ABSTRACT
Acute exacerbations and community-acquired pneumonia (CAP) are severe complications in patients with chronic obstructive pulmonary disease (COPD). In this study, we analyzed inflammatory parameters in serum including C-reactive protein (CRP), procalcitonin (PCT), and serum neopterin (NPT) to determine their potential to differentiate between patients with CAP+COPD and with acute exacerbations of COPD (AECOPD) without pneumonia. 102 (39 women and 63 men) patients were included in this retrospective study, of whom 48 presented with CAP without underlying COPD, 20 with CAP+COPD and 34 with AECOPD. CRP, PCT, and blood counts were determined by routine automated tests, and NPT concentrations were determined by ELISA. The ratios of CRP to NPT levels were calculated. Upon patient admission, CRP, PCT, and NPT levels were significantly higher in patients with CAP compared to those in AECOPD patients. CRP/NPT ratio was lower in AECOPD compared to CAP (+/−COPD) patients. Positive correlations were found between duration of hospitalization and CRP levels and the CRP/NPT ratio at study entry. Patients who were readmitted within 30 days tended to have higher NPT levels at initial presentation. Patients under ongoing corticosteroid treatment presented with lower inflammatory parameters. The CRP/NPT-ratio was suited well to discriminate between AECOPD and CAP on the basis of COPD, a CRP/NPT cutoff of 0.346 provided a sensitivity of 65% and a specificity of 79%. The combinatory use of inflammatory patterns might help to differentiate patients with AECOPD from those with CAP on the basis of COPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is an important cause of morbidity and mortality worldwide Citation(1). Acute exacerbations of COPD (AECOPD) lead to increased hospitalization rates and mortality (2,3). Triggers of AECOPD are pleiotropic: Up to 50% of exacerbations are caused by bacterial infections Citation(4), frequently isolated pathogens are Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, or Pseudomonas aeruginosa Citation(5,6). Occupational or environmental exposure to dusts, gases or vapors and air pollution in general account for 15–20% of exacerbations Citation(4), and also viral infection trigger AECOPD, a meta-analysis found a prevalence of viral respiratory infections of 34.1% Citation(7).
Community-acquired pneumonia (CAP) is a common, life-threatening complication, especially in patients aged >65 years and those with advanced COPD Citation(8). However, it is often difficult to differentiate between CAP+COPD and AECOPD—and to decide whether patients need antibiotics or not. In vitro as well as clinical studies suggest different inflammatory responses in patients with CAP+COPD and AECOPD Citation(9–11): Huerta et al. demonstrated higher concentrations of C-reactive-protein (CRP), procalcitonin (PCT), TNF-alpha, and interleukins (IL), namely IL-6, IL-1, IL-8 in CAP+COPD patients as compared to AECOPD. The same study could also show that CRP levels allowed discriminating between CAP+COPD and AECOPD.
Higher IL-6, MCP-1, IFN-gamma and TNF-alpha levels were reported in AECOPD patients with microbiologically diagnosed viral infection than in patients without confirmed viral infection Citation(12). However, the value of cytokines/IL is limited by their short half-life and rapid fluctuations in circulating concentrations. In contrast, the pteridine neopterin (NPT) is biochemically inert and allows to estimate the extent of cell-mediated immune activation easily and reproducibly in blood or urine samples Citation(13,14). The ability of CRP, PCT, and NPT to differentiate between viral and bacterial etiologies has been investigated in patients with lower respiratory tract infections by Ip et al. Citation(15).
Inflammation parameters and biomarkers might not only be useful to differentiate between viral and bacterial infection in COPD patients, but also could be useful as prognostic marker: Chang et al. described highly sensitive C-reactive protein (hs-CRP) levels at discharge as an independent predictor of readmission for AECOPD Citation(16).
The aims of our study were to analyze the impact of NPT and standard inflammatory parameters as well as combinations thereof to correctly identify COPD patients with CAP versus those with AECOPD and to evaluate their prognostic potential.
Patients and methods
Subjects
We retrospectively analyzed eligible patients with CAP, CAP+COPD, or AECOPD by reviewing the electronic medical records of patients, who were treated at the inpatient ward of the University Hospital for Internal Medicine VI, Innsbruck between 1 January 2013 and 31 October 2014.
All patients treated at this ward get an extended routine “admission lab” including CRP, complete and differential blood counts, NPT, and PCT testing (the blood samples are taken once during the stay, usually on day 2 after admission, exceptionally on day 3), liver enzymes, creatinine, hs Troponin-T, and NT-proBNP. Patients were included in analyses when serum NPT levels were tested within two days after the first medical contact, and their age was between 18 and 90 years. Exclusion criteria were pregnancy, HIV infection, organ transplantation, active hematologic or oncologic disease.
CAP patients were included if typical symptoms and a chest X-ray or (computed tomography) CT-scan confirming a pneumonic infiltrate were present. If available spirometry records from earlier admissions were reviewed, only those with an FEV1/FVC ≥ 70% were included. A history of asthma or COPD in the patients record was an exclusion criteria.
COPD patients (AECOPD as well as CAP+COPD) were included if spirometry tests from earlier admissions confirmed FEV1/FVC < 70%. Patients were classified to have AECOPD if they presented with typical symptoms and anamnestic findings according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) AECOPD definition Citation(17), but without a pneumonic infiltrate. COPD patients with a pneumonic infiltrate (confirmed by chest X-ray or CT scan) were classified as CAP+COPD.
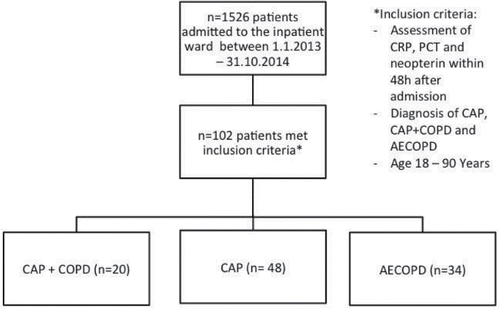
1526 patients were admitted between 1 January 2013 and 31 October 2014 to our inpatient ward. All patients who met the aforementioned inclusion criteria, who had a certified CAP or CAP+COPD or AECOPD according to the above described definition, and of whom values for CRP, PCT, and NPT (determined within 48 hours after admission) were available, were enrolled in the analysis. 102 patients met these inclusion criteria ().
In all patients, the following data were retrospectively analyzed: complete and differential blood counts, liver enzymes, CRP, PCT, NPT, creatinine, hs Troponin-T, NT-proBNP, microbiologic test results, duration of hospitalization, 30-day-mortality, readmission to our hospital until December 2014. CRP, PCT, and blood counts were determined by routine automated tests, and NPT concentrations were determined by ELISA (Brahms, Berlin, Germany). The study cohort consisted of 39 female and 63 male patients, of whom 48 patients presented with CAP without concomitant COPD [age: median (interquartile range—IQR) = 68 years (35)), 20 with CAP+COPD (age: 75 years Citation(15)), and 34 with AECOPD (age: 69 years Citation(18))]. Demographic data and baseline characteristics of patients are depicted in . 39 COPD patients had a corticosteroid treatment (36 inhaled corticosteroids, 26 systemic corticosteroids) at the time of blood sample collection.
Table 1. Baseline characteristics—demographics of the different groups.
The study was approved by the local ethic committee (approval number AN2015-0060 347/4.6).
Statistical analyses
The ratio of CRP to NPT was calculated. Baseline characteristics of the three groups were compared using chi-Square, and Fisher's exact test, where appropriate. For non-parametric data, the Mann-Whitney U test and Kruskal–Wallis test were applied. The Spearman rank correlation technique was used for analysis of associations. All tests were two-sided, and a p-value of 0.05 indicated statistical significance.
The diagnostic value of this ratio was evaluated by calculating sensitivity, specificity, and positive and negative predictive values (PPV and NPV). To find optimal cutoffs for CRP/NPT, ROC curve analysis was performed, and area under curve (AUC) of all inflammatory parameters (CRP, PCT) were calculated.
Statistical analyses were performed with the SPSS 22.0 statistical package (IBM Corp., Armonk, NY, USA). A two-sided p-value of < 0.05 was considered significant.
Results
The baseline characteristics of our cohort are shown in . The distribution of gender did not differ among the three groups (p = 0.981), while a trend toward older age was seen in patients with COPD (including AECOPD and CAP+COPD; p = 0.053). COPD stages were distributed equally in AECOPD and CAP+COPD patients (p = 0.250), and the most frequently observed COPD stage was 3 (n = 29), followed by 2 (n = 12).
Positive results of microbiologic tests were obtained in 21 patients (20.6% of all patients). Most bacterial isolates were found in bronchial secretion cultures (n = 13), while blood cultures were positive in 8 patients. The highest frequency of positive microbiologic tests was observed in the CAP+COPD group (9 of 20 patients, 45%), followed by AECOPD (5 of 34, 14.7%), and CAP (7 of 48 patients, 14.6%).
The median duration of hospitalization was 7.5 (IQR 6) days, and no differences were found among the three groups (p = 0.75), . Four patients died within 30 days (overall n = 4 3.9%; AECOPD n = 0, 0%; CAP n = 3, 6.3%; CAP+COPD n = 1, 5%), but no significant differences were observed between the three groups (p = 0.34). Re-admission to hospital occurred in 28 patients, with highest readmission rates found in CAP+COPD patients (55%, p = 0.001 compared to 32.3% in AECOPD and 12.5% in CAP).
AECOPD patients were treated more frequently with steroids (overall 79.4%, inhaled corticosteroids (ICS) 73.5%, systemic corticosteroid (SCS) 58.8%) than CAP+COPD patients (overall 60%, ICS 55%, SCS 30%), although no statistical significance was reached (p = 0.207).
Laboratory parameters
Inflammatory parameters differed significantly between patients with AECOPD, CAP+COPD, and CAP (). At admission, CRP levels were higher in CAP compared to AECOPD patients (p < 0.001; ). Similarly, PCT and NPT levels were higher in patients with CAP (PCT p = 0.006, NPT p = 0.015; ).
Table 2. Laboratory parameters at study entry.
Table 3. Clinical course of the different diagnosis groups.
Table 4. Correlation between length of hospitalization and inflammation parameters in COPD patients only.
Figure 2. Inflammatory biomarkers in the respective patient groups. a. CRP (mg/dl), b. PCT (ug/l), c. NPT (nmol/l) levels, and d. CRP/NPT ratio among AECOPD, CAP and CAP+COPD patients. The horizontal bar and box length represent the mean and SEM (standard error of the mean), respectively; § represents significance at p < 0.05.
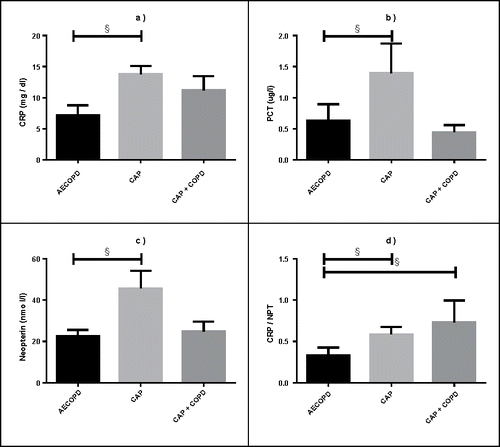
The CRP/NPT ratio was significantly lower in AECOPD compared to that in CAP (+/−COPD) patients (AECOPD vs. CAP p = 0.002; AECODP vs. CAP+COPD, p = 0.010; ). The urine NPT/creatinine did not differ among the groups (p = 0.073).
Higher hemoglobin concentrations were found in AECOPD compared to those in CAP patients (p = 0.044.) No significant differences were found in leucocyte or differential blood counts.
Higher levels of CRP (median: 9.53 mg/dl vs. 2.71 mg/dl p < 0.01), PCT (0.13 ug/l vs. 0.08 ug/l p = 0.025), and NPT (23.30 nM/l vs. 15.20 nM/l p = 0.072) were seen in patients not previously treated with corticosteroids (SCS and/or ICS, n = 63, 39 patients were treated with corticosteroids). Patients with systemic corticosteroid treatment (n = 26) presented with significantly lower CRP (p = 0.006) and PCT concentrations (p = 0.009) as well as a lower CRP/NPT ratios (p = 0.006), in patients with ICS treatment (n = 36) significantly lower values for CRP, PCT, NPT and CRP/NPT were seen.
Associations between inflammatory parameters and the outcome of patients and diagnostic value of inflammatory parameters
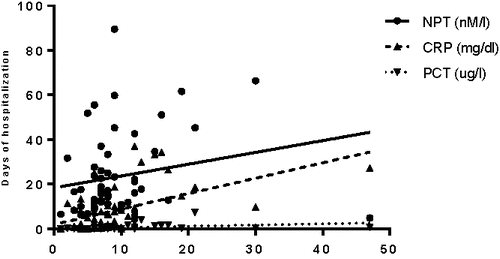
A positive correlation between length of hospitalization and inflammatory markers at admission was found for CRP (rs = 0.296, p = 0.003) and the CRP/NPT ratio (rs = 0.248, p = 0.012). In COPD only patients (AECOPD, CAP+COPD), the strength of correlation further increased, showing significant correlations for all three inflammatory parameters (Spearman's rank correlation: CRP 0.505, p < 0.001; PCT 0.376, p = 0.005; NPT 0.269, p = 0.049; see also ).
Figure 3. Correlation between NPT (nM/l), CRP (mg/dl) and PCT (ug/l) and length of hospitalization (days) in COPD patients (AECOPD and CAP+COPD). (Spearman's rank correlation = 0.305, p = 0.013).</LE3>
No significant differences were seen for CRP, PCT, or NPT in patients, who were readmitted after discharge or in patients who died within 30 days, but NPT concentrations tended to be higher in patients who had to be readmitted (median 31.7 nM vs. 16.6 nM, p = 0.052) and also in patients who died.
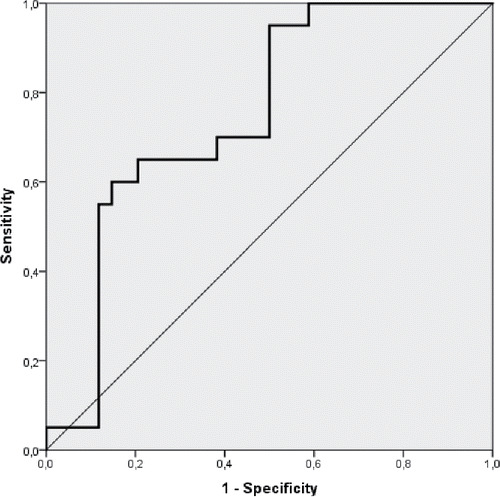
The diagnostic value of CRP/NPT ratio to discriminate between patients with AECOPD and patients with CAP+COPD was tested by calculating sensitivity, specificity, and positive and negative predictive values (PPV and NPV), and a cutoff of 0.346 was identified as having the best discriminative potential. A CRP/NPT ratio above 0.346 was indicative of CAP+COPD (PPV = 65%, NPV = 79%, sensitivity = 65% and a specificity = 79%; ) compared to AECOPD.
Discussion
In this retrospective analysis, we could demonstrate different inflammatory profiles of patients with CAP +/−COPD and AECOPD: Patients with CAP+COPD presented with significantly higher CRP values and higher CRP/NPT ratios compared to patients with AECOPD. Positive correlations were found between the duration of hospitalization and CRP as well as CRP/NPT concentrations. Patients who had to be readmitted or died within 30 days tended to have higher NPT levels.
Our study shows that inflammatory markers might not only be useful to monitor treatment response, but may also help to discriminate between patients with CAP+COPD and AECOPD. Furthermore, they seem to be related to the duration of hospitalization, and CRP and CRP/NPT values were significantly correlated with the length of stay in our population of 102 patients. In patients with underlying COPD, all inflammatory markers were higher in patients who subsequently had a longer hospital stay, possibly indicating that these patients were more critically ill.
Unfortunately, inflammatory markers were not suited well to assess the risk of patients to die or to be readmitted in our population; only NPT levels tended to be higher in patients who had to be readmitted or died. However, this result could possibly be due to the low overall early mortality in our population (n = 4). In fact, an earlier study by Lacoma et al. Citation(18) with more patients described increased PCT and NPT levels in COPD patients who died within one month after the sample collection.
CRP and PCT levels in our trial were higher in CAP (+/−COPD) patients in comparison to AECOPD patients, indicating a different inflammatory response, while NPT concentrations only tended to be higher in CAP+COPD compared to AECOPD. On the other hand, NPT levels were significantly lower in AECOPD patients compared to CAP, and calculation of a CRP/ NPT ratio enabled to better discriminate between patients with AECOPD and CAP+COPD. CRP, PCT, and NPT did not significantly differ between patients with CAP with and without COPD, although inflammatory markers tended to be higher in patients with CAP without underlying COPD—this finding could in fact be due to corticosteroid treatment in a high percentage of our COPD patients. Well in line with this hypothesis, patients without previous treatment with corticosteroids presented with higher inflammatory markers.
NPT has been described to be elevated in the setting of a viral infection Citation(15), and previous trials have already shown the potential of NPT and CRP/NPT ratio in serum to discriminate between bacterial and viral etiology in lower respiratory tract infections Citation(15) and acute respiratory tract infections Citation(19); however, in these studies, COPD as a confounder was not considered. Positive microbiologic results were found in 21% of our patients, while no investigations for viruses were performed (as serologic results are expensive and antiviral treatment is mostly no option, except for influenza).
Lacoma et al. Citation(18) showed higher PCT and CRP levels in patients with pneumonia (compared to stable COPD and AECOPD), while significantly lower NPT levels were only found in a small subgroup of AECOPD patients with bacterial isolates. One main problem in AECOPD patients is certainly that there is no gold standard to determine the cause of exacerbations, and thus, biomarkers to discriminate between viral and bacterial respiratory tract infection would be very helpful. Due to the retrospective nature of our study, we were not able to discriminate between viral and bacterial pathogens; however, it would of course be very interesting to investigate this question by further longitudinal studies also involving sputum analyses. Still, we think, that our results are promising, as they show different inflammatory profiles in patients with AECOPD and CAP+COPD, which allow to rapidly differentiate between these two pathologies—which is an important issue in a clinical setting with implications for therapy and subsequent clinical management.
This study has several limitations. First, the low number of patients is not sufficient to draw robust conclusions in terms of the predictive value of the different inflammatory parameters toward clinical prognosis and mortality. It is obvious that further studies with a prospective design including larger patient numbers will be necessary to confirm our observations. Furthermore, we cannot really estimate the role of viral pathogens as causative agents of AECOPD, and we do not know how many of our AECOPD patients were exacerbated due to unspecific triggers. However, this is a main and well-known problem in patients with AECOPD, and it cannot be solved simply and satisfactorily so far, as diagnostic possibilities are limited, methods are expensive, and virus infections mostly cannot be treated. Still, the fact that mortality of bacterial pneumonia can be significantly decreased by early antibiotic treatment Citation(20), and the fact, that “unnecessary” antibiotic treatment in patients with AECOPD is known to trigger the development of resistancies, should prompt us to find biomarkers to distinguish between patients who benefit from antibiotic therapy and those who rather benefit from other treatment options.
Conclusion
Our data confirm different inflammatory patterns in AECOPD, CAP+COPD and CAP only patients. Our findings for the first time support the hypothesis that a fast discrimination between AECOPD and CAP+COPD is practicable by determining CRP and NPT serum levels. NPT levels can further provide important prognostic information with regard to duration of hospitalization and short-term clinical prognosis.
Declaration of interest
The authors report no conflicts of interest.
References
- WHO. The top 10 causes of death 2012 [Available from: http://www.who.int/mediacentre/factsheets/fs310/en/.
- Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet 2007; 370(9589):786–796.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363(12):1128–1138.
- Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008; 359(22):2355–2365.
- Soler N, Torres A, Ewig S, Gonzalez J, Celis R, El-Ebiary M, et al. Bronchial microbial patterns in severe exacerbations of chronic obstructive pulmonary disease (COPD) requiring mechanical ventilation. Am J Respir Crit Care Med 1998; 157(5 Pt 1):1498–1505.
- Nseir S, Cavestri B, Di Pompeo C, Diarra M, Brisson H, Lemyze M, et al. Factors predicting bacterial involvement in severe acute exacerbations of chronic obstructive pulmonary disease. Respiration 2008; 76(3):253–260.
- Mohan A, Chandra S, Agarwal D, Guleria R, Broor S, Gaur B, et al. Prevalence of viral infection detected by PCR and RT-PCR in patients with acute exacerbation of COPD: A systematic review. Respirology 2010; 15(3):536–542.
- Mullerova H, Chigbo C, Hagan GW, Woodhead MA, Miravitlles M, Davis KJ, et al. The natural history of CAP in COPD patients: A population database analysis. Respir Med 2012; 106(8):1124–1133.
- Gutierrez P, Closa D, Piner R, Bulbena O, Menendez R, Torres A. Macrophage activation in exacerbated COPD with and without community-acquired pneumonia. Eur Respir J 2010; 36(2):285–291.
- Crisafulli E, Menendez R, Huerta A, Martinez R, Montull B, Clini E, et al. Systemic inflammatory pattern of patients with community-acquired pneumonia with and without COPD. Chest 2013; 143(4):1009–1017.
- Huerta A, Crisafulli E, Menendez R, Martinez R, Soler N, Guerrero M, et al. Pneumonic and nonpneumonic exacerbations of COPD: Inflammatory response and clinical characteristics. Chest 2013; 144(4):1134–1142.
- Almansa R, Socias L, Andaluz-Ojeda D, Martin-Loeches I, Bobillo F, Blanco J, et al. Viral infection is associated with an increased proinflammatory response in chronic obstructive pulmonary disease. Viral Immunol 2012; 25(4):249–253.
- Berdowska A, Zwirska-Korczala K. Neopterin measurement in clinical diagnosis. J Clin Pharm Ther 2001; 26(5):319–329.
- Fuchs D, Weiss G, Reibnegger G, Wachter H. The role of neopterin as a monitor of cellular immune activation in transplantation, inflammatory, infectious, and malignant diseases. Crit Rev Clin Lab Sci 1992; 29(3–4):307–341.
- Ip M, Rainer TH, Lee N, Chan C, Chau SS, Leung W, et al. Value of serum procalcitonin, neopterin, and C-reactive protein in differentiating bacterial from viral etiologies in patients presenting with lower respiratory tract infections. Diagn Microbiol Infect Dis 2007; 59(2):131–136.
- Chang C, Zhu H, Shen N, Han X, Chen Y, He B. Utility of the combination of serum highly-sensitive C-reactive protein level at discharge and a risk index in predicting readmission for acute exacerbation of COPD. J Bras Pneumol 2014; 40(5):495–503.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4):347–365.
- Lacoma A, Prat C, Andreo F, Lores L, Ruiz-Manzano J, Ausina V, et al. Value of procalcitonin, C-reactive protein, and neopterin in exacerbations of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2011; 6:157–169.
- Rainer TH, Chan CP, Leung MF, Leung W, Ip M, Lee N, et al. Diagnostic utility of CRP to neopterin ratio in patients with acute respiratory tract infections. J Infect 2009; 58(2):123–130.
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006; 34(6):1589–1596.