ABSTRACT
Chronic obstructive pulmonary disease (COPD) is associated with the accelerated aging of the lung. The protein klotho has been implicated in longevity, and there is some evidence that it might be involved in the pathomechanism of chronic respiratory diseases. Therefore, we aimed to examine whether the clinical condition of COPD patients is reflected in plasma klotho concentration. As plasma concentration of the protein is modulated by physiological factors that are generally improved during pulmonary rehabilitation, we hypothesized that a complex rehabilitation program may alter plasma klotho concentration. Blood samples were taken from 31 stable COPD patients. Clinical parameters such as respiratory function, 6-minute walking distance (6MWD), impact of disease (CAT), dyspnea, grip strength, chest expansion and breath holding time, smoking history, and body mass index (BMI) were evaluated. 19 patients who participated in a 3-week inpatient rehabilitation program had blood sample collection on the first, third, and last days of the program and had the above functional measurements before and after rehabilitation. Plasma klotho concentration was assessed by enzyme-linked immunosorbent assay. Klotho levels showed no correlation with clinical parameters (FEV1%, 6MWD, grip strength, CAT, smoking history, p > 0.05). Coefficient of variation of klotho measurements was 4.5% between Day 1 and Day 3. Although the rehabilitation resulted in significant improvements in 6MWD, CAT, grip strength, and chest expansion, klotho levels did not change significantly (510.1 ± 149.9 vs. 504.2 ± 139.8 pg/ml, p > 0.05). Plasma klotho concentration can be reliably measured in stable COPD; however, its levels are not correlated with clinical parameters of patients. Despite functional improvement, klotho level remains unchanged during the rehabilitation program.
Introduction
Chronic obstructive pulmonary disease (COPD) is associated with accelerated aging of the lung, involving cellular senescence, stem cell exhaustion, and increased oxidative stress Citation(1). The protein klotho is implicated in longevity; it prevents cell senescence Citation(2) and apoptosis (Citation3,4), preserves stem cells Citation(5), reduces oxidative stress (Citation4,6), and maintains endothelial integrity and function Citation(7). Klotho was first described in mice where the lack of gene expression resulted in a phenotype similar to aging and led to premature death. Apart from systemic symptoms, mice exhibited histological and functional characteristics of emphysema Citation(8).
In patients with COPD, soluble klotho mRNA expression Citation(9) as well as klotho expression in airway epithelial cells Citation(10) was found to be decreased compared to healthy individuals. However, the relation between circulating klotho and clinical parameters of patients has not been widely investigated. Therefore, we aimed to evaluate whether plasma klotho concentration reflects functional parameters of patients, in order to assess whether plasma klotho level might serve as a biomarker in COPD.
Murine and human studies have evaluated the relationship between klotho and various parameters of physical fitness. Based on these results, it was hypothesized that the skeletal muscle contractile activity modulates circulating klotho expression, and this regulation may play a role in the systemic anti-aging effects of exercise Citation(11). Klotho was found to be modified by nutritional state as well. Significantly lower plasma klotho concentrations were measured both in subjects with anorexia nervosa and obesity compared to the control group Citation(12). Chronic psychologic stress was also reported to result in reduced concentration of the protein Citation(13).
Pulmonary rehabilitation generally leads to an improvement in the above physiological factors; therefore, we hypothesized that a complex pulmonary rehabilitation program, involving not only physical training but optimized diet, medication, and psychosocial support as well might lead to an increase in plasma klotho concentration in COPD patients.
In the absence of published data, in order to interpret any long-term alteration of plasma klotho concentration correctly, we also aimed to examine the between-day variability of the protein level.
Methods
Subjects and methods
Blood samples were collected from 31 stable COPD patients (63.9 ± 7.9 years, FEV1 38.6 ± 14.1%). None of the patients suffered from accompanying asthma. Patients with chronic kidney disease and those who had any tumorous disease in the past 5 years were excluded from the study. Acute exacerbation in the past 3 months was also part of the exclusion criteria. Patients performed pulmonary function test and 6-minute walk test. They completed the COPD Assessment Test (CAT) and the modified Medical Research Council dyspnea scale (mMRC). Breath holding time, grip strength, and chest expansion were also measured. Smoking history was recorded.
19 of the above patients (63.8 ± 8.8 years, FEV1 35.7 ± 10.7%) took part in an elective 3-week inpatient pulmonary rehabilitation program, regardless of disease stage and smoking habits. They went through the above complex health assessment on the first day and the last day of the rehabilitation program, and blood samples were collected on the first, the third, and the last day. Patient characteristics are shown in
Table 1. Patient characteristics.
The complex pulmonary rehabilitation program consisted of the following key elements: daily respiratory muscle training, controlled breathing techniques, and chest wall mobilization (approx. 40 minutes); individualized endurance training by bike and/or treadmill (2 or 3 times a day); personalized diet; adjustment of medication if necessary; psychological help if needed.
Measurement of clinical parameters was performed by experienced physiotherapists. Maximum grip strength was measured on the dominant arm using a handgrip dynamometer (Kern & Sohn Gmbh, Germany). 6-minute walking test was performed according to the guideline of the American Thoracic Society Citation(14) in a 33-meter-long indoor corridor. Chest expansion is the difference of chest perimeter between maximum expiration and maximum inspiration. Values were recorded 3 times with a tape measure at the level of the xiphoid process, and average values were calculated. Breath holding time was assessed after a maximum inhalation that followed a deep exhalation. Patients wore a nose clip during the procedure.
Venous blood samples were collected into EDTA tubes. Each sample was taken in the morning at rest.
Following the immediate centrifugation of the samples (1500 rpm, 10 minutes, 4°C), supernatant was collected. Plasma samples were stored at −80°C until measurement. Plasma klotho levels were quantified using a human-soluble α-klotho enzyme-linked immunosorbent assay kit (Immuno-Biological Laboratories Co., Ltd., Takasaki, Japan). The detection range of the kit is 93.75–6000 pg/ml, and its sensitivity is 6.15 pg/ml. Klotho concentration was above the detection limit in all samples.
The study protocol was approved by the local ethics committee (19/2015), and all patients gave their written informed consent to participate in the study.
Statistical analysis
Statistical analysis of data was performed using commercially available SPSS20 and GraphPad Prism software. Between-day variability of plasma klotho was evaluated by the Bland–Altman test and by the coefficient of variation. Paired data were compared with paired t-test or Wilcoxon signed-rank test; unpaired data were compared with unpaired t-test or Mann–Whitney U test depending on the type of distribution. Data distribution was analyzed by Kolmogorov–Smirnov test. Repeated-measures analysis of variance (ANOVA) was used to compare klotho concentrations measured at the three time points. A p-value < 0.05 was considered significant. Values are given as mean ± standard deviation (SD).
Required sample size for assessing the impact of rehabilitation on klotho levels and the difference between smokers and ex-smokers was calculated using Gpower, based on plasma klotho concentrations of adults as published by Yamazaki et al. Citation(15) when first presenting the enzyme-linked immunosorbent assay (ELISA) kit chosen for the current study (562 ± 162 pg/ml). Required minimum difference was the double of the interassay variability of the ELISA kit at the prospective klotho concentration (17%). At a power of 80%, the required sample size was 19.
Results
Intra-assay repeatability and between-day variability of plasma klotho concentration in stable COPD
Plasma klotho concentration could be reliably measured; the intra-assay coefficient of variation was 3.4%.
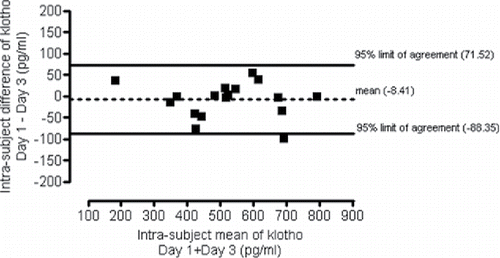
Between-day variability of klotho level was measured in plasma samples taken on the first and the third day of hospitalization. The coefficent of variation was 4.5%. The 95% limits of agreement for between-day variability were −88.35 and 71.52 pg/ml ().
Relation between plasma klotho levels and clinical parameters of stable COPD patients
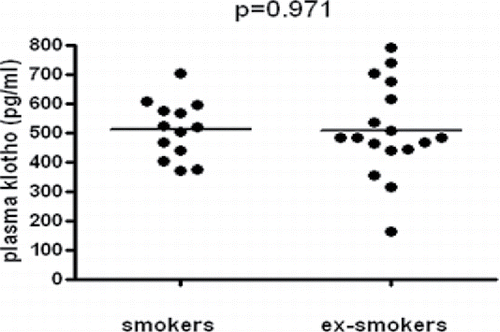
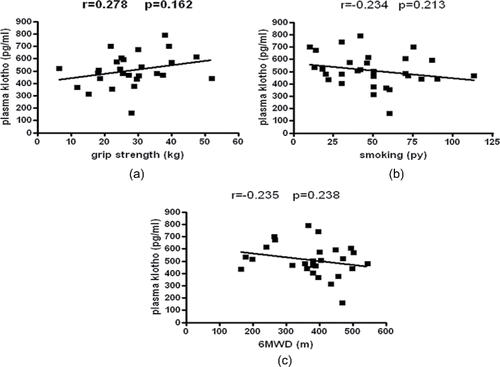
Plasma klotho level in the smoker and ex-smoker groups did not differ significantly (508.4 ± 95.2 vs. 510.2 ± 164.4 pg/ml, respectively, p > 0.05) (). The concentration of the protein showed no correlation with age, body mass index (BMI), and FEV1%, grip strength (r = 0.278; p = 0.162) (); smoking history expressed in pack-years (r = −0.234; p = 0.213) (), and 6MWD (r = −0.235; p = 0.238) ().
Improvement of clinical parameters during rehabilitation
The 3-week inpatient pulmonary rehabilitation program resulted in a significant improvement in 6MWD (373.7 ± 123.5 vs. 414.5 ± 118.6 m, p = 0.0017), grip strength (27 ± 10.9 vs. 30.2 ± 9.1 kg, p = 0.0013), chest expansion (3.59 ± 0.7 vs. 4.89 ± 1.3 cm, p = 0.0054), and CAT (14.9 ± 7.3 vs. 10.7 ± 6.5, p = 0.0042), although not every patient showed improvement in each parameter. FEV1, breath holding time, and mMRC scores did not change significantly over the course of hospitalization. Data are shown in .
Table 2. Clinical parameters of patients on the first and the last day of the pulmonary rehabilitation.
Plasma klotho levels before and after pulmonary rehabilitation
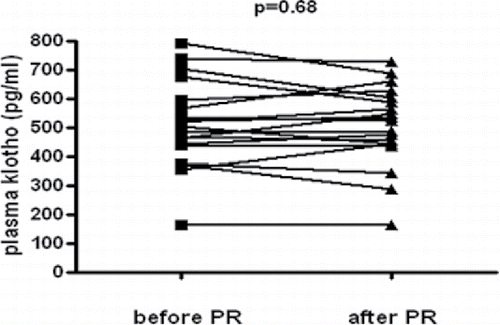
There was no significant difference in plasma klotho concentration before and after the 3-week inpatient pulmonary rehabilitation program (). Plasma klotho values on the first day of the rehabilitation were 510.1 ± 149.8 pg/ml vs. 504.2 ± 134.9 pg/ml measured on the last day of the program.
Discussion
Short- and long-term alterations of human plasma klotho level in response to different interventions have already been evaluated; however, published data about the repeatability of klotho concentration measurements are lacking. As the present study intended to assess medium-term (through a period of three weeks) changes of klotho levels, it was considered necessary to first determine the between-day variability of the protein, which was found to be as low as 4.5% (the intra-assay repeatability of plasma klotho measurement with the chosen ELISA kit was 3.4%). During the 48 hours that passed between the two blood sample collections, patients had their daily routine changed. This circumstance might have influenced the results; however, both samples were collected in the morning after at least 10 hours of rest, which minimizes the bias. Furthermore, any effect of the rehabilitation is unlikely to manifest in such a short period of time.
Among the various factors influencing klotho levels, smoking status has been the most widely examined, and the results are not unanimous. Our results, showing that soluble klotho concentration of smoker and ex-smoker COPD patients does not differ significantly, confirmed previous in vivo findings Citation(16), although others have observed lower klotho expression in airway epithelial cells in current smokers compared to non-smokers, and cigarette smoke exposure was shown to result in reduced klotho expression in vitro Citation(10).
The lack of correlation between plasma klotho levels and clinical parameters of patients reduces the potential bias in the follow-up part of our study.
The 3-week inpatient pulmonary rehabilitation program included several elements that together led to the detected changes in exercise capacity, disease-associated quality of life, grip strength, and chest expansion. We hypothesized that these effects might be reflected in plasma klotho concentrations. Beside the regular endurance training, patients participated in daily respiratory muscle training as well; they also benefited from an individual diet based on dietitian's recommendations. If needed, their medication was adjusted and psychosocial support was provided. Despite the fact that grip strength, exercise capacity, chronic stress, and nutritional state had all been reported to influence the level of klotho, the above complex program did not result in a significant change of klotho concentration in our patients.
The parameter of handgrip strength reflects total body muscle strength and is a predictor of mortality Citation(17). Two consistent results were reported regarding the relation of plasma klotho and handgrip strength in humans; both studies revealed a weak correlation between the two factors up to a certain klotho threshold, while above that level, this correlation was lost (Citation18,Citation19). Also, subjects with higher plasma klotho values had bigger knee extension strength Citation(20), and in patients with COPD, serum klotho levels correlated with quadriceps strength Citation(16).
In humans, an initial acute exercise did not result in the alteration of circulating klotho concentration (11); however, when the same subjects performed the same acute exercise after the completion of a long-term training program, their circulating klotho level significantly increased. The increase was even more expressed among young people. As age and physical fitness were shown to influence klotho response given to exercise Citation(11,21), an explanation for our negative result could be that healthy subjects and people suffering from certain diseases react in a different way. It can also be hypothesized that a 3-week period might be too short for the manifestation of alterations in resting plasma klotho concentration. The fact that the numerous, potentially influencing factors did not affect resting plasma klotho concentration may also suggest that in stable COPD, the protein level is relatively constant in the circulation.
Our findings do not contradict the results that showed an increase in plasma klotho concentration of healthy post-menopausal women following a 12-week moderate aerobic exercise training Citation(22). The ratio of the increase, although statistically significant, was less than double of the between-day variability measured in the current study.
Conclusions
Plasma klotho concentration can be reliably measured in stable COPD. The protein shows no correlation with clinical parameters; therefore, it cannot be considered as a potential biomarker in stable COPD. Despite clinical improvement, plasma klotho level remains stable during rehabilitation. In order to facilitate the interpretation of the relation between skeletal muscle activity and klotho expression, the determination of clinically relevant change is of utmost importance, and further studies that assess the dynamics of klotho-level alterations in response to acute interventions are also warranted.
Funding
This publication was supported by the Janos Bolyai Research Scholarship of the Hungarian Academy of Sciences to Andras Bikov.
Declaration of interest
The authors report no conflicts of interest.
References
- Mercado N, Ito K, Barnes P. Accelerated aging of the lung in COPD: new concepts. Thorax 2015; 70:482–489.
- Oliveira RM. Klotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett 2006; 580:5753–5758.
- Medici D, Razzaque MS, DeLuca S, Rector TL, Hou B, Kang K, et al. FGF-23—klotho signaling stimulates proliferation and prevents vitamin D-induced apoptosis. J Cell Biol 2008; 182:459–465.
- Mitobe M, Yoshida T, Sugiura H, Shirota S, Tsuchiya K, Nihei H. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol 2005; 101:e67–e74.
- Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 2007; 317:803–806.
- Yamamoto M, Clark JD, Pastor JV, Gurnani P, Nandi A, Kurosu H, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 2005; 280:38029–38034.
- Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, Suga T, et al. Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Comm 1998; 248:324–329.
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling aging. Nature 1997; 390:45–51.
- Rutten E, Gopal P, Wouters E, Franssen F, Hageman G, Vanfleteren L, et al. Various mechanistic pathways representing the aging process are altered in COPD. Chest 2016; 149:53–61.
- Gao W, Yuan C, Zhang J, Li L, Yu L, Wiegman C, et al. Klotho expression is reduced in COPD airway epithelial cells: effect on inflammation and oxidant injury. Clin Sci 2015; 129:1011–1023.
- Avin KG, Coen PM, Huang W, Stolz DB, Sowa GA, Dubé JJ, et al. Skeletal muscle as a regulator of the longevity protein, klotho. Front Physiol 2014; 5:189.
- Amitani M, Asakawa H, Amitani H, Kaimoto K, Sameshima N, Koyama KI, et al. Plasma klotho levels decrease in both anorexia nervosa and obesity. Nutrition 2013; 29:1106–1109.
- Prather AA, Epel ES, Arenander J, Broestl L, Garay BI, Wang D, et al. Longevity factor klotho and chronic psychological stress. Transl Psychiatry 2015; 5:e585.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS Statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166:111–117.
- Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, et al. Establishment of sandwitch ELISA for soluble alpha-klotho measurement: Age-dependent change of soluble alpha-klotho levels in healthy subjects. Biochem Biophys Res Commun 2010; 398:513–518.
- Patel MS, Donaldson AV, Lewis A, Natanek SA, Lee JY, Andersson YM, et al. Klotho and smoking—an interplay influencing the skeletal muscle function deficits that occur in COPD. Respir Med 2016; 113:50–56.
- Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality among healthy men. J Gerontol A Biol Sci Med Sci 2002; 57:B359–B365.
- Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Relationship of low plasma klotho with poor grip strength in older community-dwelling adults: the InCHIANTI study. Eur J Appl Physiol 2012; 112:1215–1220.
- Baldan A, Giusti A, Bosi C, Malaventura C, Musso M, Forni GL, et al. Klotho, a new marker for osteoporosis and muscle strength in β-thalassemia major. Blood Cells, Mol Dis 2015; 55:396–401.
- Semba RD, Ferrucci L, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Low plasma klotho concentrations and decline of knee strength in older adults. J Gerontol A Biol Sci Med Sci 2016; 71:103–108.
- Phelps M, Pettan-Brewer C, Ladiges W, Yablonka-Reuveni Z. Decline in muscle strength and running endurance in klotho deficient C57BL/6 mice. Biogerontology 2013; 14:729–739.
- Matsubara T, Miyaki A, Akazawa N, Choi Y, Ra SG, Tanahashi K, et al. Aerobic exercise training increases plasma klotho levels and reduces arterial stiffness in postmenopausal women. Am J Physiol Heart Circ Physiol 2014; 306:H348–H345.