ABSTRACT
During acute exacerbation of chronic obstructive pulmonary disease (AECOPD), myocardial stress may be aggravated. Sparse data exist concerning the prevalence and correlates of cardiac arrhythmias in the stable and exacerbated states of COPD. We hypothesized that AECOPD is associated with increased prevalence of cardiac arrhythmias independent of COPD-severity and co-morbidity, and explored possible mechanisms. A 24-hour Holter recording was obtained in 74 patients with stable COPD and 45 patients with AECOPD (mean age 54 years, 56% women). Any incidence of supraventricular tachycardia (SVT), frequent premature ventricular complex (PVC, >30/hour) and complex ventricular ectopy (bigeminy, trigeminy or non-sustained ventricular tachycardia) was recorded and compared between the two groups. Adjustments were made for by stable disease-related co-variates (demography, co-morbidity, COPD-severity) and by acute disease-related co-variates (heart rate, cardiac troponin T (cTnT), PO2, PCO2 and C-reactive protein (CRP)) in explorative analyses. The prevalence of SVT, frequent PVCs or complex ventricular ectopy was 40%, 27% and 33% in AECOPD, and 31%, 31% and 12% in stable COPD, respectively. Frequent PVC, but not SVT or complex ventricular ectopy, was significantly increased in AECOPD compared to stable COPD, odds ratio 3.03 (1.03–10.5, p = 0.039) when adjusted for stable disease-related co-variates. Higher heart rate, cTnT and CRP attenuated the association between AECOPD and frequent PVC to non-significant, while heart rate remained associated with frequent PVC. In conclusion, frequent PVC is more prevalent in exacerbated than in the stable states of COPD. Attenuation effects of cTnT, tachycardia and CRP suggest that cardiac stress or inflammation may be involved in mechanisms causing frequent PVC i AECOPD.
Introduction
Chronic obstructive pulmonary disease (COPD) is the third most common cause of mortality in Western countries (Citation1, Citation2). Medical co-morbidities, such as cardiovascular diseases, contribute to the overall morbidity and mortality in patients with COPD (Citation3, Citation4). The patient phenotype characterized by frequent acute exacerbations of COPD (AECOPD) has recently received attention, as it may be associated with rapid progression of COPD as well as increased short-term mortality Citation(5). Cardiovascular co-morbidity may be of particular importance during AECOPD, and the levels of circulating biomarkers of cardiac disease such as cardiac troponins (cTns) have been reported to peak during AECOPD (Citation6–9). Still, the direct causes for cTn release are not fully understood Citation(10).
Non-fatal cardiac tachyarrhythmia is another biomarker which in some, but not all, studies has been linked to increased risk of sudden cardiac death and death from cardiovascular causes (Citation11–14). Patients with COPD are often exposed to pro-arrhythmic factors such as hypoxemia, inflammation and drugs with chronotropic and pro-arrhythmic effects, particularly during AECOPD. Previously, the prevalence of atrial fibrillation/flutter and non-sustained ventricular tachycardia (NSVT) has been reported to be higher in patients with stable COPD compared to patients without COPD Citation(15). However, to our knowledge there are no studies comparing the prevalence of cardiac arrhythmias between AECOPD and stable COPD.
Accordingly, the primary aim of the present study was to test the hypothesis that the prevalence of cardiac arrhythmia is higher in patients during AECOPD than in patients with COPD in a stable state. Secondly, we sought to explore potential mechanisms that may mediate any increased risk for arrhythmias during acute disease.
Material and methods
Design and population
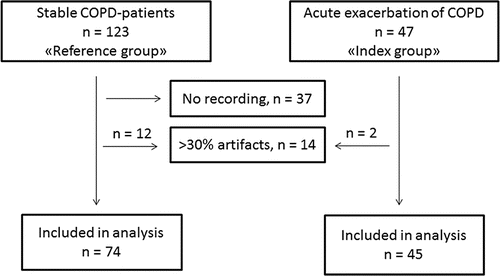
This was a cross-sectional study of patients with COPD (). The patients were between 40 and 74 years of age, they had smoked at least 10 pack-years, and they had airway obstruction verified by spirometry during stable state within the last year. The index group consisted of 47 patients hospitalized at Akershus University Hospital due to AECOPD. On a daily basis between January and September 2010, one of the authors (VS) consecutively screened patients admitted to the bed ward at the Pulmonary Department for presence of AECOPD. According to the recommendations Citation(2), patients with established COPD, ≥2 days of increase in either two of the symptoms dyspnea, cough, volume or colour of sputum were invited to participate in the study between the 3rd and 5th day of the admission. Exclusion criteria were reduced mental functioning or other psychiatric or somatic diseases hindering obtainment of an informed consent.
The reference group consisted of 123 patients admitted to a pulmonary rehabilitation clinic (Glittre), further details have been reported previously Citation(16). The first 37 consecutive patients admitted to Glittre were not included in the current analysis due to technical problems ().
Data collection
Cardiac arrhythmias
A 5-channel Holter recording (MedilogAR12, Oxford instruments Medical Ltd., Surrey, United Kingdom) was performed in 133 patients. The recordings started in the evening and had a mean length of 23.7 hours (standard deviation (SD) 3.3 hours). The digital recordings were automatically interpreted by a software engine (Medilog Darwin, ScanMed Medical, Gloucestershire, United Kingdom), and manually reviewed by two researchers (GE and RB) blinded to participant data. Recordings with more than 30% artefacts (n = 2 in index group, n = 12 in the reference group) were excluded from further analyses (). Episodes of atrial fibrillation, atrial flutter, atrial tachycardia or other supraventricular tachycardias (SVTs) with >3 complexes and >140 min−1 were defined as supraventricular tachyarrhythmia. A single ventricular extrasystole (VES) was defined as any QRS-complex >0.12 ms with a normal (N) QRS-complex before and after. In patients with known bundle branch block, a change in QRS-morphology and length was also considered to be of ventricular origin. Bigeminies and trigeminies were defined as ≥3 consecutive V-N or V-N-N couples, respectively. Ventricular tachycardia (VT) was defined as three or more consecutive V > 110 min−1, and defined as NSVT if shorter than 30 seconds. All arrhythmias were coded as dichotomous outcomes (present or absent) according to previously used cut-offs Citation(11).
Medical history, clinical examination and laboratory analyses
Details regarding age, sex, medical co-morbidities, body mass index (use of short and long-acting inhaled beta-adrenergic agonists, inhaled anticholinergic agents, inhaled corticosteroids, beta blockers and oxygen) were obtained from patient interviews and medical records' for readability. History of hypertension and diabetes mellitus were self-reported, while history of coronary heart disease (CHD) was defined as an established diagnosis of myocardial infarction, stable angina or previous coronary revascularization. Cumulative tobacco-exposure was measured in pack-years. A standard spirometry test was performed in the stable COPD group, and results recorded from a recent (<1 year) spirometry during a stable state were obtained from the medical records among patients with AECOPD. Post-bronchodilatory forced vital capacity (FVC) and forced expiratory volume during the 1st second (FEV1) were registered, and the FEV1/FVC ratio was calculated. Left ventricular hypertrophy was assessed by the Sokolow-Lyon criteria on a standard 12-lead electrocardiogrm Citation(17).
Regarding factors associated with the acute disease, the variables associated with cardiac stress, hypoxemia and systemic inflammation were recorded. Mean heart rate was calculated based on the whole Holter recording. High-sensitivity (hs) cTnT was analysed consecutively in the index group, while in the reference group, venous blood was sampled in the morning after the 24-hour Holter registration and the serum samples were frozen at −80°C pending analysis. Hs-cTnT was analysed by using COBAS Integra 400 with an enzyme-linked immunoassay (Elecsys Troponin T hs STAT, Roche Diagnostics, Basel, Switzerland). The lower limit of normal is 5 ng/L, the lowest value with 10% variability and the upper 99% percentile in adult population is 14 ng/L. Arterial blood was obtained from a puncture of the radial artery at admission for both groups, and standard analyses of pO2 and pCO2 were performed. Systemic inflammation was assessed by a high-sensitive assay of the venous concentration of C-reactive protein (CRP).
Statistical analyses
Prior to the study start, the sample size calculation assumed that median VES/h was 5 in patients with 5 in patients with TnT < 0.04 µg/L (old assay), and 10 in patients with TnT > 0.04 µg/L. With a 1:3 allocation of patients above and below these cut-offs, α = 0.05 and 1-β = 0.9 is achievable for a total of 100 patients. Continuous and categorical data are reported as mean (SD) and number (%). Continuous data were tested for normality by the Kolmogorov-Smirnov test, and log-transformed if not normally distributed. Comparisons between the two groups were performed by t-tests, Mann-Whitney U-tests or chi-square tests, as appropriate.
Co-variates were separated into stable and acute disease-related. Only stable disease-related co-variates (age, sex, pack-years, lung function, co-morbidity, body mass index, medication, left ventricular hypertrophy, oxygen treatment) were considered regarding the primary aim of the study, while acute disease-related co-variates (cTnT, heart rate, arterial blood gases and CRP) were considered in the explorative secondary aim of the study.
The primary aim was assessed in three steps. First, we compared the prevalence of each type of arrhythmia between the stable COPD and the AECOPD groups. Next, we compared the stable disease-related co-variates between the stable COPD and AECOPD groups, and the co-variates that were associated with AECOPD having p-value ≤0.2 were then analysed for univariate association with each of the arrhythmias. In the third step, multivariate logistic regression models were made for the arrhythmias that deviated significantly between the stable COPD and AECOPD groups. These were adjusted for by co-variates that were associated with both AECOPD and arrhythmias.
In assessing the second aim of the study, we used the Mantel-Haenszel test in stratified analyses by the acute disease-related co-variates that were associated (p ≤ 0.2) with AECOPD. This was performed to assess possible confounding and effect modification of the association between AECOPD and arrhythmia by the acute disease-related co-variates, and to assess whether these co-variates caused meaningful changes in the association between AECOPD and arrhythmia. Finally, we made a second explorative multivariate model, to assess the associations between arrhythmias and AECOPD per se, adjusted for the acute disease-related co-variates.
If not otherwise mentioned, a p-value <0.05 in two-way analyses was regarded statistically significant. The statistical analyses were performed with the Statistical Package for Social Sciences (SPSS), version 20.0 (IBM, Chicago, IL) and Stata version 14.1 (StataCorp LP, TX77845).
Ethical considerations
The studies were approved by the regional ethical committee and signed consent was obtained from all participants before inclusion. The studies were conducted in accordance with the Declaration of Helsinki.
Results
Characteristics of the index group (n = 45) and the reference group (n = 74) are shown in . The AECOPD group was characterized by more prevalent CHD, more frequent use of beta blockers and less use of long-acting beta agonists compared to the stable COPD patients. The patients with AECOPD also had a tendency toward higher age, lower body mass index, lower lung function, less hypertension and being more often male than stable COPD patients.
Table 1. Comparisons of demographic, clinical and biochemical variables between stable COPD patients and patients hospitalized for AECOPD.
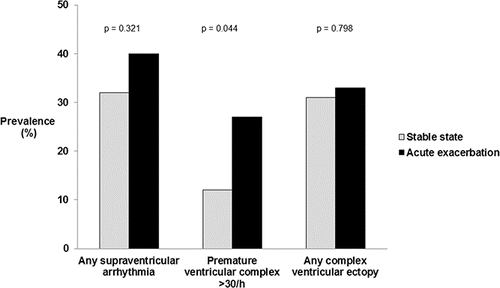
The number of patients with any incidence of supraventricular arrhythmia, frequent PVC or any complex ventricular ectopy were 41 (35%), 21 (18%) and 38 (32%), respectively. Paroxysmal short SVT (n = 37) was more common than persistent atrial fibrillation or flutter (n = 4). There were no VTs registered in any patients, while the overall prevalence of NSVT, bigeminies and trigeminies was 11 (12%), 12 (12%) and 18 (19%), respectively. The prevalence of supraventricular arrhythmias and ventricular ectopy according to COPD state is shown in . AECOPD was associated with frequent PVC (odds ratio (OR) 2.63, 95% confidence interval (CI) 1.01–6.86, p = 0.049), while there were no associations between AECOPD and the presence of supraventricular arrhythmia or complex ventricular ectopy (OR (95% CI) 1.11 (0.50, 2.45) and 1.45 (0.68, 3.20), respectively).
Figure 2. Prevalence in % of cardiac arrhythmias in patients with stable COPD (n = 75) and patients with acute exacerbation of COPD (n = 45).
The associations between stable co-variates and presence of frequent PVC are shown in . Subsequently, adjustments were made for sex, age, history of hypertension, history of CHD, body mass index, FVC, FEV1 and use of beta blockers or inhaled long-acting beta agonists in multivariate analyses (). Presence of AECOPD was still statistically significantly associated with increased prevalence of frequent PVC.
Table 2. UnivariateTable Footnote* and multivariate analyses adjusted for frequent PVC during 24 hours Holter registration (n = 119).
Regarding acute disease-related variables, there were, as expected, higher heart rate, higher concentrations of hs-cTnT, lower partial pressure of arterial oxygen and higher levels of systemic inflammation in the AECOPD group than in the stable COPD group (). We found no evidence for the effect modification of the relationship between frequent PVC and AECOPD in any of these strata (p-value for test of homogeneity range: 0.332–0.429). However, the crude association between frequent PVC and AECOPD was attenuated to the zero effect after stratification by hs-cTnT (). A modest attenuation of the relationship between PVC and AECOPD was also found after stratification by heart rate and CRP, but not by hypercapnia or hypoxemia. Thus, the multivariate adjusted model containing acute-related co-variates shows no statistically significant association between frequent PVC and AECOPD (). This model showed a consistent association between the prevalence of frequent PVC and increasing heart rate, whereas the dose-response slope between the prevalence of frequent PVC and hs-cTnT or CRP attenuated to non-significant relationships.
Table 3. Bivariate analysis of the prevalence (%) of frequent PVC among COPD patients in their stable state compared with COPD patients during exacerbation, stratified by co-variates of exacerbation severity.
Table 4. Unadjusted and adjustedTable Footnote* odds ratio of frequent PVC by AECOPD using logistic regression.
Discussion
In the present study, supraventricular and asymptomatic ventricular arrhythmias were commonly identified among COPD patients. While frequent PVC was the only type of arrhythmia that was more prevalent during AECOPD than in stable COPD, this association attenuated to the zero effect after adjustment for heart rate, hs-cTnT and CRP.
As we were not aware of previous studies considering cardiac arrhythmias in the setting of AECOPD, we tested the hypothesis that supraventricular and ventricular arrhythmias were more prevalent in the exacerbated than in the stable states of COPD. A possible clinical application of recording cardiac arrhythmias in AECOPD may be to diagnose arrhythmias causing symptoms or circulatory collapse, such as persistent rapid atrial fibrillation or sustained VT. This was not reported in our study.
Another rationale for performing the current study, was to assess the prevalence of cardiac arrhythmia as a cardiac biomarker in COPD patients, and to explore potential mechanisms related to the arrhythmia during acute disease. Although the discriminative value of ventricular ectopy for future risk in patients with established heart disease is debated, a recent population-based study suggests that ventricular ectopy is associated with increased mortality and incidence of heart failure Citation(14). In the current study, both supraventricular tachyarrhythmia and complex ventricular ectopic activity were observed in more than 1/3 of patients with COPD during a 24-hour Holter registration. This is a higher prevalence than what was obtained in general population-based studies and is more similar to that observed in patients at high cardiovascular risk, i.e. post-myocardial infarction patients (Citation12, Citation18, Citation19).
Furthermore, we found that frequent PVC, but not supraventricular tachyarrhythmia or complex ventricular arrhythmia, was more prevalent in AECOPD than in stable COPD, adjusted for medical history, medication and COPD-severity. The preferred study design for comparing cardiac arrhythmias during acute and stable phases would be longitudinal studies with repeated measurements. In the current cross-sectional design, we cannot exclude that patients in the AECOPD represent another phenotype of COPD than patients in the stable group. However, statistical adjustment by demographic factors and COPD-severity in the stable phase in both groups was made. Thus, the pathophysiologic effects of the acute disease are the main difference between the groups.
In contrast to our previous analysis of the effect of AECOPD on the cardiac biomarker cTnT Citation(8), AECOPD was not associated with frequent PVC when adjusted for indicators of the acute disease. Serum concentrations of hs-cTnT, but also higher average heart rate during 24 hours observation and CRP attenuated the effect by AECOPD on frequent PVC to non-significant. These factors indicate various pathophysiological mechanisms such as myocardial damage, higher sympathetic activity and inflammation, respectively, all of which may aggravate ventricular ectopy. Interestingly, in a different cohort of patients hospitalized for AECOPD, we found that the combination of tachycardia and cTnT elevation was associated with increased mortality Citation(5). Thus, prospective studies of ventricular ectopy during AECOPD as a biomarker for future cardiovascular events and mortality may be merited.
Besides the cross-sectional design, another limitation is the moderate sample size, which increases the risk for type II errors, particularly with the use of dichotomous variables. Other factors not accounted for in the present analyses could have influenced the prevalence of arrhythmias. Particularly, an indicator of myocardial function, such as N-terminal pro-B-type natriuretic peptide or ejection fraction would be relevant. There might also be random effects due to day-to-day variability in physical activity and psychological stress during the Holter registrations.
In conclusion, we found that patients with COPD have a higher prevalence of frequent PVC during the exacerbated state than in the stable phase. Attenuation effects of this association by cTnT, tachycardia and CRP indicate that subclinical myocardial damage, sympathetic activity and systemic inflammation are involved in pro-arrhythmic effects during AECOPD.
Declaration of interest
Takeda Nycomed, Astra Zeneca, GlaxoSmithKline, Abbot Diagnostics and Roche Diagnostics have not played any part in the data analysis, interpretation of results, drafting of the manuscript or approval for submission of the final version of the manuscript. Gunnar Einvik, Rahul Bhatnagar, Anke Neukamm, Torbjørn Omland and Vidar Søyseth are employees at Akershus University Hospital. Vidar Søyseth and Torbjørn Omland are employees at the University of Oslo. Nils Henrik Holmedahl is employed at LHL-klinikkene Glittre.
Funding
Gunnar Einvik has received grants from Astra Zeneca to perform other studies. One of the authors (Nils Henrik Holmedahl) received a grant from Takeda Nycomed to perform the part of the study which involves recruiting patients in the reference group. Professor Vidar Søyseth received research grants from Astra Zeneca and GlaxoSmithKline to buy a Holter-recording device. Torbjørn Omland has received honoraria from Abbott Diagnostics, Roche Diagnostics and Novartis, and research support via Akershus University Hospital from Abbott Diagnostics and Astra Zeneca.
Acknowledgments
Thanks to study nurses at Glittre and AHUS for assistance in data collection.
References
- Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343:269–280.
- Global Strategy for the Diagnosis, Management and Prevention of COPD. Global Initiative for Chronic Obstructive Lung Disease (GOLD), 2013. Available from http://www.goldcopd.org
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2007;176:532–555.
- MacDonald MI, Shafuddin E, King PT, Chang CL, Bardin PG, Hancox RJ. Cardiac dysfunction during exacerbations of chronic obstructive pulmonary disease. Lancet Respir Med 2016;4:138–148.
- Vestbo J. COPD: definition and phenotypes. Clin Chest Med 2014;35:1–6.
- Hoiseth AD, Neukamm A, Karlsson BD, Omland T, Brekke PH, Soyseth V. Elevated high-sensitivity cardiac troponin T is associated with increased mortality after acute exacerbation of chronic obstructive pulmonary disease. Thorax 2011;66:775–781.
- Chang CL, Robinson SC, Mills GD, Sullivan GD, Karalus NC, McLachlan JD, et al. Biochemical markers of cardiac dysfunction predict mortality in acute exacerbations of COPD. Thorax 2011;66:764–768.
- Soyseth V, Bhatnagar R, Holmedahl NH, Neukamm A, Hoiseth AD, Hagve TA, et al. Acute exacerbation of COPD is associated with fourfold elevation of cardiac troponin T. Heart 2013;99:122–126.
- Campo G, Pavasini R, Malagù M, Punzetti S, Napoli N, Guerzoni F, et al. Relationship between troponin elevation, cardiovascular history and adverse events in patients with acute exacerbation of COPD. COPD: J Chron Obstruct Pulmon Dis 2015;12:560–567.
- Fabbri LM, Beghe B, Agusti A. Cardiovascular mechanisms of death in severe COPD exacerbation: time to think and act beyond guidelines. Thorax 2011;66:745–747.
- Simpson RJ, Jr., Cascio WE, Schreiner PJ, Crow RS, Rautaharju PM, Heiss G. Prevalence of premature ventricular contractions in a population of African American and white men and women: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J 2002;143:535–540.
- Massing MW, Simpson RJ, Jr., Rautaharju PM, Schreiner PJ, Crow R, Heiss G. Usefulness of ventricular premature complexes to predict coronary heart disease events and mortality (from the Atherosclerosis Risk In Communities cohort). Am J Cardiol 2006;98:1609–1612.
- Seth N, Kaplan R, Bustamante E, Kulkarni C, Subacius H, Rosenthal JE, et al. Clinical significance of nonsustained ventricular tachycardia on routine monitoring of pacemaker patients. Pacing Clin Electrophysiol 2015;38:980–988.
- Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, et al. Ventricular Ectopy as a Predictor of Heart Failure and Death. JACC 2015;66:101–109.
- Konecny T, Park JY, Somers KR, Konecny D, Orban M, Soucek F, et al. Relation of chronic obstructive pulmonary disease to atrial and ventricular arrhythmias. Am J Cardiol 2014;114:272–277.
- Holmedahl NH, Overland B, Fondenes O, Ellingsen I, Hardie JA. Sleep hypoventilation and daytime hypercapnia in stable chronic obstructive pulmonary disease. Int J COPD 2014;9:265–275.
- Sokolow M, Lyon K. The ventricular complex in left ventricular hypertrophy as obtained by unipolar precordial and limb leads. Am J Cardiol 1949;37:161–169.
- Sajadieh A, Nielsen OW, Hein HO, Frederiksen BS, Davanlou M, Hansen JF. Ventricular arrhythmias and risk of death and acute myocardial infarction in apparently healthy subjects of age >or = 55 years. Am J Cardiol 2006;97:1351–1359.
- Khairy P, Thibault B, Talajic M, Dubuc M, Roy D, Guerra PG, et al. Prognostic significance of ventricular arrhythmias post-myocardial infarction. Can J Cardiol 2003;19:1393–1404.