ABSTRACT
The aim of this study was to identify a multivariate model to predict poor outcomes after admission for exacerbation of chronic obstructive pulmonary disease (COPD). We performed a multicenter, observational, prospective study. Patients admitted to hospital for COPD were followed up for 3 months. Relevant clinical variables at admission were selected. For each variable, the best cut-offs for the risk of poor outcome were identified using receiver operating characteristic (ROC) curves. Finally, a stepwise logistic regression model was performed. A total of 106 patients with a mean age of 71.1 (9.8) years were included. The mean maximum expiratory volume in the first second (FEV1)(%) was 45.2%, and the mean COPD assessment test (CAT) score at admission was 24.8 (7.1). At 3 months, 39 (36.8%) patients demonstrated poor outcomes: death (2.8%), readmission (20.8%) or new exacerbation (13.2%). Variables included in the logistic model were: previous hospital admission, FEV1 < 45%, Charlson ≥ 3, hemoglobin (Hb)<13 g/L, PCO2 ≥ 46 mmHg, fibrinogen ≥ 554 g/L, C-reactive protein (CRP)≥45 mg/L, leukocyte count < 9810 × 109/L, purulent sputum, long-term oxygen therapy (LTOT) and CAT ≥ 31 at admission. The final model showed that Hb < 13 g/L (OR = 2.46, 95%CI 1.09–6.36), CRP ≥ 45 mg/L (OR = 2.91, 95%CI: 1.11–7.49) and LTOT (3.07, 95%CI: 1.07–8.82) increased the probability of poor outcome up to 82.4%. Adding a CAT ≥ 31 at admission increased the probability to 91.6% (AUC = 0.75; p = 0.001). Up to 36.8% of COPD patients had a poor outcome within 3 months after hospital discharge, with low hemoglobin and high CRP levels being the risk factors for poor outcome. A high CAT at admission increased the predictive value of the model.
KEYWORDS:
Introduction
Chronic obstructive pulmonary disease (COPD) is an important cause of morbimortality throughout the world with high health-related costs Citation(1). The EPISCAN study estimated that more than two million inhabitants between 40 and 80 years old have COPD in Spain, being the cause of 18,000 deaths annually Citation(2). Its impact is largely due to exacerbations, which represent more than 70% of the health care costs incurred by this disease, particularly due to visits to emergency departments and hospitalization Citation(3). Patients with frequent exacerbations present a more rapid decrease in maximum expiratory volume in the first second (FEV1) Citation(4), a worse health status Citation(5) and have a shorter survival Citation(6).
Therefore, characterization of the risk factors to predict the outcome of an exacerbation would help to identify which patients require more intensive management in order to reduce the morbimortality and the socioeconomic impact of COPD exacerbations Citation(7).
Previous studies have shown that factors such as hypercapnia, the presence of pulmonary hypertension, the number of previous exacerbations, a low FEV1 and even a low level of physical activity (Citation8–12) are related to a greater risk of readmission after an exacerbation of COPD. However, the predictive factors identified differ according to the population included [outpatients, hospitalized or intensive care unit (ICU) patients] and the different endpoints of poor outcomes studied.
The aim of the present study was to identify variables in patients admitted to hospital for an exacerbation of COPD, which may help to predict the outcomes of these patients after hospital discharge.
Methods
Study design and population
We performed a multicenter, observational, prospective study in patients over 40 years of age admitted to 11 Spanish hospitals throughout the whole country for an exacerbation of COPD, with the aim to describe the clinical outcomes of patients admitted for an exacerbation of COPD and associated prognostic factors Citation(13). In the current analysis we aimed to identify predictor variables of poor prognosis at 3 months of follow-up. The inclusion criteria were: a) diagnosis of COPD demonstrated by spirometry in the stable phase with a post-bronchodilator FEV1/FVC < 0.7, b) history of smoking (present or past) of at least 10 pack-years, and c) severe exacerbation defined by an increase in respiratory symptoms requiring hospitalization. The exclusion criteria were: (i) patients with bronchial asthma or other chronic respiratory diseases; (ii) patients with an exacerbation due to pneumonia, pneumothorax or cardiac insufficiency; (iii) patients requiring admission to the ICU and/or non-invasive mechanical ventilation; (iv) patients who, according to the criteria of the investigator, did not present sufficient clinical or cognitive capacity to fill out the questionnaire; and (v) patients participating in another clinical trial.
The study was carried out according to the principles of the Declaration of Helsinki and the prevailing norms for performing investigation in humans. Data confidentiality was ensured according to the Law of Data Protection 15/1999. The study was approved by the Ethical Committee and Clinical Investigation of the Hospital Universitari de Girona Doctor Josep Trueta. All the participants provided signed informed consent and received the usual treatment schedule according to the criteria of the attending physician.
Study variables
Patients were consecutively included in the participating hospitals during the first 24 hours of hospitalization. We collected sociodemographic (age, sex), anthropometric (body mass index) and clinical data (smoking habit, pharmacological and non-pharmacological respiratory treatment and history of exacerbations in the previous year). On admission to the hospital, analytical variables were collected including a complete blood analysis, arterial blood gas analysis and biochemical parameters with determinations of fibrinogen and C-reactive protein (CRP) in plasma. The level of baseline dyspnea was measured using the modified scale of the Medical Research Council (mMRC) Citation(14). Co-morbidity was quantified using the Charlson index Citation(15).
The COPD assessment test (CAT) was completed by the patients during the first 24 hours of admission. The CAT is a specific questionnaire that measures the impact of disease in a patient using eight questions which evaluate cough, expectoration, dyspnea, chest tightness, patient confidence, limitations in daily activities, quality of sleep and energy. The CAT score ranges from 0 to 40, and the higher the score the worse the health status of the patient Citation(16).
After hospital discharge the patients were seen at 3 months to evaluate their evolution. The main study variable was poor patient outcome, which was defined as the presence of a moderate exacerbation, readmission or death within 3 months after discharge. A moderate exacerbation was defined as an increase in respiratory symptoms (dyspnea, changes in sputum volume and/or color) requiring treatment with systemic corticosteroids and/or antibiotics.
Statistical analysis
In order to describe the qualitative variables, absolute frequencies and percentages were used. The description of quantitative variables was performed using mean, standard deviation (SD), median and quartiles. The Kolmogorov–Smirnov test was used to assess the normality of distributions. In the case of quantitative variables, the comparison of the characteristics of the patients depending on the presence of poor outcome was carried out using the Student t-test (Mann–Whitney U-test if normality was not assumed). The Chi-squared test (Fisher test for frequencies < 5) was employed for the comparison of categorical variables.
Hospital admission within the last year, FEV1%, active smoking, Charlson score, hemoglobin (Hb) (g/dl), pCO2 (mmHg), fibrinogen, CRP (mg/L), leukocytes x109/L, long-term oxygen therapy (LTOT), purulent sputum, mMRC score and the initial CAT score were selected as potential prognostic variables of poor outcome at 3 months. Quantitative variables were transformed to binary variables before inclusion in logistic models using the best predictive cut-off point of each selected. Receiver Operating Characteristic (ROC) curve analysis, or the median values of those scores with less predictive value were used for this approach.
A final model was developed using backward logistic regression analysis including poor outcome as a dependent variable. Variables with a significance < 0.2 in the univariate analysis were included as independent variables. The results have been described with odds ratios (OR) their 95% confidence intervals (CI) and p-values. Finally, the combination of predictors from the final models was used to calculate the probabilities of poor outcome. The Hosmer–Lemeshow goodness-of-fit test was performed to assess the overall fit of the models. For all the tests, p-values < 0.05 were considered statistically significant. The Statistical Package for the Social Sciences (SPSS) (V19) was used for the statistical analyses.
Results
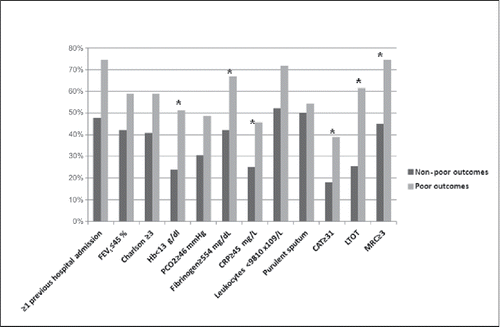
A total of 106 patients were included from January to March 2013, with a mean age of 71.1 years [SD = 9.8]. Of these, 91 (85.8%) were men, and 21.7% were active smokers. The patients presented a Charlson score of 3.7 (SD: 2.5) points, a mean post-bronchodilator FEV1(%) of 45.2% (SD: 14.7%), and 57.5% had been admitted to the hospital for an exacerbation of COPD within the last year. The mean CAT score at admission was 24.7 (SD: 7.1) and 38.7% of the patients included received LTOT. shows the remaining clinical variables of the patients at admission.
Table 1. Baseline characteristics of the patients based on the presence of poor outcome at 3 months after hospital discharge.
Poor outcomes were observed in 39 (36.8%) of the patients at 3 months after hospital discharge. Of these, 14 (13.2%) had exacerbations not requiring hospital admission, 22 (20.8%) were readmitted and 3 (2.8%) died.
Patients demonstrating poor outcomes had a lower post-bronchodilator FEV1(%) (40.9% (SD: 12.8%) vs. 47.8% (SD: 15.3%), p = 0.02), a lower post-bronchodilator FVC(%) (63% (SD: 19.1%) vs. 71.1% (SD: 17.6%), p = 0.031), had more frequently been admitted within the last year for a COPD exacerbation (74.4% vs. 47.8%, p = 0.008) and a higher percentage of these patients were treated with LTOT (61.5% vs. 25.4%, p = 0.001) compared to patients who did not present a poor outcome. In addition, patients with a poor outcome presented greater dyspnea (2.9 [SD: 1.1] vs. 2.3 [SD: 1.1], p = 0.01), lower Hb levels (12.9 [2.3] g/L vs. 14.2 [1.9] g/L, p = 0.001), and higher pCO2 (49.6 [16.1] mmHg vs. 43.7 [9.6] mmHg, p = 0.026) ().
Predictive models for poor outcome
The quantitative variables independently associated with a poor prognosis were transformed into binary variables for inclusion in the predictive models using ROC curves ().
Figure 1. Prevalence of the different variables calculated using the cut-off point with the greatest predictive power. FEV1, maximum expiratory volume in the first second; mMRC, modified Medical Research Council dyspnea scale; PCO2, partial pressure of carbon dioxide; LTOT, long-term oxygen therapy; Hb, hemoglobin; CRP, C-reactive protein; CAT: COPD assessment test. *indicates p < 0.05.
The final multivariate model showed a greater probability of presenting a poor outcome in patients with Hb values < 13 mg/L (OR = 2.46, 95% CI 1.09–6.36), CRP ≥ 45 mg/L (OR = 2.91, 95% CI: 1.11–7.49) and those receiving LTOT (3.07 95% CI: 1.07–8.82) (.
Table 2. Univariate and multivariate logistic regression models for predicting poor patient outcomes.
The probability of a poor outcome progressively increased with the number of predictors, being 15.7% for patients without any of these characteristics and 82.4% for those having all characteristics. The probabilities of poor outcome for the different combinations of risk factors are shown in
Table 3. Model 1: C-reactive protein (CRP) ≥ 45 g/L, hemoglobin (Hb) < 13 g/L and long-term oxygen therapy (LTOT) and COPD assessment test (CAT) ≥ 31.
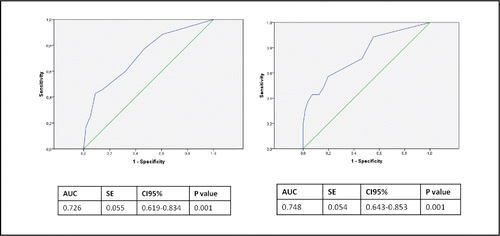
The final model showed good goodness-of-fit (Lemeshow p = 0.81) and a moderate predictive power (AUC = 0.72; 95% CI: 0.62–0.83; p = 0.001).
In order to analyze the discriminatory power of the CAT at admission, the cut-off with the greatest discriminatory power was identified (CAT ≥ 31 points). A second model included the addition of CAT ≥ 31 points to CRP ≥ 45, Hb < 13 and LTOT. As described previously, patients having all the variables increased the risk up to 91.6% (). This second model also showed good goodness-of-fit (Lemeshow p = 0.38) and an AUC of 0.75 (95% CI: 0.64–0.85, p = 0.001) ().
Figure 2. ROC analysis of significant variables derived from the logistic regression models and their capacity to predict poor outcome at 3 months. (A) Model 1 includes C-reactive protein (CRP) ≥ 45, hemoglobin (Hb) < 13 and long-term oxygen therapy (LTOT). (B) Model 2 includes C-reactive protein (CRP) ≥ 45, hemoglobin (Hb) < 13, long-term oxygen therapy (LTOT) and the COPD assessment test (CAT) ≥ 31. AUC, area under the curve; SE, standard error.
Discussion
The results of this study show that the presence of greater systemic inflammation measured by CRP, low Hb concentrations and the need for LTOT were predictive factors for presenting poor outcomes during the first 3 months after hospitalization for an exacerbation of COPD. The use of the CAT questionnaire gave the model a greater predictive power.
Frequent exacerbations of COPD have been related to a worse quality of life, a rapid decline in pulmonary function and increased risk of death in patients with COPD (Citation4–6,Citation12). At 3 months after hospital discharge, 36.8% of the patients in our study presented a poor outcome. In a similar population of patients with COPD admitted for an exacerbation, 45% presented a poor outcome (death, need for intensive care or mechanical ventilation or readmission), with 5% of death at 2 months Citation(8). In the AUDIEPOC study, including 5178 patients from centers throughout Spain, the mortality rate was 11.6% and the rate of readmission was 36% at 3 months after an exacerbation Citation(17). Our rates of poor outcome and mortality were lower than those in previous studies, probably due to the exclusion of patients admitted to ICUs.
Several variables were identified as being predictors of poor prognosis in patients admitted for an exacerbation. Frequent exacerbations (Citation10,Citation18–22) or hospital admission within the previous year Citation(19) have been related to a higher mortality following hospital discharge. The severity of obstruction is also related to a worse prognosis in patients with COPD. Thus, the lower the FEV1 the greater the rate of poor outcome after an exacerbation (Citation11,Citation22), the greater the probability of readmission (Citation12,Citation23) and the higher the mortality (Citation24–26).
In our series, Hb concentrations less than 13 g/L were an independent factor for poor outcome. Anemia is a known independent predictor of mortality in patients with severe chronic disease, and it has also been associated with greater in-hospital mortality (Citation22,Citation24,Citation27).
Similar to the results of other studies, hypoxemia Citation(28) and LTOT (Citation8,Citation21) were related to poorer patient outcomes. Hypercapnia is also an independent factor of poor outcome (Citation8,Citation29–31) and long-term mortality after admission (Citation8,Citation31). Despite observing higher pCO2 levels in patients with a poor outcome, hypercapnia was not a good predictor probably due to the exclusion of patients requiring non-invasive mechanical ventilation.
In their search for biomarkers, Mannino et al. Citation(32) observed that fibrinogen levels above 350 g/L can identify patients with COPD at risk of exacerbation and death. In addition, elevated CRP levels at discharge have been related to a greater probability of readmission Citation(33). On univariate analysis, both fibrinogen and CRP were associated with a poor prognosis. However, on multivariate analysis only CRP levels greater than 45 g/L remained associated with a poor outcome, probably due to the relationship between the two markers.
The CAT has shown to be the best clinical questionnaire to predict poor patient outcome following an exacerbation Citation(34). In a previous analysis, we observed that CAT scores at admission and at discharge after an exacerbation were not related to a worse prognosis. However, a reduction in the score of less than 4 points between admission and discharge helped to predict poor outcomes at 3 months Citation(13). To the contrary, in the study by Jing et al. Citation(33) a CAT score greater than 14 points was found to help predict readmission; however, this result was not confirmed in our series. In the present study, which was aimed at identifying prognostic factors, the CAT provided predictive value to other variables, but when used alone it was not found to be an independent factor of poor outcome.
Our model had a good goodness-of-fit and included Hb values, LTOT and CRP levels with an area under the curve (AUC) of 0.72. With the addition of CAT at admission, the goodness-of-fit of the model improved with an AUC of 0.75. Roche et al. Citation(35) used age, clinical signs of severity, and dyspnea and predicted the in-hospital mortality with an area under the receiver operating characteristic curve (AUROC) of 0.79. Ruiz-Gonzalez et al. Citation(36) observed that a model including confusion, CRP levels ≥ 50 mg/L, co-morbidities and smoking, was able to predict mortality, the need for ICU admission, or the development of heart failure with an AUROC of 0.80. Other variables that have been used in models to predict poor outcomes include the body mass index, arterial gasometry, level of dyspnea determined with the mMRC or a history of COPD exacerbations (Citation8,Citation37).
In summary, up to now most of the models proposed to predict patient outcome have suggested that the severity of the exacerbation is the main factor of in-hospital mortality, and the severity of the underlying disease is the most important factor to predict mortality of readmission after hospital discharge.
One of the limitations of our study was the exclusion of severe patients requiring ventilation support or patients admitted to the ICU. On the other hand, 85.2% of the sample were men, in accordance with the distribution of COPD by sex in Spain Citation(2). Thus, extrapolation of these results to women should be made with caution. Similar to previous studies Citation(8) we evaluated a combined outcome that included readmission, a new exacerbation or death. This approach includes the different events that can be clinically relevant and increases the precision of the model for the detection of variables associated with increased risk.
Although our cohort of COPD patients was not large, our study population was very homogeneous in severity, and we obtained robust results from the ROC curve analysis. However, these results should be confirmed and validated in large prospective studies.
In conclusion, the results of the present study demonstrate that a predictive model incorporating variables used in the usual clinical practice such as Hb, LTOT and CRP can, at admission, help to identify patients with a high probability of presenting a poor outcome during the following 3 months. The addition of an elevated CAT score at admission improves the predictive power of the model, but this score alone does not justify the use of this questionnaire as a predictor of poor outcome after an exacerbation of COPD.
Declaration of interest
Juan Luis García Rivero has received speaker's fees from Almirall, Boehringer Ingelheim, Pfizer, AstraZeneca, Chiesi, GlaxoSmithKline, Menarini, Takeda, Teva, Ferrer and Novartis, and consulting fees from Almirall, Boehringer Ingelheim and Menarini. Miriam Barecheguren has received speaker´s fees from Menarini, GlaxoSmithKline and Gebro Pharma. Marc Bonnin-Vilaplana has received speaker´s fees from Almirall, Boehringer Ingelheim, Pfizer, AstraZeneca, Chiesi, GlaxoSmithKline, Menarini, Esteve and Novartis. Alberto Herrejón has received speaker's fees from Ferrer, Menarini, Novartis, GlaxoSmithKline, Rovi and Boehringer Ingelheim. Pedro J Marcos has received speaker's fees from Pfizer, Teva, AstraZeneca, Esteve, GlaxoSmithKline, Menarini and Novartis, and consulting fees from Boehringer Ingelheim, GlaxoSmithKline and Novartis. Marc Miravitlles has received speaker's fees from Almirall, Boehringer Ingelheim, Pfizer, AstraZeneca, Chiesi, Esteve, GlaxoSmithKline, Menarini, Grifols and Novartis, and consulting fees from Almirall, Boehringer Ingelheim, Pfizer, GlaxoSmithKline, Gebro Pharma, CSL Behring, MedImmune, Takeda, Novartis and Grifols. The remaining authors have no conflicts of interest to disclosure.
ORCID
Marc Miravitlles http://orcid.org/0000-0002-9850-9520
References
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380 (9859):2095–2128.
- Miravitlles M, Soriano JB, Garcia-Rio F, Munoz L, Duran-Tauleria E, Sanchez G, et al. Prevalence of COPD in Spain: impact of undiagnosed COPD on quality of life and daily life activities. Thorax 2009; 64:863–868.
- Oostenbrink JB, Rutten-van Molken MP. Resource use and risk factors in high-cost exacerbations of COPD. Respir Med 2004; 98:883–891.
- Donaldson GC, Seemugal T, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002; 57:847–852.
- Doll H, Miravitlles M. Quality of life in acute exacerbations of chronic bronchitis and chronic obstructive pulmonary disease: a review of the literature. Pharmacoeconomics 2005; 23:345–363.
- Soler Cataluña JJ, Martínez García MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R, Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005; 60:925–931.
- Chenna PR, Mannino DM. Outcomes of severe COPD exacerbations requiring hospitalization. Semin Respir Crit Care Med 2010; 31:286–294.
- Matkovic Z, Huerta A, Soler N, Domingo R, Gabarrús A, Torres A, et al. Predictors of adverse outcome in patients hospitalised for exacerbation of COPD. Respiration 2012; 84:17–26.
- Donaldson GC, Wedzicha JA, COPD exacerbations. 1: epidemiology. Thorax 2006; 61:164–168.
- Hurst J, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363:1128–1138.
- Quintana JM, Esteban C, Garcia-Gutierrez S, Aguirre U, González N, Lafuente I, et al. Predictors of hospital admission two months after emergency department evaluation of COPD exacerbation. Respiration 2014; 88:298–306.
- García-Aymerich J, Farrero E, Félez MA, Izquierdo J, Marrades RM, Antó JM, et al. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax 2003; 58:100–105.
- Garcia-Sidro P, Naval E, Martinez-Rivera C, Bonnin-Vilaplana M, García-Rivero JL, Herrejón A, et al. The CAT (COPD Assessment Test) questionnaire as a predictor of the evolution of severe COPD exacerbations. Respir Med 2015; 109:1546–1552.
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 1999; 54:581–586.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987; 40:373–383.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J 2009; 34:648–654.
- Pozo-Rodrıguez F, Lopez-Campos JL, Alvarez-Martínez CJ, Castro-Acosta A, Agüero R, Hueto J. Clinical audit of COPD patients requiring hospital admissions in Spain: AUDIPOC study. PLoS One 2012; 7:e42156.
- Almagro P, Calbo E, Ochoa de Echaguen A, Barreiro B, Quintana S, Heredia JL, et al. Mortality after hospitalization for COPD. Chest 2002; 121:1441–1448.
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest 2007; 132:1748–1755.
- Bahadori K, FitzGerald JM. Risk factors of hospitalization and readmission of patients with COPD exacerbation—systematic review. Int J Chron Obstruct Pulmon Dis 2007; 2:241–251.
- Steer J, Gibson GJ, Bourke SC. Predicting outcomes following hospitalization for acute exacerbations of COPD. QJM 2010; 103:817–829.
- Miravitlles M, Izquierdo I, Herrejon A, Torres JV, Baro E, Borja J. ESFERA investigators: COPD severity score as a predictor of failure in exacerbations of COPD. The ESFERA study. Respir Med 2011; 105:740–747.
- Gunen H, Hacievliyagil SS, Kosar F, Mutlu LC, Gulbas G, Pehlivan E, et al. Factors affecting survival of hospitalised patients with COPD. Eur Respir J 2005; 26:234–241.
- Liu SF, Lin KC, Chin CH, Chen YC, Chang HW, Wang CC, et al. Factors influencing short-term re-admission and one-year mortality in patients with chronic obstructive pulmonary disease. Respirology 2007; 12:560–565.
- Steer J, Norman EM, Afolabi OA, Gibson GJ, Bourke SC. Dyspnoea severity and pneumonia as predictors of in-hospital mortality and early readmission in acute exacerbations of COPD. Thorax 2012; 67:117–121.
- Martínez FJ, Foster G, Curtis JL, Criner G, Weinmann G, Fishman A, et al. NETT Research Group. Predictors of mortality in patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006; 173:1326–1334.
- Alcázar B, García-Polo C, Herrejón A, Ruíz LA, De Miguel J, Ros JA, et al. Factors associated with hospital admission for exacerbation of chronic obstructive pulmonary disease. Arch Bronconeumol 2012; 48:70–76.
- Sukumalchantra Y, Dinakara P, Williams MH Jr. Prognosis of patients with chronic obstructive pulmonary disease after hospitalization for acute ventilatory failure: a three-year follow-up study. Am Rev Respir Dis 1966; 93:215–222.
- Menzies R, Gibbons W, Goldberg P. Determinants of weaning and survival among patients with COPD who require mechanical ventilation for acute respiratory failure. Chest 1989; 95:398–405.
- Pozo-Rodrıguez F, López-Campos JL, Álvarez-Martínez CJ, Castro-Acosta A, Agüero R, Hueto J. Clinical Audit of COPD patients requiring hospital admissions in Spain: AUDIPOC study. PLoS One 2012; 7:e42156.
- Bahadori K, FitzGerald JM, Levy RD, Fera T, Swiston J. Risk factors and outcomes associated with chronic obstructive pulmonary disease exacerbations requiring hospitalization. Can Respir J 2009; 16:e43–e49.
- Mannino DM, Tal-Singer R, Lomas DA, Vestbo J, Graham Barr R, Tetzlaff K, et al. Plasma fibrinogen as a biomarker for mortality and hospitalized exacerbations in people with COPD. J Chron Obstruct Pulmon Dis 2015; 2:277–278.
- Jing Z, Chun C, Mimg S, Hong Z, Bei H, Wan-shen Y. Systemic inflammatory marker CRP was better predictor of readmission for AECOPD than sputum inflammatory markers. Arch Bronconeumol 2016; 52:138–144.
- Miravitlles M, García-Sidro P, Fernández-Nistal A, Buendía MJ, Espinosa de los Monteros MJ, Esquinas C, et al. The chronic obstructive disease assessment test (CAT) improves the predictive value of previous exacerbations foor poor outcomes in COPD. Int J Chron Obsruct Pulmon Dis 2015; 10:2571–2579.
- Roche N, Zureik M, Soussan D, Neukirch F, Perrotin D, Urgence BSC. Predictors of outcomes in COPD exacerbation cases presenting to the emergency department. Eur Respir J 2008; 32:953–961.
- Ruiz-Gonzalez A, Lacasta D, Ibarz M, Martinez-Alonso M, Falguera M, Porcel JM. C-reactive protein and other predictors of poor outcome in patients hospitalized with exacerbations of chronic obstructive pulmonary disease. Respirology 2008; 13:1028–1033.
- Tsimogianni AM, Papiris SA, Stathopoulos GT, Manali ED, Roussos C, Kotanidou A. Predictors of outcome after exacerbation of chronic obstructive pulmonary disease. J Gen Intern Med 2009; 24:1043–1048.
