ABSTRACT
Chronic respiratory failure due to chronic obstructive pulmonary disease (COPD) is an increasing problem worldwide. Many patients with severe COPD develop hypoxemic respiratory failure during the natural progression of disease. Long-term oxygen therapy (LTOT) is a well-established supportive treatment for COPD and has been shown to improve survival in patients who develop chronic hypoxemic respiratory failure. The degree of hypoxemia is severe when partial pressure of oxygen in arterial blood (PaO2) is ≤55 mmHg and moderate if PaO2 is between 56 and 69 mmHg. Although current guidelines consider LTOT only in patients with severe resting hypoxemia, many COPD patients with moderate to severe disease experience moderate hypoxemia at rest or during special circumstances, such as while sleeping or exercising. The efficacy of LTOT in these patients who do not meet the actual recommendations is still a matter of debate, and extensive research is still ongoing to understand the possible benefits of LTOT for survival and/or functional outcomes such as the sensation of dyspnea, exacerbation frequency, hospitalizations, exercise capacity, and quality of life. Despite its frequent use, the administration of “palliative” oxygen does not seem to improve dyspnea except for delivery with high-flow humidified oxygen. This narrative review will focus on current evidence for the effects of LTOT in the presence of moderate hypoxemia at rest, during sleep, or during exercise in COPD.
Introduction
With recent advances both in the treatment and management of chronic obstructive pulmonary disease (COPD), mortality in patients with chronic respiratory failure has decreased over time, and end-stage disease with chronic respiratory failure has become a more evident problem Citation(1,2). End-stage COPD patients can present with gas exchange abnormalities either with hypoxemia only or with hypoxemia accompanied by hypercapnia. Long-term oxygen therapy (LTOT) has long been the standard of care for hypoxemic COPD patients. However, domiciliary non-invasive ventilation support added to LTOT may be beneficial in hypercapnic respiratory failure when daytime arterial carbon dioxide level is ≥ 45–50 mmHg in the stable period.
Approximately 7% of patients with moderate to severe COPD develop resting hypoxemia within 5 years Citation(3,4). The main risk factors for the development of hypoxemia in COPD are still not clear; however, male sex, living at a high altitude, increased body mass index, lower forced expiratory volume in one second (FEV1), increased functional residual capacity (%), presence of heart failure, pulmonary artery enlargement on computerized tomography, previous history of severe COPD exacerbation, low baseline resting oxygen saturation, and increased resting heart rate have been reported as possible risk factors Citation(3–6). Although FEV1 is an important parameter for the assessment of disease severity, there is a weak correlation between FEV1 and the degree of hypoxemia. Citation(7). This finding is also supported by the observation that lung function does not predict survival in established hypoxemic patients Citation(8). Emphysematous COPD has been reported as a risk factor for exercise-induced hypoxemia but not for resting hypoxemia Citation(9,10). In addition, recent studies suggest that race and some genetic variations (polymorphisms in chromosomes 14 and 15) may be predictive of resting hypoxemia Citation(11).
The degree of hypoxemia is directly related to mortality in COPD, and correcting hypoxemia with LTOT increases survival Citation(12). Most national and international guidelines recommend the use LTOT in the presence of resting hypoxemia, which is defined as a resting partial pressure of oxygen in arterial blood (PaO2) ≤55 mmHg (or oxygen saturation [SaO2] ≤ 88%) or resting PaO2 between 56 and 59 mmHg (or SaO2 ≤ 88%) with evidence of pulmonary hypertension, cor pulmonale, or polycythemia with a hematocrit value greater than 55% () Citation(13–18). The current recommendation is based on the results of two historical studies published in the early 1980s: the Nocturnal Oxygen Therapy Trial (NOTT) in 1980 and the Medical Research Council (MRC) study in 1981 Citation(19,20). Although both studies had relatively few patients when compared to current randomized controlled trials (RCTs; MRC study included 87 patients, NOTT study included 203 patients) and did not represent the ideal group for LTOT (e.g., 25–52% of patients were still smokers and all patients are aged < 70 years in the MRC study, smoking status was not declared in NOTT), the results showed that LTOT increased survival in hypoxemic patients, and this effect on mortality was directly correlated with the duration of LTOT use.
Table 1. Definitions.
However, approximately 20% of COPD patients were prescribed LTOT outside published guidelines, and there is still debate whether the use of oxygen therapy is beneficial during the earlier phase of the disease before the development of resting hypoxemia, especially for symptom relief Citation(21). Patients with moderate to severe COPD often have moderate hypoxemia (PaO2: 56–69 mmHg) at rest, and patients who do not have hypoxemia at rest become hypoxemic during exercise or sleep Citation(22). Although these patients are not qualified for oxygen therapy with current indications, research is still ongoing whether oxygen therapy may have potential positive effects in these patients. In this review, we touch upon the answers of some clinical questions of utmost importance regarding the effects of LTOT in moderate hypoxemia at rest and during sleep or exercise in COPD.
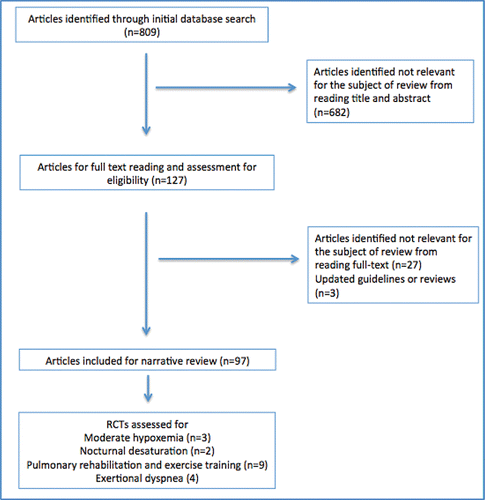
We searched Medline, EmBase, and the Cochrane database, using the keywords “COPD and oxygen therapy,” “COPD and moderate hypoxemia,” “COPD and nocturnal desaturation,” “COPD and exercise induced desaturation” for all reports of adults published up to January 2017 (). Our search was not limited to publications in English. We selected relevant reports and comprehensive reviews. We also searched the reference lists of the identified publications, selecting relevant articles with an emphasis on oxygen therapy research.
The rationale of LTOT use in COPD
Consequences of chronic hypoxemia
The main mechanisms of hypoxemia in COPD are ventilation perfusion mismatch and emphysematous destruction of the alveolocapillary membrane. Pulmonary vasoconstriction occurs in response to alveolar hypoxia in an effort to match ventilation and perfusion Citation(12). However, ongoing pulmonary vasoconstriction causes pulmonary remodeling and pulmonary hypertension, resulting in right heart failure. The degree of pulmonary hypertension is inversely correlated with the severity of resting hypoxemia. Pulmonary hypertension in COPD is also related to an increased hospitalization frequency and decreased survival Citation(23–25). From a cardiac perspective, hypoxemia causes peripheral vasodilation, which induces compensatory tachycardia with a subsequent increase in cardiac output to improve oxygen delivery Citation(24). Persistent hypoxemia may induce secondary erythrocytosis, causing polycythemia and increasing the serum viscosity. Muscle dysfunction, neurocognitive impairment, and impaired quality of life (QoL) Citation(13,25,26). All these consequences contribute to increased mortality in COPD.
Physiological and clinical effects of oxygen therapy
If hypoxemia persists, tissue hypoxia and organ dysfunction will be inevitable. Thus, the correction of hypoxemia is important for survival. Oxygen therapy may reverse pulmonary vasoconstriction and stabilize or reduce pulmonary artery pressure Citation(24,27,28). Improvement of pulmonary hemodynamics is enhanced if oxygen is used continuously, and this effect seems to be sustainable over the years Citation(19,29). Oxygen administration improves breathlessness, especially during exercise in hypoxemic subjects. Moreover, exercise tolerance in the acute setting is improved with oxygen, including in patients with mild to moderate hypoxemia Citation(30). Selinger et al. showed that positive changes induced by oxygen are reversible by the interruption of therapy Citation(31). LTOT may improve the sleep profile and quality by preventing nocturnal hypoxemia Citation(24). In addition, LTOT may be helpful for improving respiratory and general health-related QoL Citation(32).
Oxygen therapy in COPD patients with moderate hypoxemia
PaO2 has been shown to exhibit a decreasing trend that is directly related to mortality in COPD Citation(33,34). Therefore, theoretically, oxygen supplementation to achieve higher PaO2 levels in the presence of moderate hypoxemia may have a positive impact on disease outcomes. There are a limited number of studies assessing the effect of LTOT in moderately hypoxemic COPD patients (). Gorecka et al. randomized COPD patients with moderate hypoxemia into control (n = 67) and LTOT (n = 68) groups and followed them for at least three years or until death Citation(35). There were no significant differences in survival rates between patients treated with LTOT and controls. Longer oxygen use (>15 hours per day) did not improve survival. Ringbaek et al. studied 170 moderately hypoxemic COPD patients before and after 10 months of initiation of home oxygen therapy. Comparing the two periods, there were no differences in admission rates or the number of patients with at least one hospitalization Citation(36). They concluded that home oxygen therapy does not reduce hospitalization in patients with COPD without severe hypoxemia. In a trial performed in hypercapnic COPD patients with a mean PaO2 of 67 mmHg at rest, LTOT did not provide a survival benefit. However, it halted the decline in endurance time and reduced the exertional dyspnea score significantly after 1 year compared to the controls Citation(37).
Table 2. Randomized Controlled Trials For Long-Term Oxygen Therapy In COPD Patients wit Moderate Hypoxemia.
A recent multicenter study provided important insight concerning this issue Citation(38). This is the largest study to date examining the efficacy of LTOT in resting or exercise-induced moderate hypoxemic patients (n = 738). In the LTOT Trial Research Group study, patients were randomized to either supplemental oxygen or no supplementation. In the supplemental oxygen group, patients with moderate resting desaturation (Oxygen saturation with pulse oxymeter [SpO2] 89–93%) were prescribed 24-hour oxygen, and those with desaturation only during exercise (during the 6-minute walk test [6MWT], SpO2 ≥ 80% for more than 5 minutes and < 90% equal to or more than 10 seconds) were prescribed oxygen during exercise and sleep. The median follow-up time was 18 months. LTOT had no effect on time to death or time to first hospitalization. In addition, the exacerbation rate, QoL, and functional measures (depression, anxiety, and functional status) were similar between the study groups. The study also had some limitations. There is the possibility of the exclusion of patients with a greater number of symptoms because they might have declined to participate. Second, neither nocturnal desaturation nor effects of oxygen on exercise performance were assessed in the study. Finally, the estimated use was 13 hours/day in the supplemental oxygen group, and it is uncertain whether oxygen use for longer periods of time in these patients might have provided different results. This is especially important because we know that based on NOTT results, the duration of oxygen use is predictive of survival in hypoxemic COPD patients
Although theoretically initiating LTOT in the early phase of COPD, before the onset of irreversible changes, seems to be reasonable, the data reported to date have shown no benefit. Regarding current evidence, oxygen therapy is not indicated in COPD patients with moderate hypoxemia.
Oxygen therapy in COPD patients with nocturnal desaturation
Chronic obstructive pulmonary disease patients experience oxygen desaturation of clinical importance during sleep due to worsening of ventilation/perfusion mismatch and nocturnal alveolar hypoventilation Citation(39–41). Most of these patients have near normal oxygen saturation during wakefulness, and daytime PaO2 values may not be helpful for the discrimination of nocturnal desaturators Citation(41–43). The prevalence of nocturnal desaturation varies widely according to the definitions used for nocturnal desaturation and the selected patient populations in the studies. Fletcher et al. defined nocturnal desaturation as a fall in SaO2 below 90% for 5 minutes or more, reaching a nadir saturation of at least 85%, and reported that approximately one-third of patients with a PaO2 greater than 60 mmHg, have nocturnal desaturation Citation(44). Other studies used the definition of spending more than 30% of at least one of two nights with an oxygen saturation of less than 90% and showed that 50–70% of patients with mild to moderate hypoxemia have nocturnal desaturation Citation(41,45). Although nocturnal oximetry is the simplest way to show nocturnal desaturations, polysomnography is the best tool for the evaluation of sleep disorder breathing problems in COPD patients, especially for the differential diagnosis of obstructive sleep apnea Citation(46).
The impact of nocturnal desaturation on pulmonary hemodynamics is still not well understood. Levi-Valensi et al. reported that COPD patients who desaturated at night have a higher mean pulmonary artery pressure when compared to patients who did not desaturate (19 vs 17 mmHg; p < 0.05). In addition patients who developed pulmonary hypertension had higher number of desaturation dips, longer dip durations, and lower mean nocturnal arterial oxygen saturation compared with patients who desaturated but did not develop pulmonary hypertension and patients who did not desaturate Citation(42). In contrast to this finding, Chaouat and colleagues showed that the mean pulmonary artery pressure was not correlated with the degree and duration of nocturnal desaturation in COPD patients with mild daytime hypoxemia Citation(45).
The prognostic significance of nocturnal desaturation for mortality in COPD is not clear. Fletcher et al. studied non-hypoxemic COPD patients (daytime PaO2 greater than 60 mmHg) with and without nocturnal desaturation and reported that survival was significantly improved in patients without nocturnal desaturation Citation(48). They defined nocturnal desaturation based on two different definitions: episodic desaturation associated mainly with REM sleep (definition 1) and desaturation > 30% of the time spent in bed below an oxygen saturation of 90% (definition 2). When stratified for supplemental oxygen use, survival was only improved in subjects referencing definition 1. A double-blind, randomized trial evaluated the long-term effect of oxygen supplementation in COPD subjects with nocturnal desaturation () Citation(49). Patients treated with supplemental oxygen during sleep for 36 months showed a significant downward trend in pulmonary artery pressure (−3.7 mmHg) compared to patients treated with room air (+3.9 mmHg). In another RCT, nocturnal oxygen supplementation did not affect the evolution of pulmonary hypertension and mortality in COPD patients with nocturnal desaturation Citation(50). Nocturnal oxygen supplementation did not prevent initiation of conventional LTOT as well.
Table 3. Randomized Controlled Trials For Nocturnal Oxygen Therapy In COPD Patients With Nocturnal Desaturation.
Nocturnal desaturation is a known factor in sleep fragmentation and the decrease in QoL in patients with COPD. However, Lewis et al. reported that patients with nocturnal desaturation did not have worsened sleep quality, QoL, and daytime somnolence when compared to patients without desaturation Citation(41). Studies assessing the effects of the correction of desaturation with nocturnal oxygen therapy on sleep and QoL also had heterogeneous results. Nocturnal oxygen supplementation in COPD patients with isolated nocturnal hypoxemia did not improve QoL within 6 weeks Citation(51). A recent study showed that nocturnal oxygen therapy may be beneficial for improving QoL only in emphysematous but not in chronic bronchitis type COPD Citation(52). The authors explained this finding as a consequence of various responses to the hypoxemic ventilation stimulus in these two phenotypes.
Due to the paucity of data and the relatively small sample size of the studies mentioned above, the role of oxygen therapy in clinically relevant outcomes remains unclear. An ongoing study, the International Nocturnal Oxygen Trial (INOX), will address this question and facilitate our understanding of whether supplemental oxygen during sleep is effective Citation(53). This study is a three-year, multicenter, placebo-controlled, randomized trial of nocturnal oxygen therapy added to usual care, and the primary outcome is a composite of all-cause mortality or the requirement for LTOT.
Oxygen therapy in COPD patients with exercise-induced desaturation
Approximately 20% of COPD patients without resting hypoxemia develop desaturation during exercise Citation(10,54,55). Exercise-induced desaturation (EID) is defined as a fall in oxygen saturation to 88% or lower during exercise (). EID is considered an important risk factor for pulmonary hypertension, rapid decline in lung function, and recurrent exacerbations Citation(54–57). It has been reported that patients with low oxygen saturation at rest, emphysematous phenotype, and low diffusion capacity are more likely to desaturate during exercise Citation(10,58). EID diagnosis is usually confirmed by the 6MWT, and it has been shown that desaturation during the 6MWT can predict a future need for LTOT Citation(59). In addition, patients who desaturate during the 6MWT have been reported to have an approximately twofold increased risk of death compared with those who did not desaturate Citation(55,60).
Oxygen supplementation during exercise results in significant improvements in exercise tolerance both in healthy people and in COPD patients. Oxygen administration causes less breathlessness and improves exercise capacity by decreasing minute ventilation and dynamic hyperinflation, improving pulmonary hemodynamics by alleviating hypoxic vasoconstriction and increasing cardiac output and oxygen delivery in the acute setting Citation(24). It has been shown that supplemental oxygen protects against desaturation during exercise in COPD patients without severe resting hypoxemia Citation(61). Moreover, oxygen supplementation improves exercise duration, exercise endurance, and symptom perception in non-hypoxemic patients Citation(30). The likely underlying mechanism is a reduction of the hypoxemia-related increase in ventilatory demand, dynamic hyperinflation, and increased exertional breathlessness Citation(25,30).
However, evidence for the long-term use of oxygen to prevent EID during exercise in non-hypoxemic COPD patients outside the laboratory setting is limited. Moore et al. showed that ambulatory oxygen had no benefit in terms of exercise capacity Citation(62). Similar to this finding, the LTOT trial subgroup analysis showed that patients with an exercise desaturation profile (43% of patients) experienced no benefit from ambulatory oxygen supplementation with respect not only to mortality and hospitalizations but also to nadir oxygen saturation during exercise, distance walked in the 6MWT, functional status, and other measures of QoL Citation(38). In a meta-analysis, it was shown that the clinical utility and effectiveness of ambulatory oxygen in improving mortality and exercise capacity were not evident in patients who were not hypoxemic at rest Citation(63).
We can conclude that oxygen supplementation during exercise may prevent desaturation and improve exercise capacity and symptoms in the acute setting in patients who are not eligible for LTOT; however, long-term domiciliary oxygen use for EID has no effect on important outcomes such as mortality, functional capacity, and QoL.
Oxygen therapy during pulmonary rehabilitation and exercise training in COPD patients
It is well established that pulmonary rehabilitation (PR) and physical training enhance functionality and QoL in patients with COPD. It also has positive impacts such as improvements in exercise capacity and breathlessness. However, as most of these patients have limited exercise capacity because of desaturation episodes during training, supplemental oxygen during training would be beneficial. Voduc et al. evaluated the effect of supplemental oxygen during PR and showed that exercise duration increased with oxygen Citation(64). Neunhauserer et al. showed that supplemental oxygen in non-hypoxemic COPD doubled the effect of endurance training but had no effect on strength gain Citation(65). Several RCTs assessed the effects of oxygen on long-term PR and training (66–74; ). Emtner et al. studied non-hypoxemic COPD patients undergoing high-intensity endurance training with a cycle ergometer for 7 weeks while breathing either supplemental oxygen or room air and showed that the oxygen training group had an improved maximal work rate, increased endurance, and reduced exercise breathing frequency than the air group Citation(70). Dyer et al. analyzed the effects of supplemental oxygen during the PR program Citation(72). They compared the results of PR undertaken either with or without ambulatory oxygen in patients with a demonstrable benefit from oxygen at baseline (patients who walked an additional 10% or more with ambulatory oxygen in an endurance shuttle walk test). Participants who were randomized to the oxygen group were asked to use oxygen at their prescribed flow rate at home during all activities that induced dyspnea. It was shown that supplemental oxygen significantly improved the endurance walking distance.
Table 4. Randomized Controlled Trials For Long-Term Oxygen Therapy For Pulmonary Rehabilitation And Exercise Training In COPD Patients.
In contrast to these findings, some studies have reported negative results. Exercise training has been shown to result in significant improvements in exercise capacity and QoL in subjects with moderate-to-severe COPD; however, oxygen supplementation did not accentuate the benefits of training Citation(66,68,69). Spielmanns et al. used oxygen during a 24-week training program with progressively increasing loads involving the large muscle groups in a randomized controlled study, and showed that training caused statistically significant improvements in QoL, the maximal tolerated load during cycling, peak oxygen uptake, and the 6MWT. However this benefit was independent of oxygen supplementation Citation(74). Ringbaek et al. randomized normoxemic COPD patients to control or ambulatory oxygen of 2 liters/minute during exercise and reported that there was no difference between the groups in the endurance shuttle walk test, change in St George's Respiratory Questionnaire scores, or number of patients with acute exacerbation, hospital admission or dropout Citation(73). In a meta-analysis, it was reported that oxygen supplementation during training may augment the benefits of exercise; however, the available data remain inconsistent due to small sample sizes and methodological variations among the studies Citation(75).
There is insufficient evidence for the beneficial effects of oxygen supplementation during PR and exercise training. A recent trial protocol was published to understand the role of oxygen in PR (SuppORT trial). This randomized controlled study will recruit COPD patients who demonstrate oxygen desaturation lower than 90% during the 6MWT and address whether supplemental oxygen during exercise training is more effective than medical air in improving exercise capacity and QoL Citation(76). The results will help us to further understand the role of oxygen supplementation during exercise training.
Oxygen therapy for the relief of dyspnea in COPD
Relief of dyspnea during exercise
Many COPD patients experience breathlessness during small tasks even if they are not hypoxemic. Oxygen relieves dyspnea and improves recovery from breathlessness Citation(25). Oxygen supplementation may be helpful for patients to carry on their daily routine activities and therefore may improve QoL. However, the results of RCTs () have shown that the use of ambulatory oxygen therapy for daily activities have limited effects for the relief of dyspnea, exercise performance, and QoL Citation(62,77–79). Eaton et al. conducted a 12-week double-blind randomized cross-over trial in severe COPD patients with exertional desaturation Citation(78). Approximately 70% of the patients had a reduced Borg dyspnea score and improved health-related QoL with supplemental oxygen. In contrast, McDonald et al. reported that supplemental oxygen increased the walking distance in the acute setting but did not provide any long-term benefit in terms of dyspnea and QoL Citation(77). Similar results were also reported by Nonoyama et al., who showed that only a small proportion of patients with mild hypoxemia experienced a benefit from home oxygen after long-term use Citation(79). Moore et al. evaluated a 12-week, parallel, double-blinded, randomized trial of cylinder air versus cylinder oxygen, provided at 6 liters/minute for use during any activity provoking breathlessness, and no benefits were observed in terms of dyspnea or QoL Citation(62).
Table 5. Randomized Controlled Trials For Long-Term Oxygen Therapy For Exertional Dyspnea in COPD Patients.
In a recent Cochrane review, the efficacy of oxygen therapy for breathlessness and health-related QoL was assessed in COPD patients who do not meet the criteria for LTOT Citation(80). They included 44 studies encompassing 1195 participants in the review, among which 33 studies (901 participants) were included. The results showed that breathlessness during exercise or daily activities was reduced by oxygen compared with air (low-quality evidence) and that short-burst oxygen given before exercise was ineffective (low-quality evidence). Oxygen reduced breathlessness during exercise tests (moderate-quality evidence), but the effect on breathlessness in daily life was limited (low-quality evidence). There was no effect on health-related QoL (low-quality evidence). The authors concluded that oxygen can relieve breathlessness when given during exercise to mildly hypoxemic and non-hypoxemic people with COPD who would not otherwise qualify for home oxygen therapy. Most evidence is attributable to acute effects during exercise tests, and no evidence indicates that oxygen is beneficial for reducing breathlessness in daily life and improving QoL.
Relief of dyspnea for palliative purposes
End-stage COPD patients suffer from a high burden of symptoms. Dyspnea is the most debilitating symptom in advanced COPD and profoundly affects QoL. Patients who have intractable dyspnea usually have a fear of death by suffocation Citation(81), and it was shown that COPD patients usually suffer from poor symptom control including dyspnea in the last week of their life Citation(82). Supplemental oxygen is commonly used as palliative treatment in end-stage COPD patients for the relief of distress experienced by patients and their families Citation(83). Palliative oxygen therapy is defined as the use of oxygen to relieve the sensation of refractory persistent breathlessness in advanced disease or life-limiting illness irrespective of the underlying pathology, in which all reversible causes have been or are being treated optimally Citation(14). Despite the widespread use, data supporting this approach are contradictory, and there is limited evidence to support the routine use of supplemental oxygen to reduce dyspnea in non-hypoxemic patients with advanced COPD Citation(84–87). In a RCT conducted in patients with life-limiting illnesses who were ineligible for LTOT, supplemental oxygen provided no additional symptomatic benefit compared to room air Citation(86). There were no statistically significant differences between the two groups in terms of breathlessness, frequency of side effects, or change in QoL. Campbell et al. showed that when compared to air, oxygen therapy did not decrease respiratory distress in patients who were near death because of respiratory failure, as measured by the Respiratory Distress Observation Scale Citation(87). Hypoxemic patients with corrected saturation after oxygen did not experience a significant difference in symptoms between air and palliative oxygen. A meta-analysis reported that oxygen therapy reduced dyspnea compared with medical air in COPD patients who do not qualify for home oxygen Citation(88). Dyspnea was reduced by continuous oxygen during exertion but not short-burst oxygen therapy. Concerning the limited options in these patients and the relatively high expectations of patients and their relatives, we think that decisions regarding palliative oxygen should be considered on an individual basis. If palliative oxygen therapy is considered, a therapeutic trial is recommended after 3 days to assess the net clinical benefit Citation(89).
One other option for the palliative oxygen support is high-flow nasal oxygen (HFNO) administered through nasal prongs. HFNO has some advantages, such as the maintenance of mucociliary function by the delivery of warmed and humidified gas Citation(90). It also has the advantage of more reliable delivery of the desired FiO2 when the patient have high peak inspiratory flow rates due to respiratory distress Citation(91,92). Compared to standard oxygen delivery systems, HFNO improves ventilatory efficiency by washing out carbon dioxide from the anatomic dead space and causes a positive end expiratory pressure, when the mouth is closed Citation(93). It has recently been shown that HFNO reduces the work of breathing in patients with both hypoxemic and hypercapnic COPD. Citation(94,95). HFNO may reduce dyspnea successfully with greater comfort and tolerance when compared with standard conventional oxygen masks Citation(96,97). The effect of HFNO for palliative oxygen therapy is promising and merits further research.
Conclusion
The evolution of oxygen therapy in the medical field has been a long journey Citation(98). Despite relatively good evidence for the benefits of LTOT in hypoxemic COPD patients in improving survival, similar benefits have not been demonstrated in the majority of COPD patients with moderate or intermittent hypoxemia, either nocturnal or exercise-induced (). The role of oxygen in symptom relief, such as dyspnea, has also been controversial both during exercise and as a palliative tool. New studies are currently in the pipeline (the INOX and SuppORT trials), and thus, it is likely that in the next few years, the “oxygen journey” will continue.
Table 6. Summary of evidence for long-term oxygen use in in specific circumstances in COPD.
References
- Ekström MP, Wagner P, Ström KE. Trends in cause-specific mortality in oxygen dependent chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011; 183(8):1032–1036.
- Ringbaek TJ, Lange P. Trends in long-term oxygen therapy for COPD in Denmark from 2001 to 2010. Respir Med 2014; 108(3):511–516.
- Wells JM, Estepar RSJ, McDonald MN, Bhatt SP, Diaz AA, Bailey WC, et al. Clinical, physiologic, and radiographic factors contributing to development of hypoxemia in moderate to severe COPD: a cohort study. BMC Pulm Med 2016; 16(1):169.
- Vold ML, Aasebo U, Hjalmarsen A, Melbye H. Predictors of oxygen saturation ≤ 95% in a cross-sectional population based survey. Respir Med 2012; 106(11):1551–1558.
- Kim DK, Jacobson FL, Washko GR, Casaburi R, Make BJ, Crapo JD, et al. Clinical and radiographic correlates of hypoxemia and oxygen therapy in the COPDGene study. Respir Med 2011; 105(8):1211–1221.
- Saure EW, Eagan TM, Jensen RL, Bakke PS, Johannessen A, Aanerud M, et al. Predictors for PaO2 and hypoxemic respiratory failure in COPD-A three-year follow-up. COPD 2014; 11(5):531–538.
- Boutou AK, Shrikrishna D, Tanner RJ, Smith C, Kelly JL, Ward SP, et al. Lung function indices for predicting mortality in COPD. Eur Respir J 2013; 42(3):616–625.
- Law S, Boyd S, MacDonald J, Raeside D, Anderson D. Predictors of survival in patients with chronic obstructive pulmonary disease receiving long-term oxygen therapy. BMJ Support Palliat Care 2014 March 25.
- Stoller JK, Aboussouan LS, Kanner RE, Wilson LA, Diaz P, Wise R, LOTT Research Group. Characteristics of alpha-1 antitrypsin-deficient individuals in the long-term oxygen treatment trial and comparison with other subjects with chronic obstructive pulmonary disease. Ann Am Thorac Soc 2015; 12(12):1796–1804.
- Andrianopoulos V, Celli BR, Franssen FM, Pinto-Plata VM, Calverley PM, Vanfleteren LE, et al. Determinants of exercise-induced oxygen desaturation including pulmonary emphysema in COPD: Results from the ECLIPSE study. Respir Med 2016; 119:87–95.
- McDonald ML, Cho MH, Sorheim IC, Lutz SM, Castaldi PJ, Lomas DA, et al. Common genetic variants associated with resting oxygenation in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 2014; 51(5):678–687.
- Pierson DJ. Pathophysiology and clinical effects of hypoxia. Respir Care 2000; 45(1):39–51.
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2017. Available from: http://goldcopd.org.
- Hardinge M, Annandale J, Bourne S, Cooper B, Evans A, Freeman D, et al. British Thoracic Society guidelines for home oxygen use in adults. Thorax 2015; 70(Suppl 1):i1–i43.
- Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from American College of Physicians, American Collage of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011; 155(3):179–191.
- Bettoncelli G, Blasi F, Brusasco V, Centanni S, Corrado A, De Benedetto F, et al. The clinical and integrated management of COPD. An official document of AIMAR (Interdisciplinary Association of Research in Lung Disease), AIPO (Italian Association of Hospital Pulmonologists), SIMER (Italian Society of Respiratory Medicine), SIMG (Italian Society of General Medicine). Multidiscip Respir Med 2014; 9(1):25.
- Kocabaş A, Atış S, Çöplü L, Erdinç E, Ergan B, Gürgün A, et al. Kronik obstrüktif akciğer hastalığı (KOAH) koruma, tanı ve tedavi raporu 2014. Turkish Thoracic Journal 2014; (Suppl 2):68–71.
- Ortega Ruiz F, Diaz Lobato S, Galdiz Iturri JB, Garcia Rio F, Güell Rous R, Morante Velez F, et al. Continuous home oxygen therapy. Arch Bronconeumol 2014; 50(5):185–200.
- Nocturnal Oxygen Therapy Trial Group. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Ann Intern Med 1980; 93(3):391–398.
- Medical Research Council Working Party. Long-term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema: Report of the Medical Research Council Working Party. Lancet 1981; 1:681–686.
- Veale D, Chailleux E, Taytard A, Cardinaud JP. Characteristics and survival of patients prescribed long-term oxygen therapy outside prescription guidelines. Eur Respir J 1998; 12(4):780–784.
- Casanova C, Hernandez MC, Sanchez A, Garcia-Talavera I, de Torres JP, Abreu J, et al. Twenty-four hour ambulatory oximetry monitoring in COPD patients with moderate hypoxemia. Respir Care 2006; 51(12):1416–1423.
- Skjorten I, Hilde JM, Melsom MN, Hansteen V, Steine K, Humerfelt S. Pulmonary artery pressure and PaO2 in chronic obstructive pulmonary disease. Respir Med 2013; 107(8):1271–1279.
- Kim V, Benditt JO, Wise RA, Sharafkhaneh A. Oxygen therapy in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5(4):513–518.
- Criner GJ. Ambulatory home oxygen: what is the evidence for benefit and who does it help? Respir Care 2013; 58(1):48–64.
- Ambrosino N, Simonds A. The clinical management of extremely severe COPD. Respir Med 2007; 101(8):1613–1624.
- Scharf SM, Iqbal M, Keller C, Criner G, Lee S, Fessler HE, National Emphysema Treatment Trial (NETT) Group. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med 2002; 16683:314–322.
- Rowan SC, Keane MP, Gaine S, McLoughlin P. Hypoxic pulmonary hypertension in chronic lung diseases: novel vasoconstrictor pathways. Lancet Respir Med 2016; 4(3):225–236.
- Zielinski J, Tobiasz M, Hawrylkiewicz I, Sliwinski P, Palasiewicz G. Effects of long-term oxygen therapy on pulmonary hemodynamics in COPD patients: a 6-year prospective study. Chest 1998; 113(1):65–70.
- Somfay A, Porszasz J, Lee SM, Casaburi R. Dose-response effect of oxygen on hyperinflation and exercise endurance in nonhypoxaemic COPD patients. Eur Respir J 2001; 18(1):77–84.
- Selinger SR, Kennedy TP, Buescher P, Terry P, Parham W, Gofreed D, et al. Effects of removing oxygen from patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1987; 136(1):85–91.
- Eaton T, Lewis C, Young P, Kennedy Y, Garrett JE, Kolbe J. Long-term oxygen therapy improves health-related quality of life. Respir Med 2004; 98(4):285–293.
- Aanerud M, Saure EW, Benet M, Basagana X, Bakke PS, Garcia-Aymerich J, et al. Serial measurements of arterial oxygen tension are associated with mortality in COPD. COPD 2015; 12(3):287–294.
- Vold ML, Aasebo U, Wilsgaard T, Melbye H. Low oxygen saturation and mortality in an adult cohort: The Tromso study. BMC Pulm Med 2015; 15:9.
- Gorecka D, Gorzelak K, Sliwinski S, Tobiazs M, Zielinski J. Effect of long-term oxygen therapy on survival in patients with chronic obstructive pulmonary disease with moderate hypoxaemia. Thorax 1997; 52(8):674–679.
- Ringbaek TJ, Fabricius P, Lange P. The effect of home oxygen therapy on hospitalization in moderate hypoxaemic COPD. Chron Respir Dis 2005; 2(2):107–108.
- Haidl P, Clement C, Wiese C, Dellweg D, Köhler D. Long-term oxygen therapy stops the natural decline of endurance in COPD patients with reversible hypercapnia. Respiration 2004; 71(4):342–347.
- The Long Term Oxygen Treatment Trial Research Group. A randomized trial of long-term oxygen for COPD with moderate desaturation. N Engl J Med 2016; 375(17):1617–1627.
- Owens RL. Supplemental oxygen needs during sleep. Who benefits? Respir Care 2013; 58(1):32–47.
- Becker HF, Piper AJ, Flynn WE, McNamara SG, Grunstein RR, Peter JH, et al. Breathing during sleep in patients with nocturnal desaturation. Am J Respir Crit Care Med 1999; 159(1):112–118.
- Lewis CA, Fergusson W, Eaton T, Zeng I, Kolbe J. Isolated nocturnal desaturation in COPD: prevalence and impact on quality of life and sleep. Thorax 2009; 64(2):133–138.
- Levi-Valensi P, Weitzenblum E, Rida Z, Aubry P, Braghiroli A, Donner C, et al. Sleep-related oxygen desaturation and daytime pulmonary haemodynamics in COPD patients. Eur Respir J 1992; 5(3):301–307.
- Lacasse Y, Series F, Vujovic-Zotovic N, Goldstein R, Bourbeau J, Lecours R, et al. Evaluating nocturnal oxygen desaturation in COPD-revised. Respir Med 2011; 105(9):1331–1337.
- Fletcher EC, Miller J, Divine GW, Fletcher JG, Miller T. Nocturnal oxyhemoglobin desaturation in COPD patients with arterial oxygen levels above 60 mm Hg. Chest 1987; 92(4):604–608.
- Chaouat A, Weitzenblum E, Kessler R, Charpentier C, Ehrhart M, Levi-Valensi P, et al. Sleep-related O2 desaturation and daytime pulmonary haemodynamics in COPD patients with mild hypoxemia. Eur Respir J 1997; 10(8):1730–1735.
- Jen R, Li Y, Owens RL, Malhotra A. Sleep in chronic obstructive pulmonary disease: Evidence gaps and challenges. Can Respir J 2016, article ID 7947198.
- Chaouat A, Weitzenblum E, Kessler R, Schott R, Charpentier C, Levi-Valensi P, et al. Outcome of COPD patients with mild daytime hypoxaemia with or without sleep-related oxygen desaturation. Eur Respir J 2001; 17(5):848–855.
- Fletcher EC, Donner CF, Midgren B, Zielinski J, Levi-Valensi P, Braghiroli A, et al. Survival in COPD patients with a daytime PaO2 greater than 60 mm Hg with and without nocturnal oxyhemoglobin desaturation. Chest 1992; 101(3):649–655.
- Fletcher EC, Luckett RA, Goodnight-White S, Miller CC, Qian W, Costarangos-Galarza C. A double-blind trial of nocturnal supplemental oxygen for sleep desaturation in patients with chronic obstructive pulmonary disease and a daytime PaO2 above 60 mm Hg. Am Rev Respir Dis 1992; 145(5):1070–1076.
- Chaouat A, Weitzenblum E, Kessler R, Charpentier C, Enrhart M, Schott R, et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J 1999; 14(5):1002–1008.
- Orth M, Walther JW, Yalzin S, Bauer TT, de Zeeuw J, Kotterba S, et al. Influence of nocturnal oxygen therapy on quality of life in patients with COPD and isolated sleep-related hypoxemia: a prospective placebo-controlled cross-over trial. Pneumologie 2008; 62(1):11–16.
- Rizzi M, Airoldi A, Cristiano A, Frassanito F, Macaluso C, Vanni S, et al. Oxygen therapy in COPD patients with isolated nocturnal hypoxemia; comparison of quality of life and sleep between bronchitis and emphysema phenotype: A prospective observational study. Eur J Intern Med 2016; 34:78–84.
- Lacasse Y, Bernard S, Series F, Nguyen VH, Bourbeau J, Aaron S, et al. Multi-center, randomized, placebo-controlled trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease: a study protocol for the INOX trial. BMC Pulm Med 2017; 17(1):8.
- Kim C, Seo JB, Lee SM, Lee JS, Huh JW, Lee JH, et al. Exertional desaturation as a predictor of rapid lung function decline in COPD. Respiration 2013; 86(2):109–116.
- Waatevik M, Johannessen A, Gomez Real F, Aanerud M, Hardie JA, Bakke PS, et al. Oxygen desaturation in 6-min walk test is a risk factor for adverse outcomes in COPD. Eur Respir J 2016; 48(1):82–91.
- Nakahara Y, Taniguchi H, Kimura T, Kondoh Y, Arizono S, Nishimura K, et al. Exercise hypoxaemia as a predictor of pulmonary hypertension in COPD patients without severe resting hypoxaemia. Respirology 2017; 2281:120–125.
- Andrianopoulos V, Wouters EF, Pinto-Plata VM, Vanfleteren LE, Bakke PS, Franssen FM, et al. Prognostic value of variables derived from six-minute walk test in patients with COPD: Results from the ECLIPSE study. Respir Med 2015; 109(9):1138–1146.
- Knower MT, Dunagan DP, Adair NE, Chin R Jr. Baseline oxygen saturation predicts exercise desaturation below prescription threshold in patients with chronic obstructive pulmonary disease. Arch Intern Med 2001; 161(5):732–736.
- Garcia-Talavera I, Tauroni A, Trujillo JL, Pitti R, Eiroa L, Aguirre-Jaime A, et al. Time to desaturation less that one minute predicts the need for long-term home oxygen therapy. Respir Care 2011; 56(11):1812–1817.
- Casanova C, Cote C, Marin JM, Pinto-Plata V, de Torres JP, Aguirre-Jaime A, et al. Distance and oxygen desaturation during the 6-min walk test as predictors of long-term mortality in patients with COPD. Chest 2008; 134(4):746–752.
- Jolly EC, Di Boscio V, Aguirre L, Luna CM, Berensztein S, Gene RJ. Effects of supplemental oxygen during activity in patients with advanced COPD without severe resting hypoxemia. Chest 2001; 120(2):437–443.
- Moore RP, Berlowitz DJ, Denehy L, Pretto JJ, Brazzale DJ, Sharpe K, et al. A randomised trial of domiciliary, ambulatory oxygen in patients with COPD and dyspnoea but without resting hypoxaemia. Thorax 2011; 66(1):32–37.
- Ameer F, Carson KV, Usmani ZA, Smith BJ. Ambulatory oxygen for people with chronic obstructive pulmonary disease who are not hypoxaemic at rest. Cochrane Database Syst Rev 2014 Jun 24; 6:CD000238.
- Voduc N, Tessier C, Sabri E, Fergusson D, Lavallee L, Aaron SD. Effects of oxygen on exercise duration in chronic obstructive pulmonary disease patients before and after pulmonary rehabilitation. Can Respir J 2010; 17(1):e14–19.
- Neunhauserer D, Steidle-Kloc E, Weiss G, Kaiser B, Niederseer D, Hartl S, et al. Supplemental oxygen during high-intensity exercise training in nonhypoxemic chronic obstructive pulmonary disease. Am J Med 2016; 129(11):1185–1193.
- Rooyackers JM, Dekhuijzen PN, Van Herwaarden CL, Folgering HT. Training with supplemental oxygen in patients with COPD and hypoxaemia at peak exercise. Eur Respir J 1997; 10(6):1278–1284.
- Fichter J, Fleckenstein J, Stahl C, Sybrecht GW. Effect of oxygen (FiO2:0.35) on the aerobic capacity in patients with COPD. Pneumologie 1999; 53(3):121–126.
- Garrod R, Paul EA, Wedzicha JA. Supplemental oxygen during pulmonary rehabilitation in patients with COPD with exercise hypoxaemia. Thorax 2000; 55(7):539–543.
- Wadell K, Henriksson-Larsen K, Lundgren R. Physical training with and without oxygen in patients with chronic obstructive pulmonary disease and exercise induced hypoxaemia. J Rehabil Med 2001; 33(5):200–205.
- Emtner M, Porszasz J, Burns M, Somfay A, Casaburi R. Benefits of supplemental oxygen in exercise training in nonhypoxemic chronic obstructive pulmonary disease patients. Am J Respir Crit Care Med 2003; 168(9):1034–1042.
- Scorsone D, Bartolini S, Saporiti R, Braido F, Baroffio M, Pellegrino R, et al. Does a low-density gas mixture or oxygen supplementation improve exercise training in COPD? Chest 2010; 138(5):1133–1139.
- Dyer F, Callaghan J, Cheema K, Bott J. Ambulatory oxygen improves the effectiveness of pulmonary rehabilitation in selected patients with chronic obstructive pulmonary disease. Chron Respir Dis 2012; 9(2):83–91.
- Ringbaek T, Martinez G, Lange P. The long-term effect of ambulatory oxygen in normoxaemic COPD patients: a randomised study. Chron Respir Dis 2013; 10(2):77–84.
- Spielmanns M, Fuchs-Bergsma C, Winkler A, Fox G, Kruger S, Baum K. Effects of oxygen supply during training on subjects with COPD who are normoxemic at rest and during exercise: A blinded randomized controlled trial. Respir Care 2015; 60(4):540–548.
- Nonoyama M, Brooks D, Lacasse Y, Guyatt GH, Goldstein R. Oxygen therapy during exercise training in chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2007 Apr 18; (2):CD005372.
- Alison JA, McKeough ZJ, Jenkins SC, Holland AE, Hill K, Morris NR, et al. A randomised controlled trial of supplemental oxygen versus medical air during exercise training in people with chronic obstructive pulmonary disease: supplemental oxygen in pulmonary rehabilitation trial (SuppORT) (Protocol). BMC Pulm Med 2016; 16:25.
- McDonald CF, Blyth CM, Lazarus MD, Marschner I, Barter CE. Exertional oxygen of limited benefit in patients with chronic obstructive pulmonary disease and mild hypoxemia. Am J Respir Crit Care Med 1995; 152:1616–1619.
- Eaton T, Garrett JE, Young P, Fergusson W, Kolbe J, Rudkin S, et al. Ambulatory oxygen improves quality of life of COPD patients: A randomised controlled study. Eur Respir J 2002; 20(2):306–312.
- Nonoyama ML, Brooks D, Guyatt GH, Goldstein RS. Effect of oxygen on health quality of life in patients with chronic obstructive pulmonary disease with transient exertional hypoxemia. Am J Respir Crit Care Med 2007; 176(4):343–349.
- Ekström M, Ahmadi Z, Bornefalk-Hermansson A, Abernethy A, Currow D. Oxygen for breathlessness in patients with chronic obstructive pulmonary disease who do not qualify for home oxygen therapy. Cochrane Database Syst Rev 2016 Nov 25; 11:CD0006429.
- Ek K, Andershed B, Sahlberg-Blom E, Ternestedt BM. “The unpredictable death”- The last year of life for patients with advanced COPD: Relatives' stories. Palliat Support Care 2015; 13(5):1213–1222.
- Ahmadi Z, Lundström S, Janson C, Strang P, Emtner M, Currow DC, et al. End-of-life care in oxygen-dependent COPD and cancer: a national population-based study. Eur Respir J 2015; 46(4):1190–1193.
- Carlucci A, Guerrieri A, Nava S. Palliative care in COPD patients: is it only an end-of-life issue? Eur Respir Rev 2012; 21(126):347–354.
- Booth S, Wade R, Johnson M, Kite S, Swannick M, Anderson H. Expert Working Group of the Scientific Committee of the Association of Palliative Medicine. The use of oxygen in the palliation of breathlessness. A report of the expert working group of the Scientific Committee of the Association of Palliative Medicine. Respir Med 2004; 98(1):66–77.
- Marciniuk DD, Goodridge D, Hernandez P, Rocker G, Balter M, Bailey P, et al. Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J 2011; 18(2):69–78.
- Abernethy AP, McDonald CF, Frith PA, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind randomised controlled trial. Lancet 2010; 376(9743):784–793.
- Campbell ML, Yarandi H, Dove-Medows E. Oxygen is nonbeneficial for most patients who are near death. J Pain Symptom Manage 2013; 45(3):517–523.
- Uronis HE, Ekström MP, Currow DC, McCrory DC, Samsa GP, Abernethy AP. Oxygen for relief of dyspnoea in people with chronic obstructive pulmonary disease who would not qualify for home oxygen: a systematic review and meta-analysis. Thorax 2015; 70(5):492–494.
- Davidson PM, Johnson MJ. Update on the role of palliative oxygen. Curr Opin Support Palliat Care 2011; 5(2):87–91.
- Papazian L, Corley A, Hess D, Fraser JF, Frat JP, Guitton C, et al. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med 2016; 42(9):1336–1349.
- Sim MA, Dean P, Kinsella J, Black R, Carter R, Hughes M. Performance of oxygen delivery devices when the breathing pattern of respiratory failure simulated. Anesthesia 2008; 63(9):938–940.
- Wagstaff TA, Soni N. Performance of six types of oxygen delivery devices at varying respiratory rates. Anesthesia 2007; 62(5):492–503.
- Parke RL, Eccleston ML, McGuinness SP. The effects of flow on airway pressure during nasal high-flow oxygen therapy. Respir Care 2011; 56(8):1151–1155.
- Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med Dec 20.
- Pisani L, Fasano L, Corcione N, Comellini V, Musti MA, Brandao M, et al. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax 2017 Jan 19.
- Tiruvoipati R, Lewis D, Haji K, Botha J. High-flow nasal oxygen vs high-flow face mask: a randomized crossover trial in extubated patients. J Crit Care 2010; 25(3):463–468.
- Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care 2010; 55(4):408–413.
- http://museum.aarc.org/gallery/o2therapy/. Accessed in Feb 11, 2017.