ABSTRACT
The β2-adrenergic receptor (ADRB2) is an important regulator of airway smooth muscle tone in chronic obstructive pulmonary disease (COPD). Variants that impair ADRB2 function could increase disease risk or reduce the response to endogenous and inhaled adrenergic agonists in COPD. We performed a systematic review and three meta-analyses to assess whether three functional variants (Thr164Ile, Arg16Gly, and Gln27Glu) in the ADRB2 gene are associated with elevated risk of disease or reduced therapeutic response to inhaled β2-agonists in COPD. We searched the medical literature from 1966 to 2017 and found 16 relevant studies comprising 85381 study subjects. The meta-analyses found no significant association between ADRB2 genotype and COPD risk. The summary odds ratios (ORs) for COPD in Thr164Ile homozygotes and heterozygotes were 2.57 (95% confidence interval (CI): 0.54–12.4) and 1.17 (95% CI: 0.96–1.44), respectively. Corresponding summary ORs for COPD in Arg16Gly homozygotes and heterozygotes were 0.97 (95% CI: 0.76–1.22) and 1.01 (95% CI: 0.81–1.26), while summary ORs for COPD in Gln27Glu homozygotes and heterozygotes were 1.00 (95% CI: 0.80–1.25) and 0.94 (95% CI: 0.69–1.24), respectively. When stratified by ethnicity, the summary ORs for COPD did not differ from 1.0 for any of the ADRB2 variants among Asian, Caucasian, or African populations. We found no consistent associations between ADRB2 genotype and treatment response to inhaled β2-agonists in COPD. This systematic review and meta-analyses found that COPD risk and response to inhaled β2-agonists were not associated with Thr164Ile, Arg16Gly, and Gln27Glu genotypes. However, identified cases of Thr164Ile were few, and additional studies of rare ADRB2 genotypes are required.
Introduction
Chronic obstructive pulmonary disease (COPD) remains one of the leading causes of death and disability according to the World Health Organization Citation(1). Tobacco smoking is the most commonly encountered risk factor for COPD in the Western world, but not all smokers will develop the disease Citation(2). This indicates the presence of interindividual variation in response to tobacco smoke, i.e. additional risk factors that contribute to COPD development. Moreover, up to 25% of patients with COPD have never smoked Citation(2).
Previous studies have shown familial aggregation of early-onset COPD, suggesting an important role of genetics in the pathogenesis Citation(3). The β2-adrenergic receptor gene (ADRB2) encoding the cell-surface receptor involved in relaxation of smooth muscles and dilation of airways could be a candidate gene for COPD (Citation4–9). ADRB2 on smooth muscle functions to dilate and antagonise constriction of the airways, thereby protecting the lungs from long-term bronchoconstriction (Citation4–9). Variants that impair the function of the ADRB2 receptor could result in narrowing of the airways—including small airways—and increase susceptibility to COPD (Citation4–9). Functional variants in ADRB2 could also reduce the treatment response to inhaled β2-agonists in COPD such as bronchodilator response, exacerbation reduction, and tachyphylaxis during long-term treatment (Citation9–11).
The three most common variants in ADRB2, Thr164Ile (rs1800888, p.Thr164Ile, c.491C > T), Arg16Gly (rs1042713, p.Arg16Gly, c.46A > G), and Gln27Glu (rs1042714, p.Gln27Glu, c.79C > G), have been shown to influence the function of the ADRB2 (Citation9–11). The Thr164Ile variant lies within the fourth membrane spanning domain and is associated with a two- to threefold decreased affinity for agonists and some antagonists (Citation9–11). Arg16Gly and Gln27Glu reside at the amino terminus of the ADRB2 gene and play an important role in receptor downregulation (Citation9–11). In Caucasians, 1–3% carries the functional 164Ile variant, 40–50% carries the 16Gly variant, and 40–50% carries the 27Glu variant (Citation12,Citation13). Similar carrier frequencies are seen in many Asian, Arab, and African populations (Citation6,Citation14). It is therefore of interest to determine if these three functional variants are associated with elevated risk of disease or reduced therapeutic response to inhaled β2-agonists in COPD.
The primary objective of this systematic review and meta-analyses was to assess whether Thr164Ile, Arg16Gly, and Gln27Glu in ADRB2 were associated with risk of disease and response to β2-agonists in COPD. As a secondary objective, we assessed the severity of airways obstruction by ADRB2 genotype in patients with COPD.
Materials and methods
Search strategy
A systematic search of the PubMed database was performed in an attempt to identify all studies of COPD risk and treatment response as a function of ADRB2 genotype.
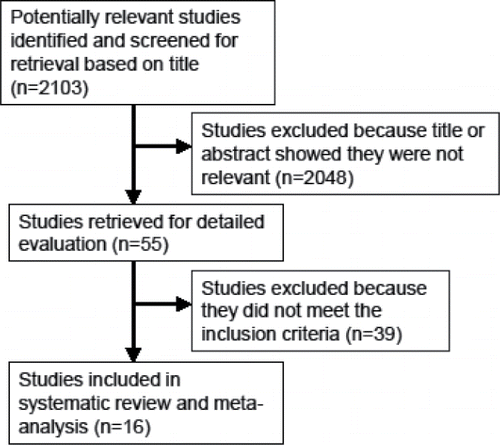
Records were searched from 1966 to March 2017 in PubMed using the broad search terms (adrenergic AND COPD) OR (adrenergic AND emphysema) OR (ADRB2 AND COPD) OR (ADRB2 AND emphysema), and the more narrow search terms (chronic obstructive pulmonary disease OR COPD) AND (beta2-adrenergic receptors OR ADRB2), (COPD OR chronic obstructive pulmonary disease) AND (adrenoceptor OR adrenergic receptor) AND (polymorphism OR mutation OR variation), (ADRB2 AND polymorphism AND Arg16Gly), (ADRB2 AND polymorphism AND Glu27Gln), (ADRB2 AND polymorphism AND Thr164Ile). The titles and abstracts of the articles were manually scanned to determine relevant studies with information on ADRB2 and COPD-related outcomes. We also checked the bibliographies of the included articles and other relevant papers in the databases. See for the flow diagram of the study selection. The meta-analyses were performed according to the PRISMA guidelines as in Citation(15) (Supplementary Table 1).
Data collection
Studies were included if they a) were case–control, clinical trials, or cross-sectional studies, b) examined association between ADRB2 Thr164Ile, Arg16Gly, and Gln27Glu genotypes and COPD risk or treatment response, c) provided information on genotype frequency in the study groups, and d) provided the criteria for establishing a COPD diagnosis (FEV1/FVC < 0.7). Only articles written in English were included.
Data analyses
Meta-analyses were performed using the MIX program version 2.0. Data from studies with information on COPD and the studied variants were combined to give a summary odds ratio (OR) using the inverse variance method. Depending on the test for heterogeneity among the studies, we used a fixed-effects model or a random-effects model for the meta-analysis. If significant heterogeneity remained after random-effects meta-regression, a trim and fill analysis was used to allow for potential heterogeneity and publication bias. Subgroup analyses were used to examine ethnicity as a potential source of heterogeneity. The ethnicity subgroups were based on studies of populations of Asian origin, Caucasian origin, and African origin. Stability of the summary risk estimate was evaluated using sensitivity analysis in which each study was individually removed and the summary OR was recalculated.
Data extraction
Data were extracted independently by two authors (AON and CJ). Disagreements were resolved by consulting a third author (MD). The following data were collected from each study: name of first author, year of publication, country of origin, ethnicity of study subjects, COPD definition, variants investigated, total number of cases and controls, primary endpoint, and p-values for comparisons. In treatment response studies, we also collected information on type of therapy used (β2-agonist as a single therapy or in combination with anticholinergic agents or glucocorticoids).
Quality score assessment
The quality of each study was independently assessed by two authors (AON and CJ). Quality scoring criteria were based on both traditional epidemiologic considerations and genetic issues as in Citation(16) (Supplementary Tables 2 and 3). The quality scores ranged from 8 to 13 in the studies examining COPD risk by ADRB2 genotype and from 8 to 10 in the studies examining response to therapy by ADRB2 genotype (Supplementary Tables 4 and 5).
Risk of bias in individual studies
We have not found any risk of bias in the individual studies that could substantially affect the overall outcome. In Ho et al. Citation(6), Hegab et al. Citation(17), Ferdinands et al. Citation(18), Brøgger et al. Citation(7), Hussein et al. Citation(19), and Ganbold et al. Citation(20), the individual subgroups had very few participants (N < 30), and study groups were not considered representative for the general population. This was allowed for in the meta-analyses where the studies only had little influence on the overall outcome, as they weighed less than 25% in total.
Results
Study characteristics
The database search resulted in 2103 potentially relevant studies. Based on titles and abstracts of the studies, 55 were found to be relevant for further detailed evaluation. Of these, 39 studies were excluded because they did not meet the inclusion criteria, and thus 16 studies of ADRB2 and COPD outcomes were included in the systematic review and meta-analyses (Citation4–7,Citation13,Citation17–27) (). Three of these articles were presenting results from more than one population (Citation13,Citation17,Citation18). The additional populations in these articles were considered separately, leaving 19 datasets for final analysis.
Six studies were based on Asian populations (Citation6,Citation17,Citation20,Citation23–25), six on Caucasians populations (Citation4,Citation5,Citation7,Citation13,Citation18,Citation22), three on African populations (Citation17–19), and three studies were multinational (Citation21,Citation26,Citation27). Detailed characteristics of the studies are presented in . Although we searched the database from 1966, the oldest study cited was published in 2001, indicating the important improvement in genetic studies over the last decades.
Table 1. Main characteristics of the studies included in the systematic review and meta-analyses.
COPD risk by ADRB2 genotype
Among studies examining COPD risk by ADRB2 genotype, one study included solely the Thr164Ile genotype Citation(13), one study included solely the Arg16Gly genotype Citation(18), seven studies included both Arg16Gly and Gln27Glu genotypes (Citation4,Citation5,Citation7,Citation17,Citation19,Citation20,Citation22), and two studies included all three variants (Citation6,Citation13) ().
Table 2. COPD risk by ADRB2 genotype.
COPD risk by Thr164Ile genotype
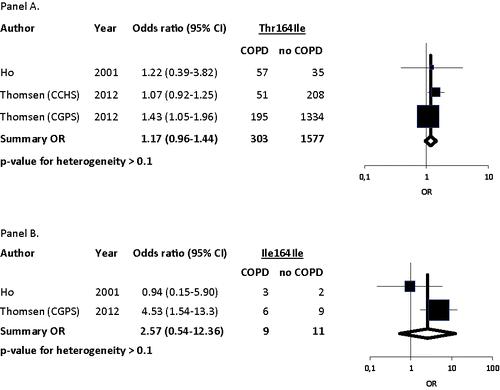
Thomsen et al. genotyped 8971 individuals for all three variants and additional 53777 individuals for the Thr164Ile variant. The authors found that Ile164Ile homozygotes had a significantly elevated OR for COPD of 4.53 (95%CI 1.54–13.3) after adjustment Citation(13). Ho et al. genotyped 65 COPD patients and 41 healthy controls for all three variants and contrary to Thomsen et al. found no significant association between Thr164Ile and risk of COPD Citation(6). The pooled analyses of the two studies showed a summary OR for COPD of 2.57 (95%CI 0.54–12.4) in homozygotes and 1.17 (95%CI 0.96–1.44) in heterozygotes ().
COPD risk by Arg16Gly genotype
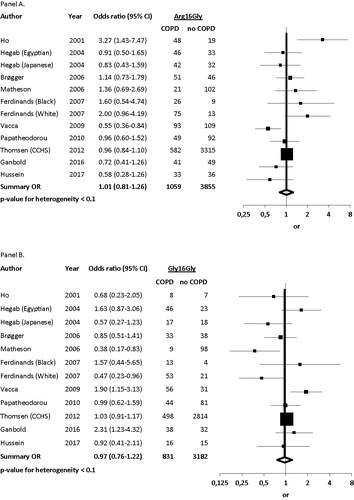
Ten studies evaluated the risk of COPD by Arg16Gly (Citation4–7,Citation13,Citation17–20,Citation22). Matheson et al. genotyped 39 COPD patients and 221 controls and found that non-carriers were at higher risk of COPD than heterozygous and homozygous individuals (OR 2.86, 95%CI 1.20–6.82) Citation(22). Similar results were found in the study by Hussein et al. who genotyped 61 patients with COPD and 54 healthy controls and found that individuals with the Arg16Arg genotype were 4.1 times more likely to develop COPD than individuals with the Gly16Gly and Arg16Gly genotypes Citation(19). In contrast, Vacca et al. found the Gly16Gly more frequently presented in 190 patients with COPD than in 172 healthy smoking controls (OR 1.90, 95%CI 1.15–3.13) Citation(4). Similar results were found in the study by Ganbold et al. who genotyped 100 patients with COPD and 100 controls and found the Gly16Gly genotype to be the most prevalent in patients with COPD (OR 2.31, 95%CI 1.23–4.32) Citation(20). In line with this, Ho et al. found homozygous Arg16 less prevalent in 65 patients with COPD than in 41 healthy controls (OR 0.28, 95%CI 0.11–0.72), suggesting that COPD patients were more likely to have at least one Gly16 allele Citation(6). The remaining five studies did not find any association between the Arg16Gly genotype and risk of COPD (Citation5,Citation7,Citation13,Citation17,Citation18).
The pooled analyses for the 10 studies showed a summary OR for COPD of 0.97 (95%CI 0.76–1.22) in homozygotes and 1.01 (95%CI 0.81–1.26) in heterozygotes (). When analyzing only those studies that were based on Asian populations (Citation6,Citation17,Citation20), the summary ORs in homozygotes and heterozygotes remained statistically nonsignificant at 0.86 (95%CI 0.49–1.52) and 1.19 (95%CI 0.51–2.78) (Suppl. Figure 1). When analyzing only the studies that were based on Caucasian populations (Citation4,Citation5,Citation7,Citation13,Citation18,Citation22), the summary ORs for COPD in homozygotes and heterozygotes were 0.90 (95%CI 0.64–1.26) and 1.01 (95%CI 0.78–1.32) (Suppl. Figure 2), respectively. When analyzing only the studies based on African populations (Citation17–19), the summary ORs for COPD in homozygotes and heterozygotes were 1.36 (95%CI: 0.85–2.16) and 0.87 (95%CI 0.54–1.38) (Suppl. Figure 3), respectively.
COPD risk by Gln27Glu genotype
Nine studies evaluated the risk of COPD by Gln27Glu (Citation4–7,Citation13,Citation17,Citation19,Citation20,Citation22). Hegab et al. genotyped 106 COPD patients and 72 controls from Egypt and found the 27Glu allele to be overrepresented in COPD patients compared with controls (OR 2.85, 95%CI 1.40–5.86) Citation(17), while Matheson et al. found a significant elevated risk for COPD in Gln27Gln vs. Gln27Glu and Glu27Glu (OR 5.34, 95%CI 1.35–21.2) Citation(22). Similar results were seen in the studies by Hussein et al. and Ganbold et al. who found ORs for the Gln27Gln genotype at 3.42 (95%CI 1.58–7.43) and 2.04 (95%CI 1.14–3.63), respectively (Citation19,Citation20). In the remaining five studies, no associations were found (Citation4–7,Citation13).
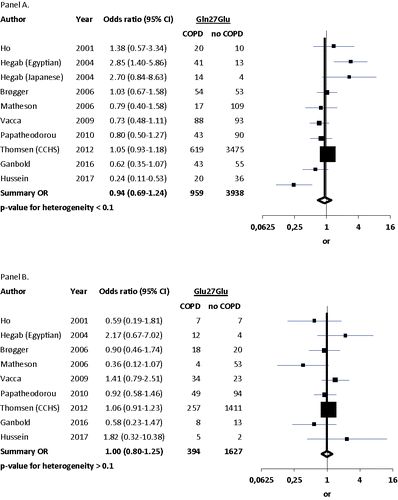
The pooled analyses for the nine studies showed a summary OR for COPD of 1.00 (95%CI 0.80–1.25) in homozygotes and 0.94 (95%CI 0.69–1.24) in heterozygotes (). When analyzing only those studies based on Asian populations (Citation6,Citation17,Citation20), the summary ORs in homozygotes and heterozygotes remained statistically nonsignificant at 0.58 (95%CI 0.28–1.20) and 1.18 (95%CI 0.50–2.75) (Suppl. Figure 4). When analyzing only the studies based on Caucasian populations (Citation4,Citation5,Citation7,Citation13,Citation22), the summary ORs for COPD in homozygotes and heterozygotes were 1.01 (95%CI 0.81–1.26) and 0.99 (95%CI 0.88–1.12) (Suppl. Figure 5). When analyzing the studies based on African populations (Citation17,Citation19), the summary ORs for COPD in homozygotes and heterozygotes were 2.22 (95%CI 0.85–5.81) and 0.84 (95%CI 0.08–9.38) (Suppl. Figure 6), respectively.
Figure 4. Meta-analyses of COPD risk by Gln27Glu genotype. Panel A shows COPD risk for Gln/Glu heterozygotes, while Panel B shows COPD risk for Glu/Glu homozygotes relative to the risk for non-carriers (Gln/Gln).
Significant heterogeneity was present in 3 meta-analyses ( and ); however, trim and fill analyses for these meta-analyses did not significantly alter the summary ORs. Egger's tests for publication bias were all negative for publication bias in the analyses (p > 0.45). To ensure that no single study skewed the overall results, each study was removed one at a time and the summary ORs recalculated. Removal of each individual study did not significantly alter the recalculated summary ORs as compared to the overall summary OR (Suppl. Figure 7).
Severity of airways obstruction by ADRB2 genotype
Because previous studies have suggested an association between lung function and ADRB2 genotype, we also evaluated this as a secondary aim. Five studies included in this systematic review evaluated the association between FEV1% predicted and ADRB2 genotype (Citation5,Citation6,Citation13,Citation18,Citation22). Thomsen et al. found that the Thr164Ile genotype was significantly associated with low FEV1% predicted and a low FEV1/FVC-ratio compared to controls, while no associations were found for the Arg16Gly and Gln27Glu genotypes Citation(13).
Ferdinands et al. found that black β-agonist users with Arg16Arg had significantly better FEV1% predicted than black β-agonist users with the Arg16Gly and Gly16Gly variants (p = 0.01). By contrast, they found no differences in FEV1% predicted according to genotypes in blacks not taking β-agonist or in whites irrespective of β-agonist use Citation(18).
Ho et al. divided the patients in their study into three groups according to their FEV1 values and found a clustering of Gln27Gln in patients with FEV1 <35% predicted (p < 0.018), suggesting that this genotype is associated with more severe COPD than the Gln27Glu and Glu27Glu genotypes Citation(6).
Papatheodorou et al. also divided the patients into three groups according to their FEV1 values, but in contrast to Ho et al. they did not find any association between the Gln27Glu genotype and severity of COPD. Instead, they showed a significant correlation between the Arg16Gly genotype and FEV1% predicted with a clustering of Gly16 homozygotes in patients with FEV1 <35% predicted (p = 0.031), suggesting that this variant is associated with severe COPD Citation(5).
Matheson et al. did not find any significant association between Gln27Glu or the Arg16Gly genotypes and FEV25–75% predicted Citation(22).
Bronchodilator response by ADRB2 genotype
Among the seven studies examining treatment response in COPD by ADRB2 genotype, one study included solely the Arg16Gly genotype Citation(21), five studies included both Arg16Gly and Gln27Glu (Citation19,Citation23–26), and one study included all three variants Citation(27) ().
Table 3. Treatment response in COPD according to ADRB2 genotype.
In the study by Bleecker et al. 2866 patients with moderate-to-very severe COPD were genotyped for the Arg16Gly genotype and were randomly treated with budesonide (synthetic glucocorticoid) alone, budesonide in combination with formoterol (long-acting β-adrenoceptor agonist, LABA), or with placebo. This study comprised two large independent datasets and found no significant interaction between genotype and treatment response for pre-dose or post-dose FEV1. The authors concluded that therapeutic response and tolerability to long-term treatment with formoterol alone or in combination with budesonide was not modified by ADRB2 Arg16Gly genotype Citation(21).
Hizawa et al. identified the Arg16Gly and the Gln27Glu geno-types at the ADRB2 gene in 264 patients with COPD. FVC, FEV1, and FEV1% predicted were measured before and 30 minutes after salbutamol (β2-agonist) administration. The results showed that patients with the Arg16 allele had lower bronchodilator responses to β2-agonist inhalation than patients homozygous for Gly16. The Gln27Glu genotype was not associated with bronchodilator responses Citation(23).
In the study by Kim et al. 104 Korean COPD patients were genotyped for the Arg16Gly, and the Gln27Glu genotypes and spirometry was performed. The immediate bronchodilator response was measured using repeated spirometry 15 minutes after inhalation of salbutamol. No significant differences were found for codon 16 variants, or for codon 27 variants. Reversibility after 12 weeks of treatment with salmeterol (LABA) in combination with fluticasone propionate (glucocorticoid) was then assessed. Similarly, no significant differences were found between the variants at this time point Citation(24).
In the study by Konno et al. 189 patients with COPD underwent assessment of bronchodilator response to either salbutamol or oxytropium bromide (anticholinergic), and the Arg16Gly and Glu27Gln genotypes were identified. They found that the mean ΔFEV1 values were similar for salbutamol and oxytropium bromide, but that the Gly16Gly variant tended to give a higher ΔFEV1 when treated with salbutamol than with oxytropium bromide. When further analyzed, the presence of Arg was associated with a preferential bronchodilator response to oxytropium, whereas the presence of Gly was associated with a preferential bronchodilator response to salbutamol Citation(25).
Rabe and his colleagues wanted to assess the risk of a first exacerbation of COPD according to treatment in 5125 patients with COPD who had at least had one exacerbation in the previous year. 2564 patients were enrolled to be treated with tiotropium, while 2561 patients were given salmeterol. They found that patients with the Arg16Arg genotype had a significantly reduced exacerbation risk compared with patients with the Arg16Gly and the Gly16Gly genotypes when treated with salmeterol. By contrast, they did not find any association between exacerbation risk and variants at codon 16 in the tiotropium group. Furthermore, no associations were found between variants at codon 27 and risk of exacerbation, independently of treatment Citation(26).
Hussein et al. genotyped 61 patients with COPD for the Arg16Gly and Gln27Glu genotypes and found that only the Gln27Glu genotype was associated with bronchodilator response to salbutamol with individuals with the Glu27Glu variant having the best response (p = 0.022) Citation(19).
Yelensky et al. genotyped 648 patients with COPD for the Arg16Gly, Gln27Glu, and Thr164Ile genotypes and evaluated lung function response to indacaterol (LABA) after 12 weeks of treatment. They found no significant association between genotypes and FEV1, exacerbations, peak flow, or transitional dyspnea index Citation(27).
In summary, only one of the seven studies included found a significant positive association between the Arg16Arg genotype and β2-agonist treatment response and exacerbation risk Citation(26), while one study found a negative association between the Arg16Arg genotype and bronchodilator response after treatment Citation(23). The remaining five studies did not find any significant association between Arg16Gly genotype and treatment response (Citation19,Citation21,Citation24,Citation25,Citation27). Only one of the seven studies found a significant association between treatment response and the Gln27Glu genotype Citation(19), while none of the studies found significant association between treatment response and Thr164Ile.
Discussion
To assess whether functional variants in the ADRB2 gene are associated with disease risk and response to therapy in COPD, we performed a systematic review and meta-analyses of 16 studies comprising a total of 85381 individuals. The analyses found no consistent association between the three genotypes and COPD risk or treatment response. Identified studies of the Thr164Ile were relatively few, and large studies of Thr164Ile and other rare ADRB2 variants are warranted.
Previous studies have evaluated the effects of ADRB2 variants on COPD risk, most of them assessing the Arg16Gly and the Gln27Glu genotypes as these are the most common genotypes. The studies have generally shown conflicting results, which could be due to differences in study designs, small sample sizes leading to under-powering of studies, or the presence of potential confounding variables such as smoking status and comorbidities. In addition, potential interactions between alleles and different variants in and around the ADRB2 gene may lead to inconsistent results because ADRB2 haplotypes have been described to occur at different frequencies based on ethnicity. There is also risk of publication bias as studies showing no associations generally tend not to be published. However, three previous meta-analyses, including a genome-wide association study, showed no association between COPD and the ADRB2-genotypes Arg16Gly and Gln27Glu (Citation28–30). This is in agreement with the study with the largest sample size and highest quality score in this review Citation(13), which found no association between the Arg16Gly and the Gln27Glu genotypes and risk of COPD. Some of the studies included in the paper had limited sample size and thus an increased risk of spurious findings. However, in meta-analyses, these studies only had little influence on the overall outcome, as they weighed less than 25% in total.
The systematic review and meta-analyses found trends toward associations of COPD and lung function with Thr164Ile, while no consistent associations were seen for Arg16Gly and Gln27Glu. This could be due to the rare Thr164Ile having more severe functional consequence for the ADRB2 receptor than the two common Arg16Gly and Gln27Glu variants. Thr164Ile is situated next to an amino acid position with predicted involvement in ligand binding Citation(13), and the variant has been associated with 3–4-fold less affinity toward ADRB2 ligands such as adenyl cyclase (Citation4,Citation13).
Many studies have been performed to assess the β2-agonist treatment response according to ADRB2 genotypes in patients with asthma (Citation31–38), while only a few have studied the treatment response in patients with COPD (Citation39,Citation40). One of the studies included in this review found a positive association between the Arg16Arg variant and treatment response Citation(26). However, this was in conflict with one other study showing a negative association between the Arg16Arg variant and treatment response Citation(23). Another study included in this review found a positive association between the Glu27Glu variant and bronchodilator response Citation(19), whereas no associations were found for the Thr164Ile genotype. Taken together, none of the three genotypes evaluated consistently affected the treatment response in patients with COPD. This is in agreement with a recently published genome-wide analysis of the response to inhaled β2-agonists in COPD. This study found no significant association between genetic variants in the ADRB2 codons 16 and 27 and bronchodilator responsiveness Citation(39).
Besides bronchodilation the ADRB2 gene may play other significant roles in regulating responses to exogenous β2-agonists, e.g. by inhibiting the proliferation of airway smooth muscle cells and neutrophil accumulation (Citation41,Citation42). The receptor has also been shown to stimulate mucociliary transport of bronchial epithelial cells and reduce the mucosal damage (Citation43,Citation44), and genetic variation in the ADRB2 gene may influence desensitization and agonist-promoted receptor downregulation (Citation4,Citation28). Further studies are needed to confirm these results and to examine their potential role in COPD.
Conclusion
This systematic review and meta-analyses found no significant association between Thr164Ile, Arg16Gly, and Gln27Glu genotypes and risk of COPD. We found no consistent difference in treatment response to β2-agonists in COPD as a function of ADRB2 genotype. Additional mechanistic and large epidemiological studies of Thr164Ile and other severe ADRB2 variants are required to conclusively determine if ADRB2 dysfunction is associated with risk of COPD development.
Declaration of interest
The sponsors of the study are public non-profit organizations and support science in general. They had no role in gathering, analyzing, or interpreting the data and could neither approve nor disapprove the submitted manuscript. The authors report no conflicts of interest.
Funding
This study was supported by the Danish Medical Research Council (No. 4183–00569B) and the Research Fund at Copenhagen University Hospital Køge (No. 13–000835).
References
- World Health Organisation. The 10 leading causes of death in the world 2012, factsheet No 310. WHO (Internet). 2014 (cited 2016 Jan 5). Available from: http://www.who.int/mediacentre/factsheets/fs310/en/. 2014 May.
- Thomsen M, Nordestgaard BG, Vestbo J, Lange P. Characteristics and outcomes of chronic obstructive pulmonary disease in never smokers in Denmark: a prospective population study. Lancet Respir Med 2013; 1(7):543–550.
- Zoeller B, Li X, Sundquist J, Sundquist K. Familial transmission of chronic obstructive pulmonary disease in adoptees: a Swedish nationwide family study. BMJ Open 2015; 5:e007310.
- Vacca G, Schwabe K, Dück R, Hlawa H-P, Westphal A, Pabst S, et al. Polymorphisms of the beta2 adrenoreceptor gene in chronic obstructive pulmonary disease. Ther Adv Respir Dis 2009; 3(1):3–10.
- Papatheodorou A, Makrythanasis P, Kaliakatsos M, Dimakou A, Orfanidou D, Roussos C, et al. Development of novel microarray methodology for the study of mutations in the SERPINA1 and ADRB2 genes–their association with Obstructive Pulmonary Disease and Disseminated Bronchiectasis in Greek patients. Clin Biochem 2010; 43(1–2):43–50.
- Ho LI, Harn HJ, Chen CJ, Tsai NM. Polymorphism of the beta(2)-adrenoceptor in COPD in Chinese subjects. Chest 2001; 120(5):1493–1489.
- Brøgger J, Steen VM, Eiken HG, Gulsvik A, Bakke P. Genetic association between COPD and polymorphisms in TNF, ADRB2 and EPHX1. Eur Respir J 2006; 27(4):682–688.
- Poller W, Meisen C, Olek K. DNA polymorphisms of the alpha 1-antitrypsin gene region in patients with chronic obstructive pulmonary disease. Eur J Clin Invest 1990; 20(1):1–7.
- Ahles A, Engelhardt S. Polymorphic variants of adrenoceptors: Pharmacology, physiology, and role in disease. Pharmacol Rev 2014; 66:598–637.
- Johnson M. Molecular mechanisms of beta(2)-adrenergic receptor function, response, and regulation. J Allergy Clin Immunol 2006; 117:18–24: quiz 25.
- Small KM, McGraw DW, Liggett SB. Pharmacology and physiology of human adrenergic receptor polymorphisms. Annu Rev Pharmacol Toxicol 2003; 43:381–411.
- Grech G, Grossman I. Preventive and Predictive Genetics: Towards Personalised Medicine. Vol. 2015. Springer; 378 p.
- Thomsen M, Nordestgaard BG, Sethi AA, Tybjærg-Hansen A, Dahl M. β2-adrenergic receptor polymorphisms, asthma and COPD: Two large population-based studies. Eur Respir J 2012; 39(3):558–566.
- Al-Balushi K, Zadjali F, Al-Sinani S, Al-Zadjali A-M, Bayoumi R. Frequencies of the Arg16Gly, Gln27Glu and Thr164Ile Adrenoceptor β2 Polymorphisms among Omanis. Sultan Qaboos Univ Med J 2015; 15(4):e486–e490.
- Nielsen AO, Quaum S, Bouchelouche PN, Laursen LC, Dahl R, Dahl M. Risk of asthma in heterozygous carriers for cystic fibrosis: A meta-analysis. J Cyst Fibrosis 2016; 15:563–567.
- Attia J, Thakkinstian A, D'Este C. Meta-analyses of molecular association studies: methodologic lessons for genetic epidemiology. J Clin Epidemiol 2003; 56(4):297–303.
- Hegab AE, Sakamoto T, Saitoh W, Massoud HH, Massoud HM, Hassanein KM, et al. Polymorphisms of IL4, IL13, and ADRB2 genes in COPD. Chest 2004; 126(6):1832–1839.
- Ferdinands JM, Mannino DM, Gwinn ML, Bray MS. ADRB2 Arg16Gly polymorphism, lung function, and mortality: results from the Atherosclerosis Risk in Communities study. PloS One 2007; 2(3):e289.
- Hussein MH, Sobhy KE, Sabry IM, El Serafi AT, Toraih EA. Beta2-adrenergic receptor gene haplotypes and bronchodilator response in Egyptian patients with chronic obstructive pulmonary disease. Adv Med Sci 2017(62):193–201.
- Ganbold C, Jamyansuren J, Puntsag O, Batjargal O, Dashtseren I, Jav S. ADRB2 and ACE gene polymorphisms in COPD susceptibility. Cent Asian J Med Sci 2016; 2016(2):127–133.
- Bleecker ER, Meyers DA, Bailey WC, Sims A-M, Bujac SR, Goldman M, et al. ADRB2 polymorphisms and budesonide/formoterol responses in COPD. Chest 2012; 142(2):320–328.
- Matheson MC, Ellis JA, Raven J, Johns DP, Walters EH, Abramson MJ. Beta2-adrenergic receptor polymorphisms are associated with asthma and COPD in adults. J Hum Genet 2006; 51(11):943–951.
- Hizawa N, Makita H, Nasuhara Y, Betsuyaku T, Itoh Y, Nagai K, et al. Beta2-adrenergic receptor genetic polymorphisms and short-term bronchodilator responses in patients with COPD. Chest 2007; 132(5):1485–1492.
- Kim WJ, Oh Y-M, Sung J, Kim T-H, Huh JW, Jung H, et al. Lung function response to 12-week treatment with combined inhalation of long-acting beta2 agonist and glucocorticoid according to ADRB2 polymorphism in patients with chronic obstructive pulmonary disease. Lung 2008; 186(6):381–386.
- Konno S, Makita H, Hasegawa M, Nasuhara Y, Nagai K, Betsuyaku T, et al. Beta2-adrenergic receptor polymorphisms as a determinant of preferential bronchodilator responses to β2-agonist and anticholinergic agents in Japanese patients with chronic obstructive pulmonary disease. Pharmacogenet Genomics 2011; 21(11):687–693.
- Rabe KF, Fabbri LM, Israel E, Kögler H, Riemann K, Schmidt H, et al. Effect of ADRB2 polymorphisms on the efficacy of salmeterol and tiotropium in preventing COPD exacerbations: a prespecified substudy of the POET-COPD trial. Lancet Respir Med 2014; 2(1):44–53.
- Yelensky R, Li Y, Lewitzky S, Leroy E, Hurwitz C, Rodman D, et al. A pharmacogenetic study of ADRB2 polymorphisms and indacaterol response in COPD patients. Pharmacogenomics J 2012; 12(6):484–488.
- Wang W, Li P, Chen Y, Yang J. Association between β2-adrenergic receptor-16Arg/Gly gene polymorphism and chronic obstructive pulmonary disease risk: Systematic review and meta-analysis. Iran J Public Health 2014; 43(7):877–888.
- Niu L-M, Liang Y, Xu M, Zhang Y-Y, Zhang Y, He B. Effect of polymorphisms in the β2-adrenergic receptor on the susceptibility and pulmonary function of patients with chronic obstructive pulmonary disease: A meta analysis. Chin Med J (Engl) 2012; 125(12):2213–2218.
- Bossé Y. Updates on the COPD gene list. Int J COPD 2012; 7:607–631.
- Palmer CNA, Lipworth BJ, Lee S, Ismail T, Macgregor DF, Mukhopadhyay S. Arginine-16 beta2 adrenoceptor genotype predisposes to exacerbations in young asthmatics taking regular salmeterol. Thorax 2006; 61(11):940–944.
- Hall IP, Blakey JD, Al Balushi KA, Wheatley A, Sayers I, Pembrey ME, et al. Beta2-adrenoceptor polymorphisms and asthma from childhood to middle age in the British 1958 birth cohort: a genetic association study. Lancet Lond Engl 2006; 368(9537):771–779.
- Migita O, Noguchi E, Jian Z, Shibasaki M, Migita T, Ichikawa K, et al. ADRB2 polymorphisms and asthma susceptibility: transmission disequilibrium test and meta-analysis. Int Arch Allergy Immunol 2004; 134(2):150–157.
- Shah NJ, Vinod Kumar S, Gurusamy U, Annan Sudarsan AK, Shewade DG. Effect of ADRB2 (adrenergic receptor β2) gene polymorphisms on the occurrence of asthma and on the response to nebulized salbutamol in South Indian patients with bronchial asthma. J Asthma Off J Assoc Care Asthma 2015; 52(8):755–762.
- Danielewicz H. What the genetic background of individuals with asthma and obesity can reveal: Is β2-adrenergic receptor gene polymorphism important? Pediatr Allergy Immunol Pulmonol 2014; 27(3):104–110.
- Petrovic-Stanojevic N, Topic A, Nikolic A, Stankovic M, Dopudja-Pantic V, Milenkovic B, et al. Polymorphisms of beta2-adrenergic receptor gene in serbian asthmatic adults: effects on response to Beta-agonists. Mol Diagn Ther 2014; 18(6):639–646.
- de Paiva ACZ, Marson FA, de L, Ribeiro JD, Bertuzzo CS. Asthma: Gln27Glu and Arg16Gly polymorphisms of the beta2-adrenergic receptor gene as risk factors. Allergy Asthma Clin Immunol Off J Can Soc Allergy Clin Immunol 2014; 10(1):8.
- Manolopoulos KN, Karpe F. ADRB2 Arg16Gly polymorphism in the LARGE trial. Lancet Lond Engl 2010; 375(9716):724–725; author reply 725.
- Hardin M, Cho M, McDonald M-L, et al. A Genome-wide analysis of the response to inhaled beta2-agonists in chronic obstructive pulmonary disease. J Pharmacogenomics 2016; 16(4):326–335.
- Hersh C. Pharmacogenetics of chronic obstructive pulmonary disease: challenges and oppportunities. Pharmacogenetics 2010; 11:237–247.
- Dekkers BGJ, Pehlić A, Mariani R, Bos IST, Meurs H, Zaagsma J. Glucocorticosteroids and β2-adrenoceptor agonists synergize to inhibit airway smooth muscle remodeling. J Pharmacol Exp Ther 2012; 342(3):780–787.
- Mirza ZN, Kato M, Kimura H, Tachibana A, Fujiu T, Suzuki M, et al. Fenoterol inhibits superoxide anion generation by human polymorphonuclear leukocytes via beta-adrenoceptor-dependent and -independent mechanisms. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol 2002; 88(5):494–500.
- Dowling RB, Johnson M, Cole PJ, Wilson R. Effect of fluticasone propionate and salmeterol on Pseudomonas aeruginosa infection of the respiratory mucosa in vitro. Eur Respir J 1999; 14(2):363–369.
- Wegner CD. Novel mechanistic targets for the treatment of sub-acute and chronic bronchitis. Curr Pharm Des 2001; 7(3):199–212.