all of them were able to terminate the study. NIMV was applied using a bilevel ventilator (BiPAP Vision, Philips Respironics) with an oronasal mask chosen according to shape and size of the patient's face. Ventilator setting was targeted to decrease breathing rate and achieve a tidal volume of about 5–6 ml/kg. Total duration of NIMV used to treat the acute episode was 4.3 ± 3.6 days. Patients' demographic and anthropometric data (BMI) together with blood gas values, recorded at the time of acute respiratory failure and at achievement of the stable state, are reported in
Table 1. Demographic, anthropometric and blood gases values of study patients at the enrollment.
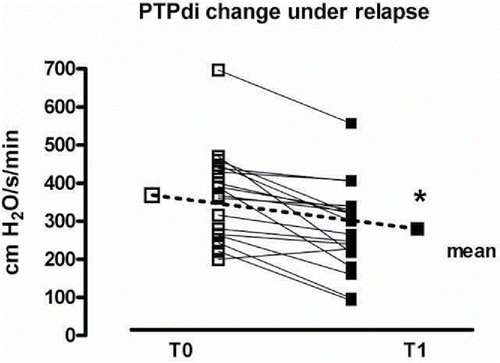
shows the changes in respiratory mechanics during the episode of acute respiratory failure and the phase of stability. The diaphragm and inspiratory muscles effort was significantly increased at T0, as well as the PTPdi and PTPes per minute and per breath. The resistive loads to breathe (i.e., PEEPi,dyn, compliance and inspiratory resistances) was also markedly increased, while the maximal pressures generated by the diaphragm and the inspiratory muscles was decreased. All these indices statistically improved in stable condition.
Table 2. Patients' respiratory mechanics during EXCOPD (T0) and stable conditions (T1).
shows the individual data improvement from T0 to T1 condition in diaphragmatic work of breathing (PTPdi).
illustrates the variation in breathing pattern and spirometry during the time course of the study. A rapid shallow breathing pattern was observed at T0, and this was reversed once a phase of clinical stability was reached. FEV1 did also significantly improve at T1.
Table 3. Patients' breathing pattern and dynamic lung volumes during EXCOPD (T0) and stable conditions (T1).
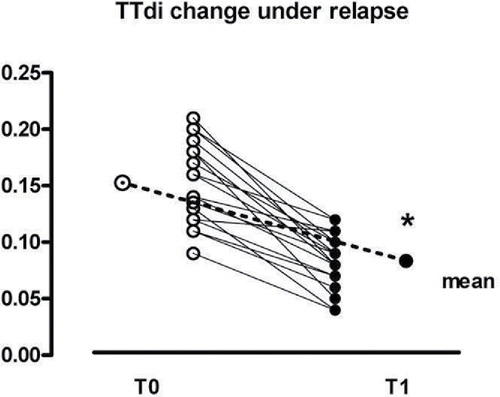
shows the individual data of the TTdi of all patients at T0 and at T1. TTdi significantly improved in all patients from the EXCOPD state to the condition of clinical stability (0.156 ± 0.04 at To vs. 0.082 ± 0.02 at T1 p < 0.001), but, as it can be seen, at T0, patients exhibited a non-univocal behaviour: half of them (n° = 9) showed a value >0.15, as expected, while the remaining 9 had a TTdi lower than 0.15. Using the stepwise logistic regression, we were unable to find any baseline variable related to these different behaviours.
Figure 2. TTdi change during exacerbation (T0) and stable state (T1). Continuous line indicates the threshold of 0.15; *p < 0.001.
Finally, ten of 18 patients (55%) experienced a paradoxical motion of the abdomen as detected by the abruptly negative deflection of Pga during the inspiration; however, the majority of them (18 patients) maintained the same kind of respiratory pattern also during the stable phase. Moreover, eleven of 18 patients (62%) showed an expiratory muscle activity that significantly decreased (or disappeared in three patients) in the stable phase (Pga,exp = 4.9 ± 2.7 vs. 1.4 ± 1.6, respectively, during T0 and T1, p < 0.01).
Discussion
In this study, we have shown for the first time that an episode of acute hypercapnic respiratory failure is associated with a significant change in pulmonary mechanics that is almost fully reversed in the recovery period. In particular, we found an increase in the inspiratory load, which induced an augmented respiratory muscle pressures (i.e., PTPdi and PTPes). This change, associated to a worsening of muscle strength, led to the capacity/load imbalance responsible for the acute respiratory failure. However, the most important and noteworthy result was that the TTdi threshold of 0.15 is not able to predict the occurrence of a rapid shallow breathing.
Moreover, in our study, no variables at enrolment were able to predict those patients who overpass or not the TTdi > 0.15 under exacerbation.
It has been already demonstrated that an imbalance between the force and the capacity of the respiratory system may be the cause of acute hypercapnic respiratory failure. Several investigations performed in intensive care unit have shown that the mechanisms responsible for acute respiratory failure during an acute exacerbation of COPD are related to a rapid shallow breathing pattern in the presence of an increased respiratory workload Citation(15–18). Only one investigation performed in patients with an acute exacerbation showed that a load/capacity imbalance, indirectly recorded noninvasively, was present in the early phases and that before discharge, most of the patients recovered functionally Citation(19).
However, this is the first study in which pulmonary mechanics between an acute and stable condition in the same patient was compared. In fact, we were able to expand our knowledge about the degree of worsening of respiratory mechanics during an acute exacerbation compared to the phase of clinical stability, when the patients were not acidotic and therefore not requiring any respiratory support.
Our study does not provide “real” evidence that the imbalance between respiratory muscle effort and load is the main mechanism leading to acute hypercapnic respiratory failure, but definitively shows that during an acute exacerbation, the patient undergoes a significant increase of respiratory load associated to a reduction of respiratory muscle strength and the ability to generate pressure, associated to a rapid shallow breathing.
What is noteworthy in our study is the non-unique behaviour of the TTdi of patients under exacerbation, particularly the fact that half of them presented a TTdi value <0.15, considered in the literature an index of “task failure”, even in the presence of a significant change in the respiratory pattern in a rapid shallow breathing.
The ability of respiratory muscles to sustain an increased inspiratory load critically depends on the respiratory duty and the mean transdiaphragmatic pressure per breath divided by maximum static transdiaphragmatic pressure Citation(3). It has been also shown that when an acute increase in inspiratory load causes the TTdi to exceed 0.15, the load cannot be sustained indefinitely, leading to the occurrence of rapid shallow breathing and hypercapnic respiratory failure Citation(3). For this reason, the finding of our study that a low TTdi during an acute exacerbation of COPD does not necessarily prevent the occurrence of rapid shallow breathing represents an original contribution to our knowledge in this field.
At the same time, the fact that no variable in stable phase is able to predict the achievement of a TTdi value > 0.15 suggests unexplored and most complex physiological reasons for the risk of “task failure.”
It is worthwhile to underline that the threshold of 0.15 usually used was actually derived from a study in normal subjects, and consequently, that a different threshold defines the “task failure” occurring in patients with chronic respiratory disease. This raises the possibility of adaptive strategies adopted by these patients to overcome the limitations of their pulmonary mechanics.
Although the TTdi has been considered an index of diaphragm fatigue, most of the studies used, as we did, experimental techniques that were not able to provide direct evidence of contractile fatigue, such as objective assessment of diaphragm contractility Citation(20). As a matter of fact, in a study performed in patients failing a weaning trial, using an objective technique to assess diaphragm function, like the phrenic nerve stimulation, the authors did not find any evidence of low-frequency fatigue, although many patients showed diaphragmatic weakness and an increased TTdi Citation(21). The same authors have recently shown in normal subjects that hypercapnia during acute loading primarily resulted from central-output inhibition of the diaphragm, suggesting that acute loading responses are controlled by the cortex rather than bulbopontine centres, and that contractile fatigue was an inconsistent finding Citation(22), questioning, at least in healthy humans, whether impaired respiratory muscle contractility per se is responsible for hypercapnia during acute loading.
One of the potential mechanisms protecting from low-frequency fatigue in the weaning failure patients was supposed to be the greater recruitment of rib cage and expiratory muscles, compared to weaning success patients Citation(23).
Actually, the novel result of our study was that in “real life”, the occurrence of inability to sustain a spontaneous breathing does not necessarily associate to the achievement of the threshold TTdi of 0.15 described by Bellemare and Grassino Citation(2,3). As a matter of fact, while a consistent number of patients (around 30% of the total) needed to be ventilated noninvasively, they were well below this threshold, and others were discharged in a phase of stability breathing very close or above this limit. The reason for this discrepancy is not clear, but it may be speculated that some COPD patients during an EXCOPD behave like those stable hypercapnic COPD patients that are unable to increase ventilation in conjunction with the increased respiratory workload and that become more hypercapnic and acidotic without overcoming the fatigue threshold Citation(24).
In our study, we did not observe any statistical difference in the ratio ΔPga/ΔPes between the acuity and stability phase. This apparent discrepancy may be well explained by the fact that in the previous study, an early reinstitution of MV was done in the weaning failure patients, not giving the time to diaphragm fatigue to develop. As a matter of fact, the same authors showed that during a fatiguing run, Pga markedly increased, while its contribution to tidal breathing was progressively decreased in the following hours, whereas Pes contribution was not affected by preceding duration of fatiguing loading Citation(25). It is therefore likely that our patients were enrolled into the study hours after the institution of NIMV, and this may have also reduced the contribution of Pga. Noteworthy, in our study, little or no contribution from Pgas to Pdi (both tidal and maximal) was evident. It was already shown that COPD patient during stable phase for a given fall in pleural pressure exhibits a smaller increase in abdominal pressure and less abdominal expansion compared to healthy subjects Citation(26). This behaviour is mainly due to a major contribution of rib cage and extra-thoracic accessory muscle to tidal breathing and/or to the expiratory muscles activity that may result in a paradoxical movement of the abdomen, as showed in two-third of our patients. As shown in our study, the abdominal paradox and the espiratory muscle activity, when present, were maintained also during the following stable phase in the majority of our patients, suggesting a poor correlation with changes in the respiratory mechanics and increased inspiratory load.
Interestingly enough, the changes in FVC and FEV1 at T1 paralleled closely what already described by Stevenson et al. Citation(27) in 22 patients recovering from an EXCOPD not requiring ventilatory support. They concluded that although respiratory system resistance and expiratory flow limitation were unchanged throughout, the changes in lung operating volumes were predominant after an acute exacerbation. In this study, minute ventilation and breathing pattern did not change, while this was not the case in the present investigation. While breathing frequency was comparable in the two studies, VT was much lower in our patients, and this may be well due to the different severities of this latter group, as shown by the values of PaCO2 at enrolment. As a matter of fact, a small VT reflects the impossibility of further increasing the pressure needed to generate higher VT, at the price of becoming severely hypercapnic.
Conversely, we were able to demonstrate a significant reduction in the resistive load (i.e., PEEPi,dyn and Raw) that may reflect on one side the amelioration of the rapid shallow breathing index, likely to minimise the effect of dynamic hyperinflation, mediated by the assistance of an additional respiratory muscle (i.e. the ventilator), and on the other side the effects of the bronchodilator therapy, together with rehabilitation.
While an imbalance between the capacity of the respiratory muscles to generate pressure and the mechanical load is a very likely contributory factor to the development of acute hypercapnic respiratory failure, other factors also must be taken into account and among these, the modulation of the respiratory drive. Unfortunately, the lack of a measure of P0.1 or respiratory muscle electromyography represents a limitation of our study.
As a conclusion, in our study, we assessed for the first time the respiratory mechanics modifications, during a severe acute exacerbation of COPD requiring NIMV compared to the stable condition, after the recovery from that. The most important result and original contribution of our study was that even if the TTdi was significantly increased during the acute phase, it did not reach the threshold of 0.15 in the half of our patients, suggesting that this threshold does not necessarily predict the occurrence of “task failure.”
Abbreviations
| PAW | = | airway pressure |
| ABG | = | arterial blood gases |
| ΔPga/ΔPes | = | DeltaPga/deltaPes |
| COPD | = | chronic obstructive pulmonary disease |
| EXCOPD | = | COPD exacerbation |
| Cdyn | = | dynamic compliance |
| Cldyn | = | dynamic lung compliance |
| Pes swing | = | esophageal swing |
| TE | = | expiratory time |
| Pga,rise | = | expiratory muscle activity |
| V' | = | flow |
| FEV1 | = | forced expiratory volume at first second |
| FVC | = | forced volume capacity |
| Rawe | = | inspiratory resistance |
| TI | = | inspiratory time |
| TI/TTOT | = | inspiratory time to total respiratory time ratio |
| PEEPi,dyn | = | intrinsic dynamic positive end-expiratory pressure |
| PEEPi | = | intrinsic positive end-expiratory pressure |
| Pes max | = | maximal esophageal pressure |
| MV | = | mechanical ventilation |
| V'E | = | minute ventilation |
| NIMV | = | noninvasive mechanical ventilation |
| Pes | = | oesophageal pressure |
| Ppl | = | pleural pressure |
| Pao | = | pressure at the airway opening |
| PTP | = | pressure time product |
| PTPes min | = | pressure time product of esophageal swing per minute |
| SpO2 | = | pulse oximetry |
| f | = | respiratory rate |
| SD | = | standard deviation |
| TTI | = | tension time index |
| VT | = | tidal volume |
| TTOT | = | total cycle duration |
| V | = | volume |
| WOB | = | work of breathing |
Declaration of interest
None of the authors have conflict of interest to declare.
References
- Cohen CA, Zagelbaum G, Gross D, Roussos C, Macklem PT. Clinical manifestations of inspiratory muscle fatigue. Am J Med 1982; 73(3):308–316.
- Bellemare F, Grassino A. Effect of pressure and timing of contraction on human diaphragm fatigue. J Appl Physiol 1982; 53:1190–1195.
- Bellemare F, Grassino A. Force reserve of the diaphragm in patients with chronic obstructive pulmonary disease. J Appl Physiol 1983; 55:8–15.
- Appendini L, Purro A, Patessio A, Zanaboni S, Carone M, Spada E, et al. Partitioning of inspiratory muscle work load and pressure assistance in ventilator-dependent patients with COPD. Am J Respir Crit Care Med 1996; 154:1301–1309.
- Vassilakopoulos T, Zakynthinos S, Roussos C. The tension-time index and the frequency/tidal volume ratio are the major pathophysiologic determinants of weaning failure and success. Am J Respir Crit Care Med 1998; 158(2):378–385.
- Purro A, Appendini L, Polillo C, Musso G, Taliano C, Mecca F, et al. Mechanical determinants of early acute ventilatory failure in COPD patients: a physiologic study. Intensive Care Med 2009; 35(4):639–647.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–365.
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding JK, Nelson NA. Antibiotic therapy in exacerbation of chronic obstructive pulmonary disease. Ann Intern Med 1987; 106(2):196–204.
- Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. PLUG Working Group (Acute Respiratory Failure Section of the European Society of Intensive Care Medicine). The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014; 189(5):520–531.
- Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the oesophageal balloon technique. Am Rev Respir Dis 1982; 126:788–791.
- Parthasarathy S, Jubran A, Laghi F, Tobin MJ. Sternomastoid, rib cage, and expiratory muscle activity during weaning failure. J Appl Physiol 2007; 103(1):140–147.
- Yan S, Sinderby C, Bielen P, Beck J, Comtois N, Sliwinski P. Expiratory muscle pressure and breathing mechanics in chronic obstructive pulmonary disease. Eur Respir J 2000; 16(4):684–690.
- Steier J, Kaul S, Seymour J, Jolley C, Rafferty G, Man W, et al. The value of multiple tests of respiratory muscle strength. Thorax 2007; 62(11):975–980.
- Tobin MJ, Van de Graaff WB. 1994. Monitoring of lung mechanics and work of breathing. In Tobin MJ, editor. Principles and Practice of Mechanical Ventilation, 1st ed. New York: McGraw-Hill, pp. 967–1004.
- Vitacca M, Porta R, Bianch L, Clini E, Ambrosino N. Differences in spontaneous breathing pattern and mechanics in patients with severe COPD recovering from acute exacerbation. Eur Respir J 1999; 13:365–370.
- Begin P, Grassino A. Inspiratory muscle dysfunction and chronic hypercapnia in chronic obstructive pulmonary disease. Am Rev Respir Dis 1991; 143:905–912.
- Rochester DF. Respiratory muscle weakness, pattern of breathing, and CO2 retention in chronic obstructive pulmonary disease. Am Rev Respir Dis 1991; 143:901–903.
- Aubier M, Murciano D, Fournier M, Milic-Emili J, Pariente R, Derenne JP. Central respiratory drive in acute respiratory failure of patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1980; 122(2):191–199.
- Gonzalez C, Servera E, Celli B, Diaz J, Marin J. A simple noninvasive pressure-time index at the mouth to measure respiratory load during acute exacerbation of COPD. A comparison with normal volunteers. Respir Med 2003 Apr; 97(4):415–420.
- Polkey MI, Kyroussis D, Hamnegard CH, Hughes PD, Rafferty GF, Moxham J, et al. Paired phrenic nerve stimuli for the detection of diaphragm fatigue in humans. Eur Respir J 1997; 10(8):1859–1864.
- Laghi F, Cattapan SE, Jubran A, Parthasarathy S, Warshawsky P, Choi YS, et al. Is weaning failure caused by low-frequency fatigue of the diaphragm? Am J Respir Crit Care Med 2003; 167(2):120–127.
- Laghi F, Shaikh HS, Morales D, Sinderby C, Jubran A, Tobin MJ. Diaphragmatic neuromechanical coupling and mechanisms of hypercapnia during inspiratory loading. Respir Physiol Neurobiol 2014; 198:32–41.
- Parthasarathy S, Jubran A, Laghi F, Tobin MJ. Sternomastoid, rib cage, and expiratory muscle activity during weaning failure. J Appl Physiol 2007; 103(1):140–147.
- Laghi F, Topeli A, Tobin MJ. Does resistive loading decrease diaphragmatic contractility before task failure? J Appl Physiol 1998; 85(3):1103–1112.
- Montes de Oca M, Celli BR. Respiratory muscle recruitment and exercise performance in eucapnic and hypercapnic severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2000; 161(3 Pt 1):880–885.
- Laghi F, and Tobin M. Disorders of respiratory muscles. State of art. Am J Respir Crit Care Med 2003; 168:10–48.
- Stevenson NJ, Walker PP, Costello RW, Calverley PMA. Lung mechanics and dyspnea during exacerbations of chronic obstructive pulmoinary disease. Am J Respir Crit Care Med 2005; 272:1510–1516.