ABSTRACT
Approximately 15–20% of patients with chronic obstructive pulmonary disease (COPD) also display characteristics of asthma. In May 2014, the asthma–COPD overlap syndrome (ACOS) was briefly addressed in the Global Initiative for Asthma (GINA) and Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy documents. We evaluated how pulmonologists diagnose and treat ACOS and how they assess its control. Pulmonologists from two university healthcare centers, having ≥ 1 year experience, treating patients with asthma, COPD, or ACOS, were invited to participate in focus groups. Two focus groups (1 hour duration) were convened with seven and five participants, respectively. According to pulmonologists from both institutions, ACOS is a new name for an existing syndrome rather than a new disease. It is characterized by incomplete reversible airflow limitations and changes in forced expiratory volume in one second over time. The pulmonologists noted that its diagnosis must be based on clinical characteristics, pulmonary function test results, and clinical intuition. To diagnose ACOS, pulmonologists must rely on their clinical judgment. They also agreed that the treatment of patients with ACOS should target the features of both asthma and COPD. Pulmonologists from both institutions used asthma control criteria to assess ACOS control. A deeper understanding would enable clinicians to establish specific criteria for the diagnosis, treatment, and follow-up of subjects with ACOS.
Introduction
Asthma is a prevalent disease that affects all age groups. It is characterized by chronic airway inflammation and is defined according to its symptoms, including cough, wheezing, shortness of breath, and chest tightness, that vary over time Citation(1). In 2015, approximately 300 million people were affected by asthma worldwide Citation(2). According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD), chronic obstructive pulmonary disease (COPD) is “a common preventable and treatable disease, is characterized by a persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases Citation(3).” The World Health Organization estimates that 65 million people worldwide suffer from moderate-to-severe COPD Citation(4).
It is increasingly recognized that asthma and COPD can coexist in the same patient. It is estimated that 15–55% of patients with airway obstruction have asthma–COPD overlap syndrome (ACOS) (Citation1,Citation5–7). Several studies have described clinical characteristics of patients diagnosed with ACOS as well as their use of healthcare services (Citation8–13), but different definitions of ACOS were used, which makes it difficult to compare the results between these studies. In May 2014, the Global Initiative for Asthma (GINA) and GOLD strategy documents included recommendations for the diagnosis and treatment of ACOS, but these recommendations remain imprecise in a clinical setting Citation(14). Although persistent airflow limitation, with several features shared between asthma and COPD is the most precise definition of ACOS available Citation(3), this definition is still imprecise. Moreover, patients with ACOS are commonly excluded from clinical trials assessing new medications Citation(15), limiting generalization of their results to this sub-population of patients. Two qualitative studies achieved in Spain have already evaluated the definition, diagnosis, and treatment of ACOS, but none assess the evaluation of ACOS control. Moreover, no qualitative study about ACOS has been realized in North America.
Therefore, the aim of this study was to evaluate how pulmonologists from university healthcare centers of the province of Quebec, Canada, define ACOS, how they diagnose and treat it, and how they assess its control. Our objective was to better understand how pulmonologists manage this syndrome.
Materials and methods
In this qualitative study, we planned two focus groups to determine how pulmonologists define ACOS, how they diagnose and treat it, and how they assess its control. One focus group was held at the Hôpital du Sacré-Coeur de Montréal, a tertiary care center affiliated with the Université de Montréal. The second focus group was held at the Centre Hospitalier Universitaire de Sherbrooke, a regional university hospital affiliated with the Université de Sherbrooke. To preserve the participants' identities, the hospitals will be numbered randomly hereafter.
Pulmonologists with ≥ 1 years' experience at their hospital's pulmonology department and who treat patients with asthma, COPD, or ACOS were invited by e-mail to participate in one of the two focus groups. To facilitate the discussions, between four and eight participants were expected to take part in each of the focus groups. A moderator and an observer were present at both focus groups to lead the discussion and take notes. In each focus group, four themes were discussed in relation to ACOS: definition, diagnosis, treatment, and assessment of control. Prior to the session, the moderator prepared questions that would be used to stimulate the discussion (). Open questions were designed so that participants could express themselves on the various topics.
Table 1. Questions for focus groups.
In both focus groups, data were collected on an audio record and later transcribed. A qualitative thematic analysis was done by two co-authors (CR and VM). Using the transcripts, both co-authors coded the data in a scheme developed to classify significant text segments of the discussions, as presented in . The percentages of pulmonologists who agreed with the opinions that emerged from the focus groups in relation to the definition, diagnosis, treatment, and assessment of ACOS control were calculated using Microsoft Excel 2013 (Microsoft Corp., Redmond, WA, USA). To characterize the focus group's participants, the baseline characteristics of the pulmonologists, including their years of practice, their affiliated hospital, and the percentage of patients with asthma, COPD, and ACOS they usually see per month, were collected by questionnaires at the end of each focus group. The percentage of men who participated in each focus group was calculated using the information collected by the moderator in each discussion group.
Ethics approval
The ethics committees at Hôpital du Sacré-Coeur de Montréal and Centre Hospitalier Universitaire de Sherbrooke approved this study.
Results
As shown in , 12 pulmonologists participated in the focus groups, with 7 at Institution 1 and 5 at Institution 2. The mean number of years' experience of pulmonologists at Institutions 1 and 2 was 19 and 25 years, respectively. The focus groups at Institutions 1 and 2 were composed of 57.1% and 80.0% men, respectively. The percentages of patients with asthma, COPD, and ACOS that pulmonologists estimated that they were treating per month were 25.7%, 54.3%, and 14.3%, respectively, at Institution 1 and were 29.2%, 46.6%, and 34.0%, respectively, at Institution 2.
Table 2. Characteristics of the participating pulmonologists.
In each focus group, the pulmonologists reported their opinions on the definition, diagnosis, treatment, and control assessment for patients with a diagnosis of ACOS. The percentages of pulmonologists who agreed with the statements suggested in the focus groups are reported in .
Figure 2. Percentage of pulmonologists who agreed with the opinions regarding the definitions of ACOS reported in the focus groups. ACOS, asthma–chronic obstructive pulmonary disease overlap syndrome; COPD, chronic obstructive pulmonary disease.
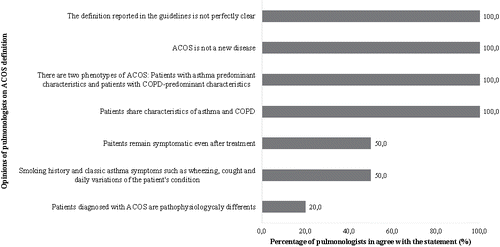
Figure 3. Percentage of pulmonologists who agreed with the opinions regarding the diagnosis of ACOS reported in the focus groups. ACOS, asthma–chronic obstructive pulmonary disease overlap syndrome; RAMQ, Régie de l'Assurance Maladie du Québec.
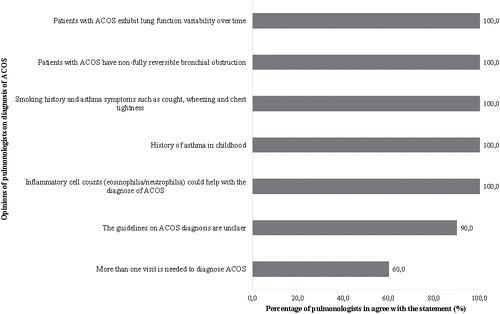
Figure 4. Percentage of pulmonologists who agreed with the opinions regarding the treatment of ACOS reported in the focus groups. ACOS, asthma–chronic obstructive pulmonary disease overlap syndrome; BD, bronchodilator; ICS, inhaled corticosteroid; LAAC, long-acting anticholinergic; LABA, long-acting beta2-agonist.
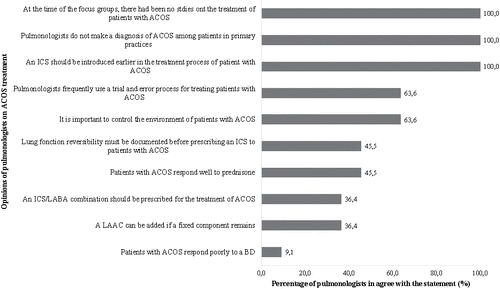
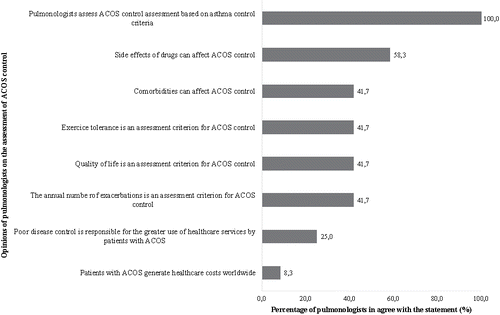
Figure 5. Percentage of pulmonologists who agreed with the opinions regarding the assessment of ACOS control reported in the focus groups. ACOS, asthma–chronic obstructive pulmonary disease overlap syndrome.
Definition of ACOS
According to the pulmonologists participating in the focus group at Institution 2, “ACOS is not a new disease, but describes a clinical entity that has existed for a long time. In recent years, this term has emerged to replace previously used terms such as asthmatic bronchitis, asthma with fixed obstruction, and COPD with a bronchospastic component.” A pulmonologist from Institution 1 agreed that ACOS was not a new disease but described the syndrome as “asthma that progresses to COPD” and said that “the name was simply changed to ACOS”.
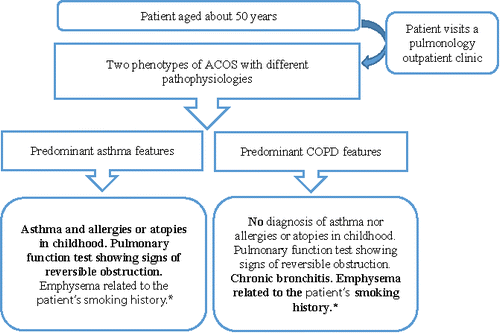
All pulmonologists reported that the definition of ACOS given in the GINA and GOLD strategy documents was not sufficiently specific. They agreed that ACOS should be defined as the presence of characteristics of both asthma and COPD. Pulmonologists defined ACOS as “an obstructive disease that shares features of both asthma and COPD. It is often two diseases that overlap, two respiratory diseases with etiological features that are somewhat different and at times additive.” As one participant said, “Asthma and COPD are two common diseases, which means that at some point one can be superimposed upon the other and a person can have both. I have the feeling that about 10% of patients with COPD also have asthma.” Fifty percent of the pulmonologists reported that the spirometric and clinical features of asthma and COPD shared by patients with ACOS included a history of smoking, expectoration, classical symptoms of asthma such as wheezing and coughing, daily changes in patient clinical status, and a history of asthma in childhood. Half of the participants also reported that “Patients with ACOS are people with symptoms similar to asthma, but they have a history of smoking, and above all, they have an obstruction that persists even after treatment.” All pulmonologists felt there were two phenotypes of ACOS (). The first phenotype of patient with ACOS is, “A patient who suffers from asthma since childhood, who unfortunately has smoked much of his life and who consults with a pulmonologist at age 50. With allergies since childhood, atopy, and smoking, the person eventually develops emphysema. It's not true asthma but it's not COPD either.” The second phenotype is a patient who “also consults at age 50 but has no history of asthma in childhood. The patient has a reversible obstruction, perhaps even meets asthma criteria and is a smoker. The patient's scan and pulmonary function tests are both show emphysema.” According to pulmonologists, the first ACOS phenotype is dominated by asthma characteristics and the second is dominated by COPD characteristics. Some reported that “The two ACOS phenotypes ACOS are probably not very different in terms of disease management, but they are different pathophysiologically.”
Figure 6. Characteristics of the two phenotypes of ACOS proposed by pulmonologists in the focus groups. *The patients share characteristics of asthma and COPD. The bottom boxes include features found in each phenotype of ACOS. Predominant features of each phenotype are indicated in bold font. ACOS, asthma–chronic obstructive pulmonary disease overlap syndrome; COPD, chronic obstructive pulmonary disease.
Diagnosis of ACOS
Most (90%) pulmonologists mentioned that the directives on the diagnosis of ACOS were imprecise, “ACOS diagnosis is complex and there are no clear guidelines”. Related to this statement, some pulmonologists of Institution 1 reported that “The diagnosis is left up to the individual pulmonologist's clinical judgment and experience.” According to 60% of the pulmonologists, more than one visit was needed to diagnose ACOS. For differential diagnosis, participant said, “Obviously pulmonologists recommend the use of spirometry testing objectives for diagnosis of asthma COPD and ACOS. An asthmatic patient's pulmonary function is reversible and can be normalized to 100%. The lung function of the COPD patient has a fixed component, less reversibility. The ACOS patient is somewhere in between. The criterion for asthma diagnosis is 12% reversibility and 200 mL improved response, but you can also have many COPD patients without asthma who have 12% reversibility with a 200 mL response. The current international consensus is that the true COPD patient needs to show an absolute response of 400 mL to say that he also have an asthmatic or bronchospastic component. In fact, patients with ACOS have more fluctuations in lung function over time, and their pulmonary function test results are between those usually obtained for patients with asthma and COPD, i.e. pulmonary dysfunction is partially reversible, but never quite returns to normal.”
The GINA and GOLD strategy documents state “It needs an improvement in forced expiratory volume in 1s (FEV1) of at least 15% and 400 mL or an improvement of 12% and 200 mL in two or more occasions, in order to diagnose a patient with ACOS” (Citation15,Citation16). Pulmonologists said that there is a gray zone in FEV1 improvement between 200 mL and 400 mL. In one of the focus groups, pulmonologists said that a patient with COPD can have a FEV1 of 12% and 200 mL without a diagnosis of asthma or ACOS. In the second focus group, pulmonologists reported that there is a gray zone between 200 and 400 mL that may be clinically significant in some patients. These people might be best treated as having ACOS, and that further investigations may be required to differentiate asthma, COPD, and ACOS.
According to the pulmonologists, even if lung function variability and emphysema are two elements that can facilitate the diagnosis of ACOS, the presence of other symptoms is required for an adequate diagnosis. The pulmonologists described representative cases to illustrate the diagnosis of ACOS (). All pulmonologists mentioned that “Ideally, beyond spirometry, we would like check the patient's sputum for the presence of eosinophilic inflammation.” Half of the pulmonologists mentioned that there is currently no billing code at the Régie de l'Assurance Maladie du Québec (RAMQ) for medical procedures associated with ACOS, which is a barrier for the investigation of this syndrome. Finally, all pulmonologists said that “It's important to develop more objective criteria for the diagnosis of ACOS, or it will become a catch-all category.”
Table 3. Examples of clinical cases reported by the pulmonologists to illustrate typical patients with ACOS.
Treatment of ACOS
All pulmonologists agreed with the fact that at the time of the focus groups, there was a lack of clinical studies on the treatment of ACOS. Some pulmonologists (63.6%) said, “Choice of therapy is a trial-and-error process.” They also noted the following: “The pulmonologist is not a first-line practitioner. Patients with ACOS come to us from their family practitioner with triple therapy.” However, all pulmonologists agreed that “inhaled corticosteroids (ICS) should be introduced earlier in the treatment of ACOS”. “If you're sure that there is an asthmatic component, you should introduce an ICS, which, together with bronchodilators, is the cornerstone of asthma treatment.” Some pulmonologists (45.5%) also mentioned that “It is imperative to document pulmonary function reversibility before prescribing a long-term ICS to an ACOS patient,” to be sure that the patient has an asthma component. “For family practitioner, if a patient has true COPD with no reversibility component, the optimal therapy is a bronchodilator, with an ICS added as a last resort. However, if the patient has a diagnosis of ACOS, ICSs play an important role.” According to pulmonologists, an ICS should be used in combination with a long-acting beta2-agonist (LABA). A long-acting anticholinergic (LAAC) should be added to the ICS + LABA combination if a fixed component remains on pulmonary function tests. Moreover, 45% of the pulmonologists reported that patients with ACOS seemed to respond well to prednisone. As mentioned by pulmonologists in the definition section, the two phenotypes of ACOS are similar in terms of treatment strategies, despite differences in their underlying pathologies. They emphasized that “It is not a grave error to prescribe triple therapy (ICS + LABA + LAAC) to a poorly diagnosed patient, at worst you're overtreating, because studies on COPD and severe asthma show that treatments converge.” Nevertheless, other pulmonologists reported that the syndrome characteristics may induce different responses to treatments. Some pulmonologists also mentioned that antileukotrienes could be used to treat patients whose ACOS symptoms shared more features with asthma than with COPD.
On a different note, some pulmonologists said that “controlling the patient's environment may be even more important in patients with ACOS than in those with COPD”. Finally, a minority of pulmonologists mentioned that patients with ACOS responded less well to bronchodilators than those with asthma.
Assessment of ACOS control
All pulmonologists based their evaluation of ACOS control on asthma control criteria. Some considered only asthma control criteria, while others included COPD criteria as well. Some pulmonologists reported specifically looking at the number of annual exacerbations, the quality of life, and exercise tolerance. According to the pulmonologists, “patients with ACOS may experience side effects from medication and comorbidities such as anxiety disorders and feelings of insecurity. In considering the treatment plan, physicians have to take into account not only severity, but these factors as well.”
The opinions of the pulmonologists on the use of healthcare services and costs associated with patients with ACOS were mixed. Overall, 25.0% of pulmonologists reported that patients with ACOS used more healthcare services and 8.3% reported that ACOS generated more costs worldwide, but other pulmonologists did not agree with these statements. One pulmonologist mentioned, “I divide COPD patients into three categories: emphysema, ACOS, and chronic bronchitis. Patients with emphysema are not too costly to the system, except for the most severe cases. Patients with ACOS may generate fewer costs than those with severe chronic bronchitis, who I think generate the greatest costs.” Another one said, “Patients with ACOS generate higher costs than patients with COPD because they are more likely to have infectious or inflammatory exacerbations.” On the other hand, one pulmonologist said, “Patients with ACOS do not use more healthcare services than patients with COPD so they do not generate higher costs.”
Other relevant topics
During the focus groups, pulmonologists discussed a few topics that were not anticipated. One such topic in both focus groups was the diagnosis of asthma, COPD, and ACOS by general practitioners. Pulmonologists said that, “In addition to unclear guidelines for the diagnosis of ACOS, most of general practitioners lack access to lung function tests (such as flow-volume loop) and other testing to accurately differentiate ACOS from asthma or COPD.”
Discussion
This is the first North American qualitative study to reveal current practice and perceptions of ACOS among respiratory experts practicing in university hospital centers. Moreover, this study is the first to investigate the methods used to evaluate ACOS control.
Definition of ACOS
Over the years, the overlap syndrome of asthma and COPD has seen considerable variation in nomenclature. In 2012, a consensus of Spanish experts called this syndrome “mixed COPD-asthma phenotype” Citation(16). In 2014, the GINA and GOLD strategy documents have used the appellation “ACOS.” In those strategy documents, ACOS is defined by “A persistent airflow limitation with several features usually associated with asthma and several features usually associated with COPD. ACOS is therefore identified by the features that it shares with both asthma and COPD Citation(15).” The ACOS definition given by pulmonologists during our study was similar to the one reported in the GOLD and GINA strategy documents. The pulmonologists also agreed that the definition of ACOS is imprecise, a problem already reported in a qualitative study published in Spain, in 2015 Citation(15). The Spanish study also suggested that the definition of ACOS used in the GINA and GOLD strategy documents could be a starting point for a better definition of the syndrome Citation(15). Even after our focus group discussions, the definition of ACOS was still ambiguous to the participating pulmonologists. According to them, the GINA and GOLD criteria to define ACOS are imprecise and difficult to use in clinical practice since they are subjective and open to interpretation. The dominant characteristics of both ACOS phenotypes mentioned in our study could be an interesting avenue to pursue in order to reach consensus on a definition of this syndrome. A Korean study published in 2015 suggests that there are four phenotypes of ACOS (A, B, C, and D), which can be divided into two groups, i.e. asthma predominant and COPD predominant. These two groups are similar to the two phenotypes described by the pulmonologists during the focus groups. In this Korean study, the asthma-predominant group includes patients with a history of asthma in childhood or severe asthma, eosinophilic inflammation, and fixed airflow obstruction, but no history of smoking. The COPD-predominant group includes patients with a history of smoking, no history of asthma (including a history of asthma in childhood), and bronchodilator reversibility Citation(17).
Diagnosis of ACOS
In 2012, a research team in Spain issued a consensus document on ACOS. Pulmonologist focus groups were organized to identify major and minor criteria for the diagnosis of ACOS. According to the pulmonologists, a diagnosis of ACOS can be made if the patient presents with two of the major criteria: very positive bronchodilator test (increase of FEV1 ≥ 15% and ≥ 400 ml over baseline), eosinophilia in sputum and personal history of asthma (history before the age of 40). Alternatively, patients could present with two minor criteria from the following list: personal history of atopy, positive bronchodilator test (increase in FEV1 ≥ 12% and ≥ 200 ml over baseline) on 2 or more occasions; and one major criterion from the previous list Citation(16).
Another study published in 2015 agreed with some of the diagnostic criteria proposed in the Spanish study: eosinophilia in sputum, history of asthma before the age of 40, and increase of FEV1 ≥ 15% and ≥ 400 ml over baseline Citation(15). However, the authors suggested that because the criteria used in the ACOS definition of the 2012 study Citation(16) were quite restrictive, the number of patients with ACOS was actually underestimated Citation(15).
The criteria that members of the focus groups reported as important for ACOS diagnosis were a history of asthma in infancy, asthma symptoms, a history of smoking, and a significant sputum eosinophil count exceeding the expected threshold. However, it seemed more difficult for pulmonologists to suggest a specific threshold criterion for the diagnosis of ACOS, because of the variability of the lung function of these patients. So, the pulmonologists used a more general description compared with the descriptions used in earlier studies (Citation15,Citation16). A study published in 2015 mentioned that the inflammatory cell count is important to determine the predominance of eosinophilic or neutrophilic cells Citation(18). Typically, patients with COPD have a greater neutrophil count, whereas patients with asthma have a greater eosinophil count (Citation19–21). Patients with ACOS should have a similar eosinophil count to patients with asthma, but greater than that of patients with COPD Citation(21). A prospective cohort study evaluating the ACOS diagnostic criteria proposed by Soler-Cataluna in 2012 reported that ACOS patients were significantly different of non-ACOS patients by blood eosinophils (p < 0,01) and by IgE (p < 0,05) Citation(14).
Thus, combined with pulmonary function measures, the sputum inflammatory cell counts could help to distinguish between asthma, COPD, and ACOS. However, in our study, no pulmonologist has mentioned that blood eosinophils counts could be an important element in the diagnosis of ACOS.
The diagnosis of ACOS seems to be made according to the pulmonologist's clinical intuition and may differ in each patient because there are no guidelines for ACOS, and the diagnostic criteria reported in the GINA and GOLD strategy documents are not specific enough. The establishment of clear guidelines could standardize the diagnosis of ACOS, enable clinicians to properly treat patients, and potentially reduce the morbidity and mortality associated with ACOS.
Treatment of ACOS
At the time of the focus groups, very few clinical studies had evaluated the safety and efficacy of pharmacological treatments for ACOS. In 2012, an expert consensus reported that an ICS should be introduced earlier in combination with a LABA in the treatment of patients with ACOS. The expert consensus also suggested that an LAAC should be added if the patient's symptoms do not improve Citation(16). Recently, a cross-sectional study reported that 69.2% of patients with ACOS were prescribed an ICS and 30.5% were prescribed a LABA/ICS combination as initial treatment Citation(22). In a qualitative study published in 2015, three clinical cases were presented to pulmonologists to discuss treatment options. Each fictitious patient received a diagnosis of ACOS, and the first treatment option suggested was a LABA/ICS combination Citation(15). As reported in a study published in 2016, patients with ACOS are treated in the same way as patients with asthma or COPD except that LAAC are used less frequently for the treatment of patients with ACOS Citation(14). Another study suggested that the inflammatory cell count is an important aspect of treatment because patients with a high eosinophil count respond better to ICS than do patients with a high neutrophil count Citation(21). They also reported that the eosinophil count in patients with ACOS is similar to that in patients with asthma and higher than that in patients with COPD Citation(21). Even though all the published studies have suggested the use of LABA/ICS for the treatment of ACOS patients, additional studies are needed to confirm the safety and efficacy of these treatments in this population.
According to the pulmonologists who participated in our study, most patients had already seen a general practitioner or family physician before being referred to a pulmonologist. Many patients were already using triple therapy (ICS + LABA + LAAC) before attending an outpatient clinic in Pulmonary Medicine, and it is very rare for pulmonologists to see patients who are not already on therapy. Consequently, pulmonologists must first check the adequacy of the patient's current medication and determine whether any change is necessary.
According to some pulmonologists, “In patients with COPD, if lung function declines dramatically in the month or two after an ICS is stopped, the presence of an asthma component is confirmed, leading to a diagnosis of ACOS.” “However, this situation is not optimal.” Pulmonologists suggested that another aspect of treatment involves controlling the patient's environment by promoting smoking cessation, immunization, respiratory rehabilitation, healthy diet, and weight loss.
Assessment of ACOS control
This qualitative study was the first to investigate the methods used by pulmonologists to evaluate ACOS control. According to them, “ACOS control is assessed using as in asthma being also attentive to COPD criteria such as number of exacerbations, quality of life, and exercise tolerance.” The pulmonologists also mentioned that comorbidities and drug side effects may affect ACOS control, and that environmental factors were as important for patients with ACOS as for those with asthma. All pulmonologists reported that they assessed ACOS control based on their clinical judgment, owing to the lack of specific guidelines.
The pulmonologists reported that ACOS control might be difficult to achieve because there were many features to evaluate, including the annual number of exacerbations, exercise tolerance, and asthma control criteria” (daytime and nighttime symptoms, physical activity, absence from work, exacerbations, need for a short-acting beta2-agonist, FEV1 or peak expiratory flow [PEF], diurnal PEF variability, and sputum eosinophil count Citation(23)). “However, once the right medication is found and good control of ACOS has been achieved, it is quite easy to maintain.”
A study reported that 17.6% of patients diagnosed with ACOS experience at least one moderate-to-severe exacerbation as compared to 9.6% of patients with non-ACOS Citation(14). Some pulmonologists participating in the present study reported that patients with ACOS were more likely to have symptoms, more frequent exacerbations and non-infectious inflammatory exacerbations. Patients were also undertreated, and their annual decline in respiratory function is rapid. These patients tended to more frequently visit an emergency department or require hospitalization, resulting in overuse of healthcare resources.
Other relevant topics
A study published in Spain in 2012 reported that information on the diagnosis of ACOS should be promulgated at the general practitioner level to increase accurate diagnosis and treatment and thus prevent morbidity and increase the quality of life Citation(16). Unlike the Spanish study, our results indicate a lack of access to the materials needed by the general practitioners for an adequate diagnosis of asthma, COPD, and ACOS. Because of the logistical differences between the healthcare systems in Spain and Quebec, it is difficult to directly implement the recommendations proposed in the Spanish study to our healthcare system, so as to decrease the morbidity of asthma, COPD, and ACOS and increase quality of life.
Strengths and limitations
This study has several strengths including the participation of expert pulmonologists in asthma and COPD. Moreover, 2 of 8 (25%) university healthcare centers and 12 of 251 pulmonologists (4.8%) participated in the focus groups and shared their opinions on ACOS. Pulmonologists from the same hospital sometimes disagreed, but subjects and issues addressed by the pulmonologists were the same in both groups, strongly suggesting that saturation of ideas was obtained after the two focus groups. The focus groups enabled us to identify consensus and disagreements between pulmonologists regarding ACOS. They also allowed us to highlight the difficulties facing physicians regarding the diagnosis and treatment of ACOS, and assessing ACOS control. The focus groups were also easy to establish and were inexpensive.
Some limitations related to the use of focus groups must be taken into account. First, the opinions of some of the pulmonologists may have been influenced by the other pulmonologists during the discussions. In addition, some pulmonologists enthusiastically participated in the discussions, whereas others were more reserved, which means the opinions of more vocal pulmonologists may dominate because of the limited time available for discussion. Finally, the generalizability of the results might be limited because pulmonologists participating in this study were affiliated to only two hospitals.
Conclusion
What is striking following the analysis of the opinions of pulmonologists working in Quebec university hospital centers is the reported lack of procedures and guidelines on the definition, diagnosis, treatment, and follow-up of ACOS patients. We believe that standardized guidelines can help avoid misdiagnosis of patients, which adversely affects outcomes. We hope that our findings will encourage the respiratory community worldwide to undertake further studies on ACOS to help in the development of international guidelines to provide clearer and more precise guidance to physicians.
Acknowledgments
The authors are grateful to Danielle Buch, medical writer, for assisting in the English translation of the quoted focus group transcripts.
Declaration of interest
CR received a scholarship from the Fonds de Recherche du Québec en Santé (FRQ-S) and from Unité Soutien-SRAP, during the conduct of the study and personal fees from Novartis, outside the submitted work. LB reports grants and personal fees from GlaxoSmithKline, grants and personal fees from AstraZeneca, grants and personal fees from Pfizer, grants from Novartis, outside the submitted work. MFB has received speaker fees and research grants from AstraZeneca, Boehringer Ingelheim, and Novartis. CL reports grants and personal fees from GlaxoSmithKline, personal fees from AstraZeneca, personal fees from TEVA, outside the submitted work. P.L. has sponsored research agreements with Boehringer Ingelheim, Novartis, and Sanofi; he has performed consultancy for Boehringer Ingelheim and Merck, and has received lecture fees from AstraZeneca, Boehringer Ingelheim, Merck, and Novartis. VM has nothing to disclose.
Funding
This work was supported by Fonds de Recherche du Québec – Santé; Unité Soutien-SRAP.
References
- Boulet LP, FitzGerald JM, Reddel HK. The revised 2014 GINA strategy report: opportunities for change. Curr Opin Pulm Med 2015; 21(1):1–7.
- FitzGerald JM, Bateman ED, Boulet LP, Cruz AA, Haahtela T, Levy ML, et al. Global Strategy for asthma management and prevention. 2015.
- Decramer M, Vestbo J, Bourbeau J, Celi BR, Hui DS, Lopez Varela MV, et al. Global Strategy for Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease (update 2014) 2014.
- World Health Organization. Chronic respiratory disease [website]. [cited 2016 17–02). Available from: http://www.who.int/gard/publications/chronic_respiratory_diseases.pdf
- Weatherall M, Travers J, Shirtcliffe PM, Marsh SE, Williams MV, Nowitz MR, et al. Distinct clinical phenotypes of airways disease defined by cluster analysis. Eur Respir J 2009; 34(4):812–818.
- Soriano JB, Davis KJ, Coleman B, Visick G, Mannino D, Pride NB. The proportional Venn diagram of obstructive lung disease: two approximations from the United States and the United Kingdom. Chest 2003; 124(2):474–481.
- Marsh SE, Travers J, Weatherall M, Williams MV, Aldington S, Shirtcliffe PM, et al. Proportional classifications of COPD phenotypes. Thorax 2008; 63(9):761–767.
- Blanchette CM, Gutierrez B, Ory C, Chang E, Akazawa M. Economic burden in direct costs of concomitant chronic obstructive pulmonary disease and asthma in a Medicare Advantage population. J Manag Care Pharm 2008; 14(2):176–185.
- Gerhardsson de Verdier M, Andersson M, Kern DM, Zhou S, Tunceli O. Asthma and chronic obstructive pulmonary disease overlap syndrome: Doubled costs compared with patients with asthma alone. Value Health 2015; 18(6):759–766.
- Kim MA, Noh CS, Chang YJ, Hong YK, Lee JS, Lee SW, et al. Asthma and COPD overlap syndrome is associated with increased risk of hospitalisation. Int J Tuberc Lung Dis 2015; 19(7):864–869.
- Andersen H, Lampela P, Nevanlinna A, Saynajakangas O, Keistinen T. High hospital burden in overlap syndrome of asthma and COPD. Clin Respir J 2013; 7(4):342–346.
- Menezes AM, Montes de Oca M, Perez-Padilla R, Nadeau G, Wehrmeister FC, Lopez-Varela MV, et al. Increased risk of exacerbation and hospitalization in subjects with an overlap phenotype: COPD-asthma. Chest 2014; 145(2):297–304.
- Rhee CK, Yoon HK, Yoo KH, Kim YS, Lee SW, Park YB, et al. Medical utilization and cost in patients with overlap syndrome of chronic obstructive pulmonary disease and asthma. Copd 2014; 11(2):163–170.
- Cosio BG, Soriano JB, Lopez-Campos JL, Calle-Rubio M, Soler-Cataluna JJ, de-Torres JP, et al. Defining the asthma-COPD overlap syndrome in a COPD cohort. Chest 2016; 149(1):45–52.
- Miravitlles M, Alcazar B, Alvarez FJ, Bazus T, Calle M, Casanova C, et al. What pulmonologists think about the asthma-COPD overlap syndrome. Int J Chron Obstruct Pulmon Dis 2015; 10:1321–1330.
- Soler-Cataluna JJ, Cosio B, Izquierdo JL, Lopez-Campos JL, Marin JM, Aguero R, et al. Consensus document on the overlap phenotype COPD-asthma in COPD. Arch Bronconeumol 2012; 48(9):331–337.
- Rhee CK. Phenotype of asthma-chronic obstructive pulmonary disease overlap syndrome. Korean J Intern Med 2015; 30(4):443–449.
- Barnes PJ. Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J Allergy Clin Immunol 2015; 136(3):531–545.
- George L, Brightling CE. Eosinophilic airway inflammation: role in asthma and chronic obstructive pulmonary disease. Ther Adv Chronic Dis 2016; 7(1):34–51.
- Papaiwannou A, Zarogoulidis P, Porpodis K, Spyratos D, Kioumis I, Pitsiou G, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis 2014; 6 Suppl 1:S146–S151.
- Kitaguchi Y, Komatsu Y, Fujimoto K, Hanaoka M, Kubo K. Sputum eosinophilia can predict responsiveness to inhaled corticosteroid treatment in patients with overlap syndrome of COPD and asthma. Int J Chronic Obstruct Pulmon Dis 2012; 7:283–289.
- Barrecheguren M, Monteagudo M, Ferrer J, Borrell E, Llor C, Esquinas C, et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir Med 2015.
- Lougheed MD, Leniere C, Ducharme FM, Licskai C, Dell SD, Rowe BH, et al. Canadian Thoracic Society 2012 guideline update: Diagnosis and management of asthma in preschoolers, children and adults: executive summary. Can Respir J 2012; 19(6):e81–e88.