ABSTRACT
Introduction: Night-time respiratory symptoms have a considerable impact on sleep and life quality in patients with chronic obstructive pulmonary disease (COPD). Lack of awareness of night-time symptoms can lead to worsened COPD control. Automated long-term monitoring of respiratory symptoms with LEOSound enables assessment of nocturnal wheezing and cough. Methods: In this observational study we investigated the prevalence and severity of cough and wheezing in patients with stable COPD [Global Initiative for Chronic Obstructive Lung Disease (GOLD) II–IV] disease for two consecutive nights with the LEOSound system. 48 patients (30 males, 63%) were eligible for inclusion, median age was 67 years, and body mass index (BMI) was 25.3 kg/m2. Results: In 15 out of 48 patients (31%), we found wheezing periods for at least 10-minute duration. Wheezing periods >30 minutes were monitored in seven patients and wheezing periods >60 minutes were monitored in three patients. The maximum duration of wheezing was 470 minutes in one patient with COPD II. The median wheezing rate differed between the COPD stages and between active and non-active smokers. Cough was found in 42 patients (87.5%) with a range of 1–326 events. The cough-period-index in night one was 0.83 n/hour (P25:0.33||P75: 2.04) and night two 0.97 n/hour (P25:0.25||P75: 1.9). Most of the cough events were non-productive with a median of 0.86. Conclusions: Night-time symptoms are common in COPD patients. LEOSound offers an opportunity to evaluate objectively night-time symptoms like wheezing and cough in patients with COPD which remain otherwise unnoticed. We found a high incidence of night-time wheezing in these patients, which was related to persistant smoking.
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease with numerous pulmonary and extrapulmonary manifestations, which represents a significant public health problem worldwide. Common symptoms of COPD are chest tightness, chronic cough, wheezing, and phlegm. Sleep quality in patients with COPD is often poor and night-time symptoms seem to be responsible for sleep disturbances (Citation1–4). Symptoms of COPD differ in the morning and at night (Citation5–10). Lack of awareness of night-time respiratory symptoms can lead to worsened COPD control, disturbed sleep, and impaired daytime performance. Respiratory symptoms during night time have been reported in a significant number of patients and may affect sleep (Citation1, Citation2, Citation4, Citation11). Night-time symptoms are defined as those between the time of going to bed and the time of getting up to start the day, and morning symptoms are those between the time of getting out of bed and approximately 11 am. Objective long-term recordings of respiratory symptoms are necessary to analyze their frequency and severity.
LEOSound is a mobile system used for automated long-term recording and analysis of respiratory sounds Citation(12). The present study focuses on description of frequency, severity, and characteristics of night-time symptoms like cough and wheezing in patients with stable COPD stage II–IV within two consecutive nights.
Methods
In this observational study, we investigated the prevalence and severity of night-time symptoms like wheezing and cough in patients aged 40–80 years with stable COPD II–IV according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) free from current or recent exacerbation. The presence of asthma was excluded in all patients. Additional exclusion criteria were the refusal of participation and any acute respiratory or serious infectious disease. Health-related quality of life and the impact on the daily life were measured with the St. George`s Respiratory Questionnaire (SGRQ) and the COPD Assessment Test (CAT). The CAT is an eight-item scale used to assess the impact of COPD symptoms on health status. Higher scores indicate a greater impact of COPD on health status. The protocol was approved by the Marburg Ethics Committee before study initiation and written informed consent was obtained in all cases.
All patients were recruited in a pneumological outpatient medical center. Lung function and blood gas data, not older than 3 months, were collected from the patients’ files. Patients underwent an ambulant long-term recording of night-time symptoms with the LEOSound system for two consecutive nights (Citation12, Citation13). Night-time symptoms of COPD were defined as those taking place between bed time and getting up in the morning. As a consequence, long-term recording of lung sounds started at 10 pm and was finished after getting up from bed in the morning.
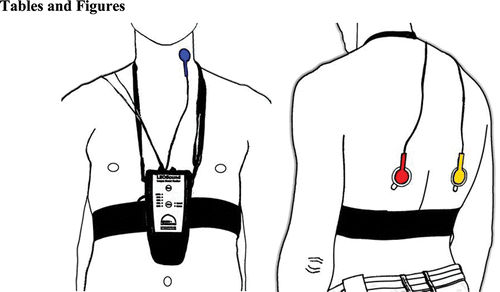
LEOSound is a commercially available, automated lung sound monitor that enables the continuous recording of lung sounds by three small bio-acoustical sensors attached to the trachea and to the back (two sensors) of the patient (). An ambient microphone is integrated, to distinguish lung sounds from speech and other ambient sounds. The validated system Citation(12) works like a ‘long-term stethoscope’ and allows 24-hour lung sound auscultation at the patients’ home or at the hospital. The LEOSound analyzer software automatically evaluates the data for the presence of cough and wheezing. It stores raw data and the results in a database. The records, as well as the automatic ratings, are shown on a graphic illustrated user interface. Additionally, the user can listen to all three channels, to verify the automated analysis. The analyzer software gives the opportunity to scope segments with a duration of 30 seconds, hereafter referred to as epochs. Wheezing and cough events are marked by the system's analyzer software and manually verified by trained medical experts. The present study determined frequency, severity, and duration of cough and wheezing events only during night time and not in the morning hours.
Figure 1. LEOSound recorder with connected trachea microphone (left) and two lung microphones (right).
Wheezing: A wheezing period consists of at least two or more consecutive epochs with detected wheezing events. Mean rate of wheezing describes the mean percentage of wheezing found in the whole recording. Wheezing was defined as clinically relevant with a minimum duration of 10 minutes.
Cough: Cough events within an interval shorter than 15 seconds were condensed to one cough period and the cough-period-index shows the mean periods per hour. Medical experts extract specific patterns from the cough sound signals to distinguish cough quality in an automated manner. Quality of cough, namely the discrimination between non-productive and productive cough, was also analyzed.
Statistics
Data analysis was performed by means of SPSS V. 22 (IBM Corporation). The distribution of all variables was tested for normality with the Kolmogorov–Smirnov test. For p ≤ 0.05, a significant deviation from normality was assumed. All lung sound variables and the majority of other variables exhibited deviations from normality including high values for skewness and kurtosis. Therefore it was decided to use robust non-parametric methods for descriptive and inference statistics.
Median and quartiles (Q25 and Q75) were used for descriptive purposes. Interdependence between variables was analyzed with the Spearman Rho rank correlation coefficient (r). Differences between three groups (e.g. COPD severity groups) were assessed by means of the Kruskal–Wallis test. Subsequent pairwise comparisons and all comparisons of two groups were done with the Mann–Whitney U test. Results were considered statistically significant for p ≤ 0.05 (α ≤ 0.05).
Due to the explorative character of this study, no adjustment of the alpha risk for non-independent testing was performed. This means that significant results should be interpreted with caution. Significant results from this study need to be replicated before they can be generalized. Results from this study can be used to facilitate sample size determinations for future studies. In this manuscript, the description of the median and quartiles will follow the syntax: “median (Q25 || Q75).”
Results
66 patients were enrolled in the study of whom 48 (30 males, 63%) were eligible for inclusion in the analysis set. Only patients with two recording nights with sufficient data quality were included. 18 patients dropped out because of a false initiation of the LEOSound system, a failure to connect the microphones correctly to the monitor, or interfering noise making data analysis impossible. Demographics, baseline characteristics, and data of lung function are listed in . 18 patients had COPD stage II, 19 COPD III, and 11 COPD IV. 37.5% received triple therapy (LABA, LAMA, and corticosteroids) and 43.8% received dual therapy, either inhaled corticosteroids with long-acting beta agonists (12.5%) or long-acting-muscarinic agents with long-acting beta-agonists (31.3%). Median age was 67 (64.25 || 73), BMI was 25.3 (21.91 || 30.88), and number of pack-years was 40 (30 || 53.75) with a percentage of active smokers of 29.2% (n = 14). All patients completed the CAT and SGRQ with a resulting median CAT Score of 18.5 and an SGRQ Score of 44.5.
Table 1. Demographics, baseline characteristics, and data of lung function.
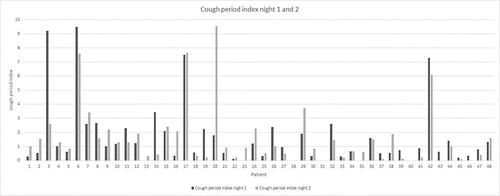
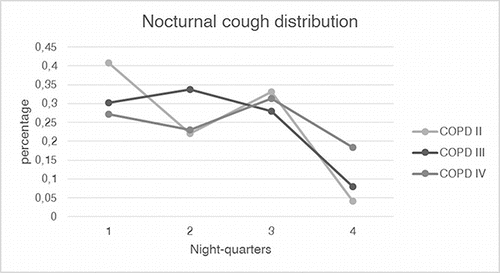
Cough was found in 42 patients (87.5%) with a range of 1–326 events. The cough-period-index in night one was 0.83/hour (0.33 || 2.04) and night in two 0.97/hour (0.25 || 1.9) (). Most of the cough periods were non-productive with a median of 0.86 (86%), only 8% (night 1) -11% (night 2) were productive. The percentage of productive cough during night one is positively correlated with CAT Score and SGRQ Score (CAT: r = 0.309, p = 0.046; SGRQ: r = 0.307, p = 0.048). This correlation was slightly lower in night two (CAT: r = 0.28, p = 0.069; SGRQ: r = 0.284, p = 0.065). By dividing the nights into quarters, cough occurred primarily in the first three parts of the night. The distribution of cough differed in COPD stages, with patients in COPD stage IV having a smaller deviation over the night than patients in the other two stages ().
First and second night of recordings showed a moderate variability of the frequency of respiratory symptoms, however patients with cough and/or wheezing periods in night one had also cough and/or wheezing periods in night two. shows details of the results from the recordings of night-time symptoms. For further analysis, patients were divided into three COPD severity groups [COPD II n = 18 (10 males); COPD III n = 19 (12 males); and COPD IV n = 11 (8 males)]. We found significantly different CAT/SGRQ Scores for the severity groups with COPD, GOLD IV typically being the most affected (CAT p = 0.038; IV > III; SGRQ p = 0.008, IV > III, IV > II). Between severity of wheezing and the questionnaires CAT and SGRQ, we did not find any significant relationship. However there was a slight tendency towards a relation between CAT and occurrence of long wheezing periods.
Table 2. Nocturnal respiratory symptoms.
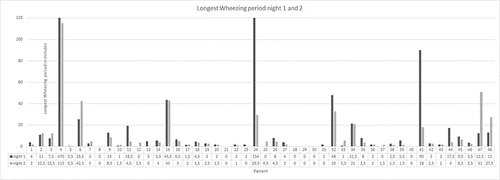
In 15 out of 48 patients (31%) wheezing periods for at least 10-minutes duration were found. Wheezing periods >30 minutes could be monitored in seven patients, and wheezing periods >60 minutes could be monitored in three patients (). The maximum duration of wheezing was 470 minutes in one patient with COPD II. By dividing the collective in two groups differentiated by the presence of clinically relevant wheezing (wheezing periods ≥10 minutes in at least one of both nights), we found a significant difference between these groups for forced expiratory volume in 1 second (FEV1) (p = 0.03) with higher FEV1 values in the group of wheezing patients. Patients with relevant wheezing (>10 minutes) were significantly more often active smokers than patients with no relevant wheezing (p = 0.013).
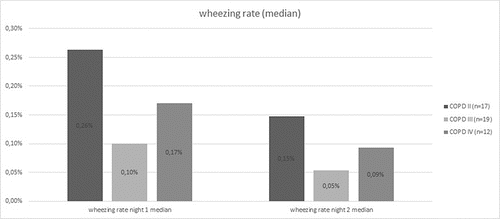
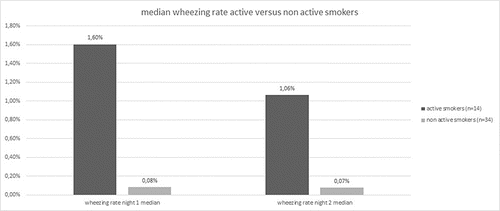
We also calculated the median wheezing rate which differed between the COPD stages (II = 0.26% in night one, 0.15% in night two; III = 0.1% in night one, 0.05% in night two; and IV = 0.17% in night one, 0.09% in night two) () and clearly differentiated between active smokers and non-active smokers ().
Discussion
Long-term recording of night-time symptoms is a useful tool for objective diagnosis of cough and wheezing in patients with COPD. As a main result of this study we found night-time wheezing in 31% of our COPD patients with a maximum duration of 470 minutes. Cough was found in 88% with a range of 1–326 events. Patients with long night-time wheezing phases had on average better lung function and higher CAT scores, and were mostly active smokers.
Respiratory symptoms are particularly disruptive during the night time and/or early morning, contributing to sleep disturbance, limited morning activities, and poor health status (Citation1–8). Nocturnal symptoms are often underreported and not considered in the clinical management. Morning has been reported as the worst time of day by COPD patients especially among those with severe COPD Citation(5). Physicians underappreciate the importance of nocturnal respiratory symptoms in patients with COPD for patients’ well-being. The potential link between night-time symptoms, sleep disturbance, and long-term clinical outcome is still unknown Citation(1).
In a pan-European cross-sectional study, Kessler et al. Citation(5) analyzed the symptom variability in patients with severe COPD. Breathlessness was the most common symptom (73%) in 2258 patients, followed by phlegm, cough, wheezing, and chest tightness. Morning hours were described as the worst time of day. Symptoms of COPD patients were found to vary over day and week Citation(5). Patients with night-time or early morning symptoms have a significantly worse health status, more sleep disturbances, and higher health-care resource utilization than patients without the symptoms (Citation3, Citation14). Previous studies have shown an association between morning and night-time symptoms and disease severity (Citation6–8, Citation14–16). Tsiligianni et al. Citation(17) analyzed morning and night-time symptom prevalence and correlation with health status and severity of COPD. Out of 2269 primary care patients (58% male) a total of 1159 (52%) reported morning symptoms and 879 (39%) night complaints. Patients with morning and night-time symptoms were mostly smokers with a poorer lung function.
Objective measurements of respiratory symptoms and sleep are necessary to examine the relationship between night-time symptoms and sleep disturbances. Sleep disturbances in COPD patients include daytime sleepiness, difficulty in maintaining sleep, early morning awakenings, and struggle against sleep during daytime (Citation2, Citation4, Citation11). The occurrence of respiratory symptoms during day and night is usually captured by diaries or questionnaires. It is obvious that data captured by diaries do not reflect the real occurrence of night-time symptoms, especially wheezing Citation(1). Therefore computerized cough and wheeze monitoring is a practical and non-invasive method for assessing respiratory symptoms during wakeness and sleep.
This study shows for the first time that long periods of wheezing can be found in patients with stable COPD disease during sleep. Long-term recordings of night time lung sound are the only way to identify nocturnal wheezing. Regarding the etiology of wheezing in our patients, we initially have assumed that it is the cause of an asthma-COPD overlap syndrome. However, in none of the patients with night-time wheezing, there was evidence for the presence of an accompanying asthma. Interestingly, all patients with severe night-time wheezing belonged to the group of active smokers. Thus, it is likely that the persisting nicotine consumption maintains mucosal swelling and hypersecretion and a persistent inflammatory reaction. During sleep in supine position these mechanisms can lead to an increasing bronchial obstruction with consecutive wheezing. Tsiligianni et al. Citation(17) also report a higher proportion of smokers in patients with severe morning and night symptoms, which matches ours findings. In our opinion, the finding of night-time wheezing is of great importance because of possible negative consequences sleep quality and daytime performance. In case of an overlap syndrome, anti-obstructive or anti-inflammatory therapy must be intensified, and in case of continued nicotine consumption nicotine abstinence should be recommended. Up to now we do not know, if periods with wheezing lead to sleep-structure interfering arousals.
This study shows the importance of objective long-term monitoring of night-time symptoms. Bronchial obstruction with the clinical sign of wheezing during sleep does not correspond to the criteria of a well-adjusted COPD therapy. It is necessary to clarify whether the cause of night-time wheezing in patients with COPD is triggered by an overlap syndrome, a persistent nicotine consumption, or an insufficient anti-obstructive therapy.
Cough is an important symptom in COPD patients because it predicts disease severity and a poor prognosis. Since there is no accepted threshold for clinical significance of cough, there is need for further research and definitions.
More closer examination of cough events in our patients shows an individual cough pattern. While some patients had rather few but long bursts of cough, others showed many short bursts over the whole night. This pattern could be reproduced in both nights. So every patient seems to have an individual strategy for lung clearance. Most of the cough periods were non-productive with a median of 86%. Further, patients with high amount of productive cough subjectively describe a worse quality of life (SGRQ) and higher impact of COPD symptoms on health status (CAT). This is an interesting finding and correlates with the known fact that productive cough is mostly found in the morning hours after getting up from bed.
In summary, night-time symptoms in COPD patients are prevalent and often not considered in the clinical work because of a lack of objective measurement opportunities. Typically, patients with COPD do not report nigh-time symptoms to their physician or the physician does not ask for these symptoms. LEOSound offers a chance to evaluate night-time wheezing and cough, which might otherwise stay unnoticed. The relatively high incidence of night-time wheezing in mildly affected COPD patients might be a potential indicator for active smoking.
Funding
This study was funded by GlaxoSmithKline.
References
- Agusti A, Hedner J, Marin JM, Barbe F, Cazzola M, Rennard S. Night-time symptoms: a forgotten dimension. Eur Respir Rev 2011; 20:183–194.
- McSharry DG, Ryan S, Calverley P, Edwards JC, McNicholas WT. Sleep quality in chronic obstructive pulmonary disease. Respirology 2012; 17:1119–1124.
- Stephenson JJ, Cai Q, Mocarski M, Tan H, Doshi JA, Sullivan SD. Impact and factors associated with nighttime and early morning symptoms among patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2015; 10:577–586.
- Nunes DM, Mota RM, de Pontes Neto OL, Perreira ED, de Bruin VM, de Bruin PFl. Impaired sleep reduces quality of life in chronic obstructive pulmonary disease. Lung 2009; 187:159–163.
- Kessler R, Partridge MR, Miravitlles M, Cazzola M, Vogelmeier C, Leynaud D, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J 2011; 37:264–272.
- Lange P, Marott JL, Vestbo J, Nordestgaard BG. Prevalence of night-time dyspnoea in COPD and its implications for prognosis. Eur Respir J 2014; 43:1590–1598.
- Roche N, Chavannes NH, Miravitlles M. COPD symptoms in the morning: impact, evaluation and management. Respir Res 2013; 14:112.
- Roche N, Small M, Broomfield S, Higgins V, Pollard R. Real World COPD: Association of morning symptoms with clinical and patient reported outcomes. COPD 2013; 10:679–686.
- Miravitlles M, Worth H, Cataluna JJ, Price D, De Benedetto F, Roche N, et al. Observational study to characterize 24-hour COPD-symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res 2014; 15:122.
- Soler-Cataluna JJ, Sauleda J, Valdes L, Marin P, Aguero R, Perez M, et al. Prevalence and perception of 24 h-symptom patterns in patients with stable chronic obstructive pulmonary disease in Spain. Arch Broncopneumol 2016; 52:308–315.
- Jen R, Li Y, Owens RL, Malhotra A. Sleep in chronic obstructive pulmonary disease: Evidence gaps and challenges. Can Respir J. 2016; 2016:7947198.
- Koehler U, Brandenburg U, Weissflog A, Sohrabi K, Gross V. LEOSound, an innovative procedure for acoustic long-term monitoring of asthma symptoms (wheezing and coughing) in children and adults. Pneumologie 2014; 68:277–281.
- Gross V, Reinke C, Dette F, Koch R, Vasilescu D, Penzel T, et al. Mobile nocturnal long-term monitoring of wheezing and cough. Biomed Tech (Berl) 2007; 52 (1):73–76.
- Price D, Small M, Milligan G, Higgins V, Gil EG, Estruch J. Impact of night-time symptoms in COPD: a real-world study in five European countries. Int J Chron Obstruct Pulmon Dis 2013; 8:595–603.
- Partridge MR, Karlsson N, Small IR. Patient insight into the impact of chronic obstructive pulmonary disease in the morning: an internet survey. Curr Med Res Opin 2009; 25:2043–2048.
- Lopez-Campos JL, Calero C, Quintana-Gallego E. Symptom variability in COPD: a narrative review. Int J Chron Obstruct Pulmon Dis 2013; 8:231–238.
- Tsiligianni I, Metting E, van der Molen T, Chavannes N, Kocks J. Morning and night symptoms in primary care COPD patients: a cross-sectional and longitudinal study. An UNLOCK study from the IPCRG. NPJ Prim Care Respir Med 2016; 26: 16040.