ABSTRACT
Bronchiectasis is a long-neglected disease currently experiencing a surge in interest. It is a highly complex condition with numerous aetiologies, co-morbidities and a heterogeneous disease presentation and clinical course. The past few years have seen major advances in our understanding of the disease, primarily through large real-life cohort studies. The main outcomes of interest in bronchiectasis are symptoms, exacerbations, treatment response, disease progression and death. We are now more able to identify clearly the radiological, clinical, microbiological and inflammatory contributors to these outcomes. Over the past couple of years, multidimensional scoring systems such as the Bronchiectasis Severity Index have been introduced to predict disease severity and mortality. Although there are currently no licensed therapies for bronchiectasis, an increasing number of clinical trials are planned or ongoing. While this emerging evidence is awaited, bronchiectasis guidelines will continue to be informed largely by real-life evidence from observational studies and patient registries. Key developments in the bronchiectasis field include the establishment of international disease registries and characterisation of disease phenotypes using cluster analysis and biological data.
The ‘new age’ of bronchiectasis
Bronchiectasis, which is characterised by irreversible widening of the bronchi and/or branches along with inflammation and chronic bacterial infection, is a long-neglected disease currently experiencing a surge in interest Citation(1). The increasing incidence and prevalence of bronchiectasis, possibly related to population ageing and/or greater diagnostic awareness, is a significant concern for healthcare systems in view of the excess morbidity and mortality Citation(2,3) and high utilisation of healthcare resources Citation(4).
There are no licensed treatments specifically for bronchiectasis. Most therapies are extrapolated from either chronic obstructive pulmonary disease (COPD)/asthma (e.g., bronchodilators and inhaled corticosteroids) or cystic fibrosis (e.g., long-term antibiotics and mucoactive therapies) Citation(1). At the clinical level, identifying patients at highest risk of exacerbations or poor quality of life is important to intensify treatment. Similarly, identifying patients at low risk of unfavourable outcomes is important to avoid overuse of antibiotics and promote antimicrobial stewardship Citation(5). At the research level, many clinical trials have failed to meet their primary endpoints because of heterogeneous treatment responses and the inherent difficulty of identifying patients likely to respond (6−8). As a result, the concepts of phenotyping and disease stratification are now of paramount importance in bronchiectasis and across the spectrum of airway diseases Citation(9,10).
Phenotypes and endotypes
The term ‘phenotype’ is defined in general terms as a set of observable characteristics of an individual resulting from the interaction of its nature (genetics) and the environment. For persons with COPD, Han and colleagues have proposed a variation, which is: ‘a single or combination of disease attributes that describe differences between individuals with COPD as they relate to clinically meaningful outcomes (symptoms, exacerbations, response to therapy, rate of disease progression, or death)’ Citation(11). This definition applies equally well to patients with COPD, bronchiectasis or alpha-1-antitrypsin deficiency (AATD). In practical terms, phenotype describes aspects of the patient that influence clinical decision-making (e.g., the need for close monitoring because of a worse prognosis) or, perhaps more importantly, describes how a patient should be treated based on a specific response to a therapy. Phenotypes are clinically useful; for example, a COPD patient with a history of exacerbations is more likely to be an exacerbator in future and can thus be classed as an exacerbator phenotype Citation(12). Alternatively, a simple clinical phenotype ‘label’ can mask significant complexity. Patients may exacerbate for multiple reasons including eosinophilic or neutrophilic inflammation, immunodeficiency, co-morbidity, genetic susceptibility or microbial dysbiosis among others Citation(9,13). Insight into the underlying biology is therefore key to determining how to treat a ‘phenotype’. Bronchiectasis phenotypes are currently emerging. The ultimate aim of disease stratification studies is to define endotypes, which are subtypes of a condition defined by distinct functional and pathobiological mechanisms. As the pathophysiology of bronchiectasis continues to be poorly understood, it is not yet possible to base treatment decisions on endotypes.
Disease characteristics and outcomes in bronchiectasis
Describing bronchiectasis phenotypes requires integration of many pieces of information derived from various clinical domains. Each domain is associated to a greater or lesser degree with the clinically meaningful outcomes of symptoms, exacerbations, response to therapy, rate of disease progression, and death.
Radiology
Radiological findings in bronchiectasis are associated closely with aetiology, symptoms, exacerbation frequency and risk of death. Bronchiectasis shows considerable radiological heterogeneity. The most common pattern is lower lobe bronchiectasis, which is characteristic of idiopathic bronchiectasis but may also be associated with COPD, infection or aspiration. Bronchiectasis of the middle lobes is classically associated with nontuberculous mycobacteria (NTM) infection or primary ciliary dyskinesia. Upper lobe bronchiectasis is suggestive of cystic fibrosis; hence, all patients presenting with upper lobe predominant disease should be screened for cystic fibrosis. Central bronchiectasis is less common and is typically a manifestation of allergic bronchopulmonary aspergillosis (ABPA) or tracheobronchomegaly (Mounier-Kuhn syndrome).
In a study from the United Kingdom, more extensive bronchiectasis in terms of the number of lobes involved or the presence of cystic bronchiectasis was independently associated with severe exacerbations (hazard ratio [HR] 1.48; 95% confidence interval [CI]: 1.02−2.15), but was not an independent predictor of mortality Citation(14). The finding was in agreement with the work of Loebinger and colleagues who showed, in 91 patients, that the extent of bronchiectasis, severity of dilation, bronchial wall thickness, mucus plugging, mosaicism and emphysema were all associated with mortality on univariate analysis; however, none were independently associated with mortality on multivariate analysis Citation(15).
Microbiology
Microbiology is a major contributor to bronchiectasis phenotype. The absence/presence of bacteria and species type influence patients' symptomatology, drive a large proportion of exacerbations, and are independently associated with an increased risk of death.
Pseudomonas aeruginosa is the second most common organism isolated in bronchiectasis after Haemophilus influenzae and has the greatest impact on clinical outcomes. The derivation and validation study for the Bronchiectasis Severity Index, a predictive tool that identifies patients at risk of future mortality, hospitalisation and exacerbations, demonstrated a clear association between bacteriology and exacerbation frequency Citation(14). The mean number of annual exacerbations increased from 1.29 ± 0.9 in patients without regular bacterial colonisation in their sputum, to 2.04 ± 1.4 in the presence of some common respiratory pathogens, to 2.85 ± 1.5 in the presence of P. aeruginosa (). The negative prognostic impact of P. aeruginosa colonisation was also evident from a meta-analysis in which data were pooled from 21 observational cohort studies involving 3683 adult patients with bronchiectasis Citation(16). Compared to patients without P. aeruginosa colonisation, those with P. aeruginosa colonisation had a threefold higher risk of mortality (p < 0.0001), a sevenfold greater risk of hospital admission (p < 0.0001), an average of one additional exacerbation per year (p < 0.0001), a FEV1 15% lower than predicted, and significantly (p < 0.0001) worse quality of life as measured by the St. George's Respiratory Questionnaire (). Most differences persisted even after adjustment for factors such as baseline FEV1. Pseudomonas colonisation is by far the best described phenotype of bronchiectasis. There is also a degree of evidence suggesting that P. aeruginosa infection represents an ‘endotype’ since patients have distinct inflammatory profiles, with markedly elevated levels of neutrophil markers such as elastase, myeloperoxidase and matrix metalloproteinases (MMP) Citation(17,18). Some studies suggest that susceptibility to P. aeruginosa may have a genetic basis Citation(19) or may be related to the production of aberrant blocking antibodies Citation(20).
Figure 1. Impact of microbiology on annual exacerbation frequency in bronchiectasis Citation(14). MRSA, Methicillin-resistant S. aureus.
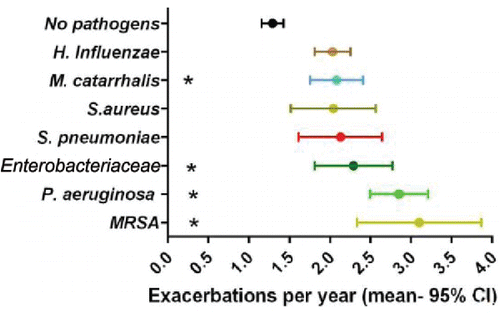
Table 1. Outcomes in bronchiectasis patients with versus without Pseudomonas aeruginosa colonisation: meta-analysis of 21 observational cohort studies comprising 3683 patients.
Bacteriology in bronchiectasis is becoming increasingly complex. Traditional culture methods are largely being replaced, at least for research purposes, with the lung microbiome, a technology that uses next-generation sequencing to produce a DNA profile of the diverse bacterial communities present in the lung Citation(21). Data on lung microbiota composition in bronchiectasis is thus far limited but suggest that less bacterial diversity correlates with lower lung function and higher levels of inflammation, which can be determined by measuring neutrophil markers such as MMPs Citation(18). Bronchiectasis patients with dominance of a single species, particularly Pseudomonas sp., have been shown to have worse symptoms and more inflammation than those with other airway pathogens Citation(22). Determining whether bronchiectasis phenotypes/endotypes can be defined based on microbiota profiles will require larger studies.
Aetiology
An important initial step in managing patients with bronchiectasis is to identify the underlying aetiology. Numerous diseases can lead to bronchiectasis, and the specific aetiology influences clinical manifestations and outcomes.
The European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) registry is a European Respiratory Society initiative aimed at collecting more data through a prospective, pan-European observational study of patients with bronchiectasis Citation(23). The study received central ethical approval in January 2015 and, as of September 2016, had participants from 232 registered centres in 40 countries. The registry currently holds data on more than 4000 patients and aims to recruit 10,000 patients by March 2020.
The most recent data from the EMBARC registry, which was presented at the European Respiratory Society (ERS) Congress 2016, reported on 2031 patients from 23 European countries Citation(24). Mean age of patients with bronchiectasis in Europe was 63 years, and 58% were women Citation(24). The most common aetiology was idiopathic bronchiectasis (39%), followed by post-infective bronchiectasis (27%). A relevant proportion of patients had diagnoses requiring a specific treatment, including immunodeficiency diseases, ABPA, NTM infection and AATD. For best outcomes, such patients must be identified from the general bronchiectasis population and treated appropriately (e.g., corticosteroids with or without antifungal treatment for ABPA). The demographic and disease-related characteristics of the population were similar to other cohorts reported from Europe Citation(25,26). In contrast, there are marked differences in the presentation of bronchiectasis internationally. For example, the bronchiectasis population described in Guangzhou, China, had a similar gender balance (62% female) but was considerably younger (mean age 44 years) than the European cohorts. Aetiology was mainly idiopathic (46%), followed by post-infectious (27%), and immunodeficiency (9%) Citation(27). The EMBARC registry has recently expanded to include non-European centres including India. First data from the Indian registry were presented at ERS 2016. In India (n = 552), the bronchiectasis population was 63% male and had a mean age of 51 years. The most frequent aetiologies were post-tuberculosis bronchiectasis (29%), idiopathic bronchiectasis (27%) and post-infective (non-TB) bronchiectasis (21%). A different pattern of bronchiectasis again is observed in the United States. First analysis of the United States Bronchiectasis Research Registry (n = 1826) indicated a mean age similar to European cohorts (64 years), but with greater female predominance (79%) and a high proportion of patients (63%) with a history of NTM infection or NTM isolated at the time of baseline evaluation into the registry Citation(28).
Insight into local patterns of bronchiectasis is of considerable clinical importance due to the direct influence of aetiology on treatment decisions. For example, in Europe, about 20% of bronchiectasis patients are treated with macrolide antibiotics. In the United States, macrolide use would need to be approached with extreme caution to avoid inducing NTM resistance. Demographic and clinical heterogeneity between bronchiectasis populations also influence the ability to perform clinical trials and the extent to which results from any given region would apply elsewhere. Observational studies and patient registries represent the actual population in a given location and are thus essential in terms of understanding the true complexity and heterogeneity of this disease.
Understanding prognosis in bronchiectasis
Multidimensional scoring systems developed for COPD, such as the BODE (Body mass index [BMI], airflow Obstruction, Dyspnea and Exercise capacity) Index and GOLD (Global initiative for chronic Obstructive Lung Disease) classification system, have increased our understanding of disease heterogeneity. In the past few years, similar grading systems have also been introduced in bronchiectasis.
As mentioned above, the Bronchiectasis Severity Index was developed as a tool to determine disease severity by identifying independent risk factors for mortality, exacerbations, hospitalisations, and quality of life Citation(14). The scoring system was derived initially using data from 608 patients enrolled in a prospective cohort study (Edinburgh, UK). The instrument was subsequently validated in independent patient cohorts from Dundee, UK (n = 218), Leuven, Belgium (n = 253), Monza, Italy (n = 105) and Newcastle, UK (n = 126). The Bronchiectasis Severity Index comprises eight factors: age; BMI; FEV1; hospital admissions; exacerbations; breathlessness; P. aeruginosa colonisation or colonisation with other bacteria; and radiological severity. Scores range from a minimum of 0 to a maximum of 25. Based on disease severity, it is possible to describe bronchiectasis phenotypes that relate to clinically relevant outcomes.
Elsewhere, a Spanish group has developed a multidimensional scoring system to predict mortality in patients with bronchiectasis Citation(29). Data used to construct the FACED score were derived from a retrospective cohort study of 819 patients with bronchiectasis diagnosed by high-resolution computed tomography (CT); 397 patients were used to construct the score, and the remaining 422 patients were used to validate the score. The system incorporates five dichotomised variables associated with poor outcomes: FEV1 (F, cut-off 50%, maximum value 2 points); age (A, cut-off 70 years, 2 points); colonisation with P. aeruginosa (C, yes/no, 1 point); radiological extension (E, number of affected lobes, cut-off 2 lobes, 1 point); and dyspnoea (D, cut-off grade II on the Medical Research Council scale, 1 point). Patients with higher scores (5–7 points) have a significantly higher risk of mortality compared to those with lower scores.
A series of large studies comparing these scoring systems confirmed that, while their ability to predict mortality may be similar, there are also some important differences. The Bronchiectasis Severity Index accurately reflects disease severity and disease impact such as exacerbation frequency, hospital admissions, quality of life, exercise capacity and symptoms such as cough Citation(30,31). In contrast, the FACED scoring system lacks this same ability, possibly because it is heavily weighted by age. Two points are awarded for age, with the cut-off being 70 years. Thus, in patients aged < 70 years (the majority of bronchiectasis populations), all other factors must be present for a patient to be classified as high risk. Many patients with high disease impact are thus classified as mild or moderate risk. The relevance of this difference between the scoring systems is illustrated by examining patients receiving lung transplantation for bronchiectasis, the classic example of a patient subset regarded universally as ‘severe’. In a recent series of 34 patients who underwent lung transplantation for bronchiectasis Citation(32), the Bronchiectasis Severity Index identified 100% of patients as severe, whereas more than half of patients were identified as mild or moderate by the FACED score.
Thus, for optimal result with these scoring systems, each instrument must be used solely for its intended purpose. The Bronchiectasis Severity Index has been designed to predict severity and quality of life across a range of outcomes, whereas the FACED score was developed to predict mortality alone.
Multidimensional clustering
Multidimensional clustering has been proven to be a highly successful approach to understanding heterogeneity in COPD and asthma Citation(33,34). The method involves applying complex statistical methods to a large data set to determine which demographic and clinical variables are inter-related, and then using these ‘clusters’ to describe phenotypes. To this end, a secondary analysis was conducted of five European databases involving 1145 prospectively enrolled adult patients with bronchiectasis Citation(35). Principal component and cluster analyses were performed using demographics, co-morbidities, and clinical, radiological, functional and microbiological variables collected from patients during the stable state. Data on exacerbations, hospitalisations and mortality recorded during 3-year follow-up were also included in the analysis. Four bronchiectasis phenotypes were identified based on colonisation status and daily sputum production:
| • | severe Pseudomonas infection (16%); | ||||
| • | other chronic infections (e.g. Haemophilus) (24%); | ||||
| • | daily sputum production without colonisation (33%); | ||||
| • | dry bronchiectasis (27%). | ||||
During follow-up, patients in the four clusters showed significant differences in terms of their quality of life, exacerbations, hospitalisations and mortality. There was also clear evidence of increased neutrophilic inflammation in the infection-driven clusters Citation(35).
While the analysis has a certain degree of value in terms of informing future research (e.g., focused treatment by phenotype), it can also be criticised for not extending current knowledge Citation(5). Similar analyses conducted in other world regions have provided some interesting variations.
In China, hierarchical cluster analysis was performed using demographic data, as well as clinical variables relating to lung function, sputum bacteriology, aetiology, radiology, disease severity, quality of life, cough scale, capsaicin sensitivity, exercise tolerance, health care use and frequency of exacerbations in 148 patients with bronchiectasis Citation(36). Four distinct clusters were described:
| • | mild and idiopathic bronchiectasis in young patients; | ||||
| • | severe patients with post-infective bronchiectasis and P. aeruginosa; | ||||
| • | late onset severe idiopathic bronchiectasis; | ||||
| • | elderly patients with moderate disease. | ||||
The only consistency between the Chinese and European analyses was the Pseudomonas phenotype, which is not surprising given the demographic and aetiological differences in bronchiectasis populations between these two regions. The relatively small patient sample in the Chinese study may also have contributed to variation in the proposed phenotypes.
Cluster analysis was also performed in Spain using data from the same retrospective cohort study used to derive the FACED score Citation(37). The available population (n = 468) was 58% female with a mean age of 63 years. Significant variables used for this analysis included age, gender, BMI, smoking habit, dyspnoea, macroscopic appearance of sputum, number of exacerbations, chronic colonisation with P. aeruginosa, FEV1, number of pulmonary lobes affected, idiopathic bronchiectasis and associated COPD. Once again, four distinct phenotypes with different prognoses were identified:
| • | young women with mild disease; | ||||
| • | overweight elderly women with mild bronchiectasis; | ||||
| • | elderly men with severe disease, chronic infection (mainly with P. aeruginosa), airflow obstruction and exacerbations; | ||||
| • | elderly patients with ‘severe’ disease but infrequent exacerbations. | ||||
The take-home message to emerge from examining these three separate analyses, which incorporated different approaches (i.e., variables considered significant for analysis) but used the same statistical method, is that the phenotypes proposed by each group may or may not be true phenotypes. Only the P. aeruginosa infection, frequently exacerbating phenotype was consistent across all analyses and, indeed, is the most robust phenotype identified to date in bronchiectasis Citation(16). A challenge for future may be to look beyond phenotypes and aim towards identifying endotypes.
Co-morbidity
On account of the relatively advanced average age of bronchiectasis patients in Europe and other Western regions (e.g. 60−70 years), the presence of co-morbidities apart from the underlying aetiological disease/s is common. In a multicentre cohort analysis of 986 patients with bronchiectasis from four European centres, the average patient had four co-morbid conditions Citation(38). An independent relationship was demonstrated between the number of co-morbidities and long-term mortality. Co-morbidities independently associated with mortality were malignant disease (including haematological malignancy), COPD, cognitive impairment, inflammatory bowel disease, liver disease, connective tissue diseases (e.g. rheumatoid arthritis), iron deficiency anaemia, diabetes, asthma, pulmonary hypertension, peripheral vascular disease and ischaemic heart disease. Other groups have been hinting at these relationships for some time, with individual studies showing the importance of co-morbid COPD Citation(39), asthma Citation(40) and cardiovascular diseases Citation(41) on the risk of poorer outcomes.
The study of McDonnell and co-workers is particularly relevant for having been first to demonstrate the link between co-morbidities and aetiologies, which were then used to construct the Bronchiectasis Aetiology and Co-morbidity Index (BACI) Citation(38). The BACI predicted 5-year mortality (p < 0.0001), hospital admissions (p < 0.0001), exacerbations (p = 0.03) and quality of life (p < 0.0008) for all strata of bronchiectasis severity as assessed by the Bronchiectasis Severity Index Citation(14). P. aeruginosa colonisation was also linked to co-morbidities. Co-morbidities predicted mortality risk with a higher accuracy than markers of bronchiectasis severity, emphasising the importance of incorporating aetiologies and co-morbidities into multidimensional phenotyping of patients with bronchiectasis.
Inflammation and elastin breakdown
COPD, AATD and bronchiectasis all share in common the presence of neutrophilic inflammation, although each condition is characterised to a differing degree by bacterial infection. In bronchiectasis, higher airway bacterial loads are associated with airway and systemic inflammation and greater risk of exacerbation Citation(42). Lungs of bronchiectasis patients show active proteolytic damage similar to that observed in COPD with high levels of neutrophil elastase, MMPs and other markers. Although the damage may be ameliorated to some degree with antibiotic therapy, it tends to return soon after the antibiotics are discontinued Citation(42).
Neutrophil elastase, a serine protease released from primary neutrophil granules, is linked to disease severity in bronchiectasis. Tsang and colleagues showed a clear association between elastase in sputum and extent of bronchiectasis and FEV1 Citation(43), while Goeminne and co-workers showed that elastase was responsible for 82% of the total gelatinolytic activity of bronchiectasis sputum Citation(44). Elastin breakdown in patients with bronchiectasis is evident in lung histology early in the disease and increases with worsening severity. Proteolytic breakdown can be measured indirectly (serum desmosine) through the generation of unique cross linking amino acids that are released when elastin is degraded. Recent data in 386 patients from the United Kingdom showed that elevated sputum neutrophil elastase activity was associated with shorter time to next exacerbation, more rapid lung function decline, higher risk of hospitalisation, and mortality Citation(45). Desmosine was also significantly associated with the risk of severe exacerbations (HR 2.7; 95% CI: 1.42−5.29; p = 0.003). Neutrophilic inflammation is thus a key driver of disease progression in bronchiectasis. Patients with accelerated elastin breakdown may represent a bronchiectasis endotype Citation(45).
Treatment of bronchiectasis
It remains uncertain, at present, whether bronchiectasis phenotypes described to date will be able to inform treatment. Data regarding bronchiectasis treatment, and more so phenotype-directed treatment, are limited. Primary goals of treatment continue to be to reduce exacerbations and improve quality of life. Bronchiectasis management is expected to undergo considerable change over the next 5 years as the results of ongoing clinical trials (e.g., multiple antibiotics, anti-inflammatory agents, extrapolation of therapies used in cystic fibrosis) become available. Nevertheless, we remain without robust evidence for many widely used treatments. A lack of research funding and the ‘orphan nature’ of the disease, together with heterogeneity in aetiology and presentation, are likely to preclude the design and conduct of large-scale randomised controlled trials for many therapies. For the foreseeable future, it is expected that core knowledge will continue to come from real-life clinical data and registries. Treatment will remain largely empirical using best clinical judgement.
A consensus statement from the EMBARC Clinical Research Collaboration has identified key research priorities in the field of bronchiectasis Citation(46). The 55 recommendations provide a roadmap for improving our understanding of the disease and providing better outcomes for patients.
Conclusions
Bronchiectasis is a rapidly developing field. Although disease phenotyping is in its infancy, analyses performed to date suggest enormous heterogeneity in disease severity and presentation as well as potential to identify populations with greater likelihood of treatment response and varying prognoses. Many patients with bronchiectasis experience long diagnostic delays, and underdiagnosis continues to be common. While new therapies are welcomed, their introduction will bring the associated challenge of identifying patients likely to gain the most benefit. Most potential new therapies are antibiotic-related and thus carry the risk of antibiotic resistance. Registries, networks and greater collaborative efforts are essential elements of generating real-life data to inform clinical guidelines.
Declaration of interest
James D Chalmers currently holds research grants from Aradigm Corporation, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, European Union Innovative Medicines Initiative, GlaxoSmithKline, Insmed, Medical Research Council, Pfizer, Polyphor, Scottish Government, and Wellcome Trust. He has received fees for consultancy or speaking from AstraZeneca, Bayer Healthcare, Chiesi, Grifols, Napp, and Pfizer.
Funding
Editorial assistance was provided by Content Ed Net (Madrid, Spain) with funding from Grifols SA (Barcelona Spain).
References
- Chalmers JD, Aliberti S, Blasi F. Management of bronchiectasis in adults. Eur Respir J 2015; 45(5):1446−1462.
- Roberts HJ, Hubbard R. Trends in bronchiectasis mortality in England and Wales. Respir Med 2010; 104(7):981–985.
- Quint JK, Millett ER, Joshi M, Navaratnam V, Thomas SL, Hurst JR, et al. Changes in the incidence, prevalence and mortality of bronchiectasis in the UK from 2004 to 2013: a population-based cohort study. Eur Respir J 2016; 47(1):186−193.
- Ringshausen FC, de Roux A, Diel R, Hohmann D, Welte T, Rademacher J. Bronchiectasis in Germany: a population-based estimation of disease prevalence. Eur Respir J 2015; 46(6):1805−1807.
- Smith DJ. Phenotyping bronchiectasis: is it all about sputum and infection? Eur Respir J 2016; 47(4):1037−1039.
- O'Donnell AE, Barker AF, Ilowite JS, Fick RB. Treatment of idiopathic bronchiectasis with aerosolized recombinant human DNase I. rhDNase Study Group. Chest 1998; 113(5):1329−1334.
- Bilton D, Tino G, Barker AF, Chambers DC, De Soyza A, Dupont LJ, et al. Inhaled mannitol for non-cystic fibrosis bronchiectasis: a randomised, controlled trial. Thorax 2014; 69(12):1073−1079.
- Barker AF, O'Donnell AE, Flume P, Thompson PJ, Ruzi JD, de Gracia J, et al. Aztreonam for inhalation solution in patients with non-cystic fibrosis bronchiectasis (AIR-BX1 and AIR-BX2): two randomised double-blind, placebo-controlled phase 3 trials. Lancet Respir Med 2014; 2(9):738−749.
- Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016; 47(2):410−419.
- Barnes NC, Sharma R, Lettis S, Calverley PM. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J 2016; 47(5):1374−1382.
- Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010; 182(5):598−604.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363(12):1128−1138.
- Wang Z, Bafadhel M, Haldar K, Spivak A, Mayhew D, Miller BE, et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J 2016; 47(4):1082−1092.
- Chalmers JD, Goeminne P, Aliberti S, McDonnell MJ, Lonni S, Davidson J, et al. The Bronchiectasis Severity Index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189(5):576−585.
- Loebinger MR, Wells AU, Hansell DM, Chinyanganya N, Devaraj A, Meister M, Wilson R. Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J 2009; 34(4):843−849.
- Finch S, McDonnell MJ, Abo-Leyah H, Aliberti S, Chalmers JD. A comprehensive analysis of the impact of pseudomonas aeruginosa colonization on prognosis in adult bronchiectasis. Ann Am Thorac Soc 2015; 12(11):1602−1611.
- Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2012; 186(7):657−665.
- Chalmers JD, McHugh BJ, Doherty C, Smith MP, Govan JR, Kilpatrick DC, Hill AT. Mannose-binding lectin deficiency and disease severity in non-cystic fibrosis bronchiectasis: a prospective study. Lancet Respir Med 2013; 1(3):224−232.
- Taylor SL, Rogers GB, Chen AC, Burr LD, McGuckin MA, Serisier DJ. Matrix metalloproteinases vary with airway microbiota composition and lung function in non-cystic fibrosis bronchiectasis. Ann Am Thorac Soc 2015; 12(5):701−707.
- Wells TJ, Whitters D, Sevastsyanovich YR, Heath JN, Pravin J, Goodall M, et al. Increased severity of respiratory infections associated with elevated anti-LPS IgG2 which inhibits serum bactericidal killing. J Exp Med 2014; 211(9):1893−1904.
- Tunney MM, Einarsson GG, Wei L, Drain M, Klem ER, Cardwell C, et al. Lung microbiota and bacterial abundance in patients with bronchiectasis when clinically stable and during exacerbation. Am J Respir Crit Care Med 2013; 187(10):1118–1126.
- Rogers GB, van der Gast CJ, Cuthbertson L, Thomson SK, Bruce KD, Martin ML, et al. Clinical measures of disease in adult non-CF bronchiectasis correlate with airway microbiota composition. Thorax 2013; 68(8):731–737.
- Chalmers JD, Aliberti S, Polverino E, Vendrell M, Crichton M, Loebinger M, et al. The EMBARC European Bronchiectasis Registry: protocol for an international observational study. ERJ Open Res 2016; 2(1):pii: 00081–2015. eCollection 2016.
- Haworth CS, Johnson C, Aliberti S, Goeminne PC, Ringhausen F, Boersma W, et al. Management of bronchiectasis in Europe: data from the European bronchiectasis registry (EMBARC). Eur Respir J 2016; 48(Suppl 60):OA273. DOI: 10.1183/13993003.congress-2016.OA273.
- Lonni S, Chalmers JD, Goeminne PC, McDonnell MJ, Dimakou K, De Soyza A, et al. Etiology of non-cystic fibrosis bronchiectasis in adults and its correlation to disease severity. Ann Am Thorac Soc 2015; 12(12):1764−1770.
- Pasteur MC, Helliwell SM, Houghton SJ, Webb SC, Foweraker JE, Coulden RA, et al. An investigation into causative factors in patients with bronchiectasis. Am J Respir Crit Care Med 2000; 162(4 Pt 1):1277−1284.
- Guan WJ, Gao YH, Xu G, Lin ZY, Tang Y, Li HM, et al. Aetiology of bronchiectasis in Guangzhou, southern China. Respirology 2015; 20(5):739–748.
- Aksamit TR, O'Donnell AE, Barker A, Olivier KN, Winthrop KL, Daniels ML, et al. Adult bronchiectasis patients: a first look at the United States Bronchiectasis Research Registry. Chest 2016 Nov 23; pii: S0012–3692(16)62354–1.
- Martínez-García MÁ, de Gracia J, Relat MV, Girón RM, Carro LM, de la Rosa Carrillo D, et al. Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014; 43(5):1357−1367.
- McDonnell MJ, Aliberti S, Goeminne PC, Dimakou K, Zucchetti SC, Davidson J, et al. Multidimensional severity assessment in bronchiectasis: an analysis of seven European cohorts. Thorax 2016 Aug 11; pii: thoraxjnl-2016-208481. doi: 10.1136/thoraxjnl-2016-208481. [Epub ahead of print].
- Ellis HC, Cowman S, Fernandes M, Wilson R, Loebinger MR. Predicting mortality in bronchiectasis using bronchiectasis severity index and FACED scores: a 19-year cohort study. Eur Respir J 2016; 47(2):482−489.
- Rademacher J, Ringshausen FC, Suhling H, Fuge J, Marsch G, Warnecke G, et al. Lung transplantation for non-cystic fibrosis bronchiectasis. Respir Med 2016; 115:60−65.
- Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med 2011; 184(6):662−671.
- Amelink M, de Nijs SB, de Groot JC, van Tilburg PM, van Spiegel PI, Krouwels FH, et al. Three phenotypes of adult-onset asthma. Allergy 2013; 68(5):674−680.
- Aliberti S, Lonni S, Dore S, McDonnell MJ, Goeminne PC, Dimakou K, et al. Clinical phenotypes in adult patients with bronchiectasis. Eur Respir J 2016; 47(4):1113−1122.
- Guan WJ, Jiang M, Gao YH, Li HM, Xu G, Zheng JP, et al. Unsupervised learning technique identifies bronchiectasis phenotypes with distinct clinical characteristics. Int J Tuberc Lung Dis 2016; 20(3):402−410.
- Martínez-García MÁ, Vendrell M, Girón R, Máiz-Carro L, de la Rosa Carrillo D, de Gracia J, et al. The multiple faces of non-cystic fibrosis bronchiectasis. A cluster analysis approach. Ann Am Thorac Soc 2016; 13(9):1468−1475.
- McDonnell MJ, Aliberti S, Goeminne PC, Restrepo MI, Finch S, Pesci A, et al. Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med 2016; 4(12):969−979.
- Goeminne PC, Nawrot TS, Ruttens D, Seys S, Dupont LJ. Mortality in non-cystic fibrosis bronchiectasis: a prospective cohort analysis. Respir Med 2014; 108(2):287−296.
- Mao B, Yang JW, Lu HW, Xu JF. Asthma and bronchiectasis exacerbation. Eur Respir J 2016; 47(6):1680−1686.
- Navaratnam V, Millett ER, Hurst JR, Thomas SL, Smeeth L, Hubbard RB, et al. Bronchiectasis and the risk of cardiovascular disease: a population-based study. Thorax 2017; 72(2):161–166.
- Chalmers JD, Smith MP, McHugh BJ, Doherty C, Govan JR, Hill AT. Short- and long-term antibiotic treatment reduces airway and systemic inflammation in non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2012; 186(7):657−665.
- Tsang KW, Chan K, Ho P, Zheng L, Ooi GC, Ho JC, Lam W. Sputum elastase in steady-state bronchiectasis. Chest 2000; 117(2):420−426.
- Goeminne PC, Vandooren J, Moelants EA, Decraene A, Rabaey E, Pauwels A, et al. The Sputum Colour Chart as a predictor of lung inflammation, proteolysis and damage in non-cystic fibrosis bronchiectasis: a case-control analysis. Respirology 2014; 19(2):203−210.
- Chalmers JD, Moffitt KL, Suarez-Cuartin G, Sibila O, Finch S, Furrie E, et al. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med 2016 Dec 2. [Epub ahead of print].
- Aliberti S, Masefield S, Polverino E, De Soyza A, Loebinger MR, Menendez R, et al. Research priorities in bronchiectasis: a consensus statement from the EMBARC Clinical Research Collaboration. Eur Respir J 2016; 48(3):632−647.
