ABSTRACT
Patients with chronic obstructive pulmonary disease and pulmonary hypertension (PH–COPD) have an increased risk of hospitalizations and death compared to COPD alone. Identifying PH in COPD is challenging because performing right heart catheterization, the gold standard for PH diagnosis, is invasive and not routinely performed. Clinical characterization of COPD patients at risk who are progressing toward PH will aid therapeutic development at earlier stages of progressively fatal PH–COPD. We studied the records of 5,45,086 patients in a large Veterans Affairs healthcare network (2000–2012) with a primary discharge diagnosis of COPD based on encounters' ICD-9 codes and further stratified into those who received an additional ICD-9 code for a PH diagnosis. Patients with PH–COPD were assigned to one of the four subgroups: those with (a) no history of exacerbation or hospital admissions, (b) history of exacerbations but no hospital admissions, (c) hospital admissions unrelated to COPD and (d) history of COPD exacerbation-related hospital admissions. We also examined the COPD and COPD-PH cohorts for associated comorbidities such as cardiac disease and the presence of obstructive sleep apnea (OSA). A regression analysis revealed that patients with COPD exacerbation-related hospital admissions had 7 × higher risk of having a concomitant clinical diagnosis of PH compared to non-hospitalized patients. COPD-PH patients had higher rates of cardiac comorbidities (89% vs. 66%) and OSA (34% vs. 16%) compared to COPD alone. We conclude that COPD patients hospitalized for COPD exacerbations are at a higher risk for developing PH, and hospitalized COPD patients with cardiac comorbidities and/or OSA should be screened as at-risk population for developing PH.
Introduction
Pulmonary hypertension (PH) is a condition in which elevated pulmonary artery pressures lead to exertional dyspnea, progressive right heart failure, and often death Citation(1). It is confirmed as having a mean resting pulmonary artery pressure (mPAP) of greater than or equal to 25 mm Hg when measured by right heart catheterization (RHC) Citation(2). PH confers an increased risk of mortality in the veteran population Citation(3).
Chronic obstructive pulmonary disease (COPD) is marked by persistent airflow limitation and chronic inflammation of the airways Citation(4). It is a common and morbid condition: as of 2013, COPD is the third leading cause of death in the United States Citation(5). Patients with COPD can develop PH (PH–COPD). PH–COPD is classified under group 3 PH according to the World Health Organization (WHO) classification scheme Citation(6). Mortality is high in patients with COPD who develop PH (Citation7–11). Additionally, the presence of PH in patients with COPD is a risk factor for the development of acute exacerbations of COPD Citation(12–14).
Identifying COPD patients who have developed PH is difficult due to insufficient diagnostic modalities, poor understanding of the pathophysiology of the disease, and lack of strong predictive models. Symptoms such as dyspnea on exertion are common to both disease processes. Although trans-thoracic echocardiography (TTE) is often used as a screening test for the presence of PH, the use of this study in patients with structural lung disease is not well validated and sometimes inaccurate Citation(15,16). RHC is the gold standard of diagnosis for PH Citation(2), but in practice, this study is not routinely used in patients with COPD Citation(17).
Another factor complicating the diagnosis of PH in COPD is that the development of elevated pulmonary artery pressures is not necessarily associated with the traditional measures of COPD severity, such as airflow limitations. For some patients, the degree of PH in COPD can be mild to moderate in severity, and develop slowly over time Citation(18). In contrast, a subset of patients appears to have severe PH that is “out-of-proportion” to the expected degree of elevation in pulmonary pressures based on lung parenchymal abnormalities Citation(19). Severe PH has even been found in a subset of COPD patients who have imaging showing emphysematous parenchymal changes, but normal spirometry Citation(20). The reason for the development of PH in COPD is not well understood, but factors including chronic hypoxemia, inflammation, and toxicity from nicotine exposure are thought to contribute to the vascular remodeling, endothelial dysfunction, and destruction of capillary beds that are seen in this disease process Citation(21).
Interventions effective in PH do not improve outcomes in PH–COPD and raise safety concerns. It is important to identify patients with PH–COPD not only because they have worse outcomes, but also because they should be studied to see if targeted PH therapies could be beneficial. Research focusing on therapies for PH in patients with COPD is needed Citation(11,22). Our study was designed to identify factors predicting the presence of a clinical diagnosis of PH in patients with COPD.
Methods
This was a retrospective cohort study which longitudinally followed patients from an inception time point to a set end time point. It was approved by the Institutional Review Board of Baylor College of Medicine (H-30464), the Research and Development Committee of Michael E. DeBakey Veterans Affairs Medical Center, and Veterans Health Administration Corporate Data Warehouse.
Patients
All patients were members of the South Central Veteran's Affairs Healthcare Network (VISN 16) subset of the Veterans Health Administration Corporate Data Warehouse during fiscal years (FY) 2000–2012. Patients who received their care at multiple VA facilities during the study period were excluded. This database was queried using ICD-9 codes (refer to in the supplement for the ICD-9 codes used in this study). Patients were included in the study either by having one hospitalization in which COPD was the primary discharge diagnosis or by having two outpatient encounters in which COPD was the primary diagnosis. Inception time was defined as either the first admission with a primary discharge diagnosis of COPD or the second outpatient encounter with a primary diagnosis of COPD.
Table 1. Clinical characteristics of PH–COPD and COPD-alone patients.
With recent COPD guidelines emphasizing COPD exacerbation as the main determinant of severity and important clinical outcomes like mortality, we divided COPD cohort into subgroups based on the history of COPD exacerbation and hospital admission Citation(4). The groups included patients with (a) no history of exacerbation or hospital admission, (b) a history of exacerbations but no hospital admission, (c) non-COPD hospital admission but no exacerbations, and (d) a history of exacerbation-related hospital admissions. COPD exacerbation was defined as any hospital admission with COPD as the primary discharge diagnosis, and any ER visit or outpatient clinic visit with COPD as the primary diagnosis that was associated with prescribing a course of antibiotic or systemic corticosteroid. Exacerbations were considered as one episode if they occurred within two weeks of each other.
The main COPD cohort was divided into two subgroups using ICD-9 codes, based on whether the patient had also undergone a diagnosis of PH during the time period between the inception and the endpoint. The two groups were therefore Citation(1) patients having diagnoses of COPD and PH (PH–COPD cohort) and Citation(2) patients having COPD with no PH diagnosis (COPD cohort). Data of the patients were collected from the inception time until death or end of FY 2012.
Data collection
We extracted variables related to the pre-inception period, including demographic data (age, sex, and race), body mass index (BMI), and the presence of co-existing illnesses via assessment of concomitant cardiac diagnoses (Supplement ), presence of obstructive sleep apnea (OSA), and the Charlson comorbidity index Citation(23). Post-inception data from the study period (FY 2000–2012) were also obtained: these included mortality; annual number of all-cause hospitalizations, emergency room visits, and outpatient encounters; and respiratory and cardiac medications.
Statistical analysis
Data analysis was performed using STATA SE version 12.1 (StataCorp, College Station, TX, USA). The primary analysis compared all patients in the two study cohorts. All study variables, including baseline and outcome measures, were analyzed descriptively. Percentages and counts were provided for dichotomous and polychotomous variables. Means and standard deviations were provided for continuous variables. For dichotomous variables, chi-square tests were used to evaluate the statistical significance of differences, and Student's t-test was used for the means of continuous variables. We used a multivariate logistic regression analysis for inpatient and 30-day mortality and Cox regression analysis for overall mortality. p value <0.01 was considered significant in this large population database.
Results
During the study period, a total of 5,45,086 patients from VISN 16 database were reviewed: these patients were sorted by a primary diagnosis of COPD, which identified 54,008 patients with COPD. This represented 10% of the patient population. This cohort was divided into two groups based on whether they had a diagnosis of PH at any time during the study period. Four percent of the patients with COPD carried a diagnosis of PH, encompassing 2,176 patients (PH–COPD cohort); the remaining 51,832 patients comprised the COPD-alone cohort.
shows demographic data of the two groups. The PH–COPD group was older and had a higher mean BMI. The PH–COPD carried a much-effective diagnosis of OSA, various cardiac comorbidities and had higher Charlson comorbidity index indicating more chronically ill population compared to COPD alone. A higher percentage of the PH–COPD group received cardiac medications including beta blocker, statins and anticoagulation and aspirin.
Incidence and prevalence of PH
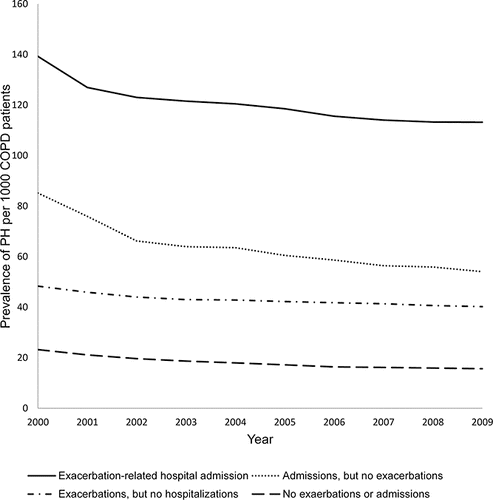
The data showed that the yearly incidence of PH–COPD ranged from 0.39% to 0.57%. Prevalence increased from 1.4% to 4.9% over the study period. The prevalence of PH was then evaluated based on characteristics of COPD. Patients with COPD were stratified into four groups based on the presence of COPD exacerbations, hospitalizations for any cause, and exacerbation-related hospitalizations. shows the prevalence of PH in the four COPD groups for FY 2000–2009. Patients with COPD-related admissions had the highest prevalence of PH diagnosis, while patients with no history of COPD exacerbation and or admission were diagnosed least with PH.
shows the results of regression analysis that was performed on the COPD cohort to evaluate the factors predicting the diagnosis of PH. Patients with a higher number of COPD exacerbations or a higher number of any type of admissions were more likely to have PH diagnosis. Not surprisingly, higher BMI and the presence of OSA predicted higher chance of PH diagnosis. Investigating medication exposures showed that prescription for beta blocker or anticoagulation/aspirin was associated with a higher chance of being diagnosed with PH. In contrast, prescription for statins predicted a lower chance of being diagnosed with PH.
Table 2. Predictors of developing pulmonary hypertension in patients with COPD.
Mortality
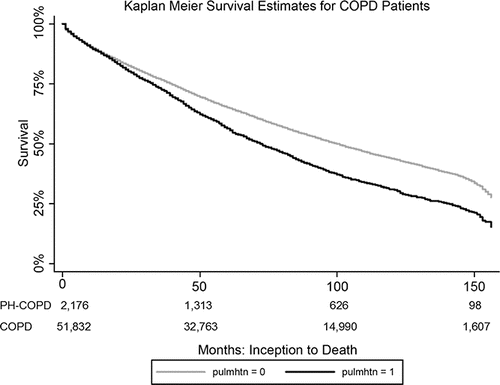
Survival of patients with PH–COPD was reduced over the study period, as illustrated by the Kaplan–Meier curve shown in . A diagnosis of PH–COPD is associated with increased odds of overall mortality, as noted in . Undergoing the diagnosis of PH–COPD also increases the odds of having more health care utilization: this population had a 22% increase in outpatient encounters, a 32% increase in inpatient hospitalizations, and a 27% increase in emergency room visits.
Table 3. Presence of PH in veterans with COPD is associated with increased mortality and health care utilization.
Discussion
This study is the first large-scale, long-term, retrospective assessment of the veteran population with clinical diagnoses of COPD and PH. No similar large-scale studies evaluating the topic of PH–COPD have been done, and our work represents a step toward better characterization of these patients. We found that patients with COPD exacerbation-related hospital admissions had the highest prevalence of PH among this large cohort of COPD patients. Moreover, the annual number of COPD exacerbations was found to be a factor predicting the diagnosis of PH–COPD. Based on these results, we propose that patients with COPD who are hospitalized for an exacerbation should be evaluated for PH and/or the presence of diastolic and/or systolic left heart dysfunction.
Our study has some key limitations. First, the veterans with both COPD and PH were identified using ICD-9 codes. Using this strategy gives false-positive and false-negative diagnoses: it can fail to identify patients with PH, and alternatively could include patients who were incorrectly labeled with PH. Our study is thus dependent on clinical diagnoses based on physician definitions and provider coding practices rather than precise definitions of disease. Given that RHCs are not routinely performed and we do not have accurate numbers on whether patients in this cohort had this invasive procedure, it is a strong possibility that many patient diagnoses of PH were made based on echocardiogram reports, which can generate inaccurate results in patients with lung disease, as discussed previously. However, echocardiograms as a screening test are commonly used in a high-risk population such as veterans, and despite its limitations, our results reflect the importance of identifying those patients that merit a more discerning screening and assessment for PH besides echocardiogram, and perhaps clinical phenotypes offer that option.
Another limitation of using ICD-9 codes is that the definition used to identify COPD exacerbations could inadvertently include patients who were admitted for pneumonia, and we acknowledge that this is a shortcoming. Additionally, our methodology confers an ascertainment bias, as patients with increased healthcare utilization would be more likely to undergo procedures such as echocardiography that would result in them obtaining a diagnosis of PH. Another limitation in the study lies in the fact that although the study is longitudinal, we cannot make assumptions on the time intervals between the diagnosis of COPD, and the occurrence of PH and COPD-related hospital admissions. Thus, no causation can be confirmed. Hypoxemia and long-term oxygen treatment were not assessed in the scope of this study but would be informative in further stratifying a clinical phenotype since these are independent risk factors for the development of COPD-associated PH.
Identifying the PH–COPD population is important because future research could find that PH-specific therapy could be beneficial. Currently, the only therapy that shows clear evidence of reducing progression of PH in COPD is long-term oxygen therapy Citation(24). Results from studies evaluating other PH-specific therapies have been less robust. There have been equivocal results regarding sildenafil Citation(25–28), tadalafil Citation(29), bosentan Citation(30,31), and iloprost Citation(32). Intriguingly, a recent study has evaluated patients with PH that is “out-of-proportion” to their obstructive pulmonary disease: although small and retrospective in nature, treatment with PH-specific medications produced some promising changes in pulmonary hemodynamics Citation(33). The use of PH-specific medications in the PH–COPD population merits further investigation Citation(22). We believe that our data provide important information about how to identify this subgroup of patients so that they can be further studied.
Prior research has suggested that patients with frequent exacerbations of COPD comprise a distinct phenotype of the disease Citation(34). These patients have worsened quality of life indicators compared to the generalized COPD population, and display higher rates of healthcare utilization and hospitalization Citation(35). An analysis of the ECLIPSE database showed that although the rates of exacerbation increased with the higher GOLD stage, there was a subset of patients who were at an increased risk for exacerbations irrespective of disease severity as measured by spirometry Citation(36). We suggest that the presence of PH could be a factor contributing to this independent susceptibility phenotype.
We found that approximately 4% of the patients with a diagnosis of COPD also carried a diagnosis of PH. Comparing this prevalence to results found in other studies is challenging due to differences in how the diagnosis of PH is established. Our study is limited in that it is a database query that relies on ICD-9 diagnostic codes. Presumably, the diagnosis of PH–COPD is made on the basis of TTE, which itself has limitations as noted previously. A subset of patients likely did had RHC that assisted in making this diagnosis, but data on this topic were not obtainable from our database. The prevalence of PH–COPD increased over our study period. It is unclear whether this is due to increased physician recognition of the condition, or perhaps due to increased survival due to initiation of COPD-appropriate therapies.
Our data provide other information regarding PH–COPD. Having a diagnosis of PH–COPD was associated with increased mortality and healthcare utilization. This is in agreement with previous research in the veteran population, which also suggests that the presence of PH in COPD confers mortality risks Citation(13). In our study, patients with PH–COPD were more likely to have cardiac comorbidities and an elevated BMI; additionally, the use of cardiac-related medications predicated the diagnosis of PH–COPD.
One finding in our study was that patients who carried a diagnosis of PH were more likely to have cardiac comorbidities. We acknowledge that this finding represents a potential confounder, since cardiac disease itself is a driver of PH, and is classified as group 2 PH Citation(37). A recent study which assessed all veterans who had undergone RHC nationally between 2007 and 2012 noted that many patients had both cardiac and pulmonary abnormalities Citation(3). Other prevalence studies looking at PH in COPD have noted a large amount of pulmonary venous hypertension secondary to left ventricular disease Citation(10,38,39). Even in the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL), which looked at the outcomes of patients with pulmonary arterial hypertension, around 20% of the patients with this clinical diagnosis also have evidence of airway obstruction Citation(40). This information highlights the limitations in the current classification schema, namely that many patients with PH have multiple contributors.
Patients with PH–COPD were more likely to have OSA. In many regards, this is unsurprising because OSA itself can increase pulmonary pressures. The prevalence of PH in OSA ranges from approximately 20% to 40% depending on the method used for the diagnosis and the population being studied Citation(41–44). The treatment of OSA using continuous positive airway pressure has been shown to improve pulmonary hemodynamics Citation(45,46). The presence of sleep apnea in COPD, much like the presence of cardiac comorbidities, serves as an additive and possibly modifiable risk factor that contributes to the development of PH.
Conclusions
Patients who have COPD exacerbations requiring hospitalization have more incidence of being diagnosed with PH. We propose that all patients with COPD exacerbations requiring hospitalization should be screened for PH and/or left heart failure. This clinical rule of thumb could facilitate the identification of this important subset of patients.
Abbreviations
| COPD | = | chronic obstructive pulmonary disease |
| PH | = | pulmonary hypertension |
| ICD | = | International Classification of Disease |
| mPAP | = | mean pulmonary artery pressure |
| RHC | = | right heart catheterization |
| WHO | = | World Health Organization |
| TTE | = | trans-thoracic echocardiography |
| VISN 16 | = | South Central Veteran's Affairs Healthcare Network |
| FY | = | fiscal years |
| BMI | = | body mass index |
| OSA | = | obstructive sleep apnea |
Declaration of interest
The authors have no conflicts of interest.
Supplemental_Table_1.docx
Download MS Word (13.8 KB)Acknowledgments
This work was supported by a Career Development Award #IK20BX002410 (LMP) from the United States Department of Veterans Affairs, Biomedical Laboratory Research and Development.
The authors thank the Center for Translational Research on Inflammatory Diseases (CTRID) at the Veterans Affairs Medical Center in Houston, Texas, for their scholastic support and advice in the preparation of this manuscript.
References
- McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 2009; 119(16):2250–2294.
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46(4):903–975.
- Maron BA, Hess E, Maddox TM, Opotowsky AR, Tedford RJ, Lahm T, et al. Association of borderline pulmonary hypertension with mortality and hospitalization in a large patient cohort: insights from the veterans affairs clinical assessment, reporting, and tracking program. Circulation 2016; 133(13):1240–1248.
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2016; http://www.goldcopd.org
- Heron M. Deaths: leading causes for 2013. Natl Vital Stat Rep 2016; 65(2):1–95.
- Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62(25 Suppl):D34–D41.
- Weitzenblum E, Hirth C, Ducolone A, Mirhom R, Rasaholinjanahary J, Ehrhart M. Prognostic value of pulmonary artery pressure in chronic obstructive pulmonary disease. Thorax 1981; 36(10):752–758.
- Oswald-Mammosser M, Weitzenblum E, Quoix E, Moser G, Chaouat A, Charpentier C, et al. Prognostic factors in COPD patients receiving long-term oxygen therapy. Importance of pulmonary artery pressure. Chest 1995; 107(5):1193–1198.
- Chaouat A, Bugnet AS, Kadaoui N, Schott R, Enache I, Ducoloné A, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2005; 172(2):189–194.
- Cuttica MJ, Kalhan R, Shlobin OA, Ahmad S, Gladwin M, Machado RF, et al. Categorization and impact of pulmonary hypertension in patients with advanced COPD. Respir Med 2010; 104(12):1877–1882.
- Dweik RA, Rounds S, Erzurum SC, Archer S, Fagan K, Hassoun PM, et al. An official American Thoracic Society Statement: pulmonary hypertension phenotypes. Am J Respir Crit Care Med 2014; 189(3):345–355.
- Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159(1):158–164.
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest 2007; 132(6):1748–1755.
- Terzano C, Conti V, Di Stefano F, Petroianni A, Ceccarelli D, Graziani E, et al. Comorbidity, hospitalization, and mortality in COPD: results from a longitudinal study. Lung 2010; 188(4):321–329.
- Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. Am J Respir Crit Care Med 2003; 167(5):735–740.
- Fisher MR, Criner GJ, Fishman AP, Hassoun PM, Minai OA, Scharf SM, et al. Estimating pulmonary artery pressures by echocardiography in patients with emphysema. Eur Respir J 2007; 30(5):914–921.
- Blanco I, Piccari L, Barbera JA. Pulmonary vasculature in COPD: The silent component. Respirology 2016; 21(6):984–994.
- Kessler R, Faller M, Weitzenblum E, Chaouat A, Aykut A, Ducoloné A, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med 2001; 164(2):219–224.
- Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J 2008; 32(5):1371–1385.
- Adir Y, Shachner R, Amir O, Humbert M. Severe pulmonary hypertension associated with emphysema: a new phenotype? Chest 2012; 142(6):1654–1658.
- Pugliese SC, Poth JM, Fini MA, Olschewski A, El Kasmi KC, Stenmark KR. The role of inflammation in hypoxic pulmonary hypertension: from cellular mechanisms to clinical phenotypes. Am J Physiol Lung Cell Mol Physiol 2015; 308(3):L229–L252.
- Celli BR, Decramer M, Wedzicha JA, Wilson KC, Agustí A, Criner GJ, et al. An Official American Thoracic Society/European Respiratory Society Statement: Research questions in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015; 191(7):e4–e27.
- Frenkel WJ, Jongerius EJ, Mandjes-van Uitert MJ, van Munster BC, de Rooij SE. Validation of the Charlson Comorbidity Index in acutely hospitalized elderly adults: a prospective cohort study. J Am Geriatr Soc 2014; 62(2):342–346.
- Weitzenblum E, Sautegeau A, Ehrhart M, Mammosser M, Pelletier A. Long-term oxygen therapy can reverse the progression of pulmonary hypertension in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis 1985; 131(4):493–498.
- Blanco I, Gimeno E, Munoz PA, Pizarro S, Gistau C, Rodriguez-Roisin R, et al. Hemodynamic and gas exchange effects of sildenafil in patients with chronic obstructive pulmonary disease and pulmonary hypertension. Am J Respir Crit Care Med 2010; 181(3):270–278.
- Blanco I, Santos S, Gea J, Güell R, Torres F, Gimeno-Santos E, et al. Sildenafil to improve respiratory rehabilitation outcomes in COPD: a controlled trial. Eur Respir J 2013; 42(4):982–992.
- Rao RS, Singh S, Sharma BB, Agarwal VV, Singh V. Sildenafil improves six-minute walk distance in chronic obstructive pulmonary disease: a randomised, double-blind, placebo-controlled trial. Indian J Chest Dis Allied Sci 2011; 53(2):81–85.
- Lederer DJ, Bartels MN, Schluger NW, Brogan F, Jellen P, Thomashow BM, et al. Sildenafil for chronic obstructive pulmonary disease: a randomized crossover trial. COPD 2012; 9(3):268–275.
- Goudie AR, Lipworth BJ, Hopkinson PJ, Wei L, Struthers AD. Tadalafil in patients with chronic obstructive pulmonary disease: a randomised, double-blind, parallel-group, placebo-controlled trial. Lancet Respir Med 2014; 2(4):293–300.
- Stolz D, Rasch H, Linka A, Di Valentino M, Meyer A, Brutsche M, et al. A randomised, controlled trial of bosentan in severe COPD. Eur Respir J 2008; 32(3):619–628.
- Valerio G, Bracciale P, Grazia D'Agostino A. Effect of bosentan upon pulmonary hypertension in chronic obstructive pulmonary disease. Ther Adv Respir Dis 2009; 3(1):15–21.
- Dernaika TA, Beavin M, Kinasewitz GT. Iloprost improves gas exchange and exercise tolerance in patients with pulmonary hypertension and chronic obstructive pulmonary disease. Respiration 2010; 79(5):377–382.
- Calcaianu G, Canuet M, Schuller A, Enache I, Chaouat A, Kessler R. Pulmonary arterial hypertension-specific drug therapy in COPD patients with severe pulmonary hypertension and mild-to-moderate airflow limitation. Respiration 2016; 91(1):9–17.
- Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med 2010; 182(5):598–604.
- Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med 2013; 11:181.
- Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med 2010; 363(12):1128–1138.
- Fang JC, DeMarco T, Givertz MM, Borlaug BA, Lewis GD, Rame JE, et al. World Health Organization Pulmonary Hypertension group 2: pulmonary hypertension due to left heart disease in the adult – a summary statement from the Pulmonary Hypertension Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2012; 31(9):913–933.
- Scharf SM, Iqbal M, Keller C, Criner G, Lee S, Fessler HE, National Emphysema Treatment Trial (NETT) Group. Hemodynamic characterization of patients with severe emphysema. Am J Respir Crit Care Med 2002; 166(3):314–322.
- Andersen KH, Iversen M, Kjaergaard J, Mortensen J, Nielsen-Kudsk JE, Bendstrup E, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant 2012; 31(4):373–380.
- Poms AD, Turner M, Farber HW, Meltzer LA, McGoon MD. Comorbid conditions and outcomes in patients with pulmonary arterial hypertension: a REVEAL registry analysis. Chest 2013; 144(1):169–176.
- Sanner BM, Doberauer C, Konermann M, Sturm A, Zidek W. Pulmonary hypertension in patients with obstructive sleep apnea syndrome. Arch Intern Med 1997; 157(21):2483–2487.
- Sajkov D, Cowie RJ, Thornton AT, Espinoza HA, McEvoy RD. Pulmonary hypertension and hypoxemia in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 1994; 149(2 Pt 1):416–422.
- Chaouat A, Weitzenblum E, Krieger J, Oswald M, Kessler R. Pulmonary hemodynamics in the obstructive sleep apnea syndrome. Results in 220 consecutive patients. Chest 1996; 109(2):380–386.
- Sajkov D, McEvoy RD. Obstructive sleep apnea and pulmonary hypertension. Prog Cardiovasc Dis 2009; 51(5):363–370.
- Arias MA, García-Río F, Alonso-Fernández A, Martínez I, Villamor J. Pulmonary hypertension in obstructive sleep apnoea: effects of continuous positive airway pressure: a randomized, controlled cross-over study. Eur Heart J 2006; 27(9):1106–1113.
- Sajkov D, Wang T, Saunders NA, Bune AJ, Mcevoy RD. Continuous positive airway pressure treatment improves pulmonary hemodynamics in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2002; 165(2):152–158.