ABSTRACT
Inhaled corticosteroids (ICSs) are the cornerstone of the treatment of asthma, but their role in COPD is limited. Several guidelines recommend their use in patients with severe airflow limitation, frequent exacerbations and asthma-COPD overlap (ACO), while the previous GOLD document recommended ICS for patients with high risk of exacerbations and a high level of symptoms (group D). Following the changes in the GOLD document 2017 update, in which impaired lung function is no longer considered as a determinant of exacerbation risk, a high number of COPD patients can now be labeled as group B (low risk of exacerbations and high level of symptoms) instead of D, and hence, no longer fulfill the indication for ICS. Since long-term therapy with ICS can entail secondary effects, the withdrawal of this treatment should be considered in this group of patients. In this article, we summarize the evidence for discontinuation of ICS in this subgroup of patients and provide suggestions for clinicians on the appropriate use on ICS in patients moving from D to B.
Introduction
Inhaled corticosteroids (ICSs) are the cornerstone of the treatment of asthma, but have a limited role in the management of stable COPD, especially after the advent of efficient long-acting bronchodilators (long-acting beta-agonists—LABA and long-acting muscarinic antagonists—LAMA). The current approved indication of ICS in COPD is in patients with severe airflow limitation and frequent exacerbations despite treatment with bronchodilators. However, many patients have been prescribed ICS without this indication, and in these cases, the potential risks of the therapy may outweigh the benefits Citation(1). The 2011 update of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommended treatment with ICS plus a LABA as the first choice treatment in patients classified in group D; i.e. those with a high risk of exacerbations and a high level of symptoms Citation(2). In that classification, high risk was defined as either frequent previous exacerbations, a reduced FEV1 or both. Consequently, in 2017, GOLD changed the classification criteria and the risk of exacerbations is now defined exclusively by the history of exacerbations in the previous year Citation(3). With this change, some patients previously classified as D because of impaired lung function alone will now be classified as B (low risk, high symptoms), in which ICSs are not indicated. As an example, in a recent report of the large POPE cohort, up to 35% of patients switched from D to B according to the new GOLD 2017 criteria Citation(4). This reclassification may have important implications for patient management, because ICSs are no longer indicated in patients newly classified as B.
Since long-term use of ICS may be associated with potentially severe adverse effects, it is important to carefully select patients who should continue with ICS after reclassification from D to B. The new GOLD strategy does not mention any recommendation, but recent evidence derived from clinical trials and observational studies may help to guide clinicians. In this article, we summarize the evidence for discontinuation of ICS in this subgroup of patients and provide suggestions for clinicians on the appropriate use on ICS in patients moving from D to B.
Indication for ICS in COPD
In COPD, ICSs in combination with LABA were initially approved for the treatment of patients with a FEV1 <50% and a history of repeated exacerbations. In the GOLD 2011, LABA/ICS or LAMA was recommended as the first-line therapy in group D (high level of symptoms, high risk of exacerbations). In the updated 2017 version, patients are to be grouped based on symptoms and history of exacerbations only, which leads to a large shift from group D to group B and therefore to a reduction in the number of patients in whom ICSs are indicated Citation(4). Thus, the initial recommended therapy for group D patients is now LABA/LAMA, and ICS should be added only in patients who do not adequately respond to dual long-acting bronchodilator therapy, further reducing the number of patients with an indication for ICS.
On the other hand, several national guidelines for the management of COPD use a phenotypic approach towards the selection of pharmacotherapy Citation(5–7). In these guidelines, LABA/ICS are indicated for exacerbators and patients with asthma-COPD overlap (ACO). As an example, in the Spanish guideline, patients are divided into non-exacerbators, ACO, exacerbators with emphysema and exacerbators with chronic bronchitis Citation(5). Although there are no universally accepted diagnostic criteria for ACO, the diagnostic algorithm included patients with a concomitant diagnosis of COPD and asthma or COPD patients with characteristics of asthma such as a very reversible bronchodilator test and/or blood eosinophil levels higher than 300 cells/µl Citation(8).
A recent review of national guidelines for the management of COPD in Europe showed agreement between documents in that ICSs are indicated in combination with bronchodilators in patients with high risk for exacerbations and/or with a history of two or more exacerbations in the previous year, and ACO (recognized as a phenotype in the Czech Republic, Finland, Russia, Spain and Sweden) Citation(9).
Evidence for discontinuation of ICS in COPD
The use of ICS in COPD does not come without risks Citation(10). Some of the important adverse effects of ICS are increased risks of pneumonia Citation(11), osteoporotic fractures Citation(12), poor control of diabetes Citation(13) and local side effects (candidiasis, sore throat, etc), among others. It is thus important not to use ICS outside the approved indication or in patients who will likely not benefit from this treatment. Since the use of ICS is widespread, it is necessary to assess the possible implications of discontinuation of ICS in patients in whom they are not considered to be useful or who are at increased risk of adverse effects.
Several studies have addressed the possible effect of discontinuation of ICS in COPD on the exacerbation rate. Two randomized controlled studies (COPE and WISP) showed a higher risk of exacerbation for placebo versus patients who continued with ICS (fluticasone proprionate) Citation(14, 15). On the other hand, in the studies using active comparators instead of placebo, the picture was less clear. The COSMIC trial studied the withdrawal of ICS from the LABA/ICS combination (salmeterol/fluticasone proprionate) in exacerbating patients (two or more moderate/severe exacerbations per year and FEV1 30–70%). In the withdrawal group, there was an increase of mild exacerbations (defined as increase in the use of a short-acting bronchodilator) but no differences in moderate and severe exacerbations Citation(16). Patients who discontinued ICS were more symptomatic in terms of dyspnea and cough and had a decrease in FEV1 of about 50 mL. These effects were not observed in the INSTEAD trial, which included only patients without exacerbations in the previous year and FEV1 50–80% who were on LABA/ICS (salmeterol plus fluticasone proprionate) for at least 3 months before enrolment. Patients were randomized to continue with LABA/ICS or switched to LABA (indacaterol) Citation(17). Interestingly, there was no increase in exacerbations in the INSTEAD trial as well as symptoms, and there was no impairment in quality of life after discontinuation of ICS. The results of the INSTEAD trial have been replicated in two real-life studies. The OPTIMO trial included patients with FEV1 >50% and less than 2 exacerbations in the previous year Citation(18). The decision to withdraw ICS was made freely by a treating physician upon inclusion. After 6 months, there were no differences in exacerbations rates or deterioration of symptoms or lung function between groups. In the second study (DACCORD) conducted in Germany, 236 patients with a mean FEV1 of 67.4% predicted were discontinued from ICS based on the decision of the treating physician, and it was found that their frequency of exacerbations was no different from that of the group of 1,022 patients who continued with ICS Citation(19).
The largest withdrawal study to date is WISDOM, which included patients with FEV1 <50% and one or more exacerbations in the previous year Citation(20). In this trial, patients were given ICS/LABA/LAMA in the run-in period. Then they were randomized to ICS continuation or stepwise discontinuation groups. There was no difference in moderate and severe exacerbations between groups, and similar to the COSMIC trial, a mean and non-progressive decline in FEV1 of 43 mL was noted.
Evidence related to the risk of exacerbations after ICS withdrawal can also be extracted from the FLAME trial Citation(21). While it was not a withdrawal study in design, 56.3% of the patients included were receiving ICS before inclusion. The patients were randomized into LABA/LAMA and LABA/ICS groups. The subgroup analysis of ICS users before inclusion showed significantly fewer exacerbations and better lung function in those who were switched to LABA/LAMA, and therefore were discontinued from ICS.
Although there is significant heterogeneity in the ICS withdrawal studies, they show that in many groups of patients, ICS can safely be withdrawn, especially if dual long-acting bronchodilator therapy is used instead.
Eosinophils as markers of response to ICS in COPD
Sputum eosinophilia (defined as sputum eosinophils ≥3%) is seen in about 1/3 of patients with stable COPD (consistent with histological findings) and has been proposed as a marker of risk for exacerbations and response to ICS Citation(22, 23). Since there are limitations to its everyday use, blood eosinophil counts (BECs) were alternatively proposed as a biomarker. Indeed, significant correlation was found between BEC and sputum eosinophils Citation(24). The specificity of BEC for predicting sputum eosinophilia was 76% for a cut-off value of 300/µL and 92% for a cut-off of 400/µL. The sensitivity was 60% for a cut-off value of 300 cells/µL and 91% for a cut-off of 200 cells/µL. In other words, patients were unlikely to have eosinophilic airway inflammation if the BECs were below 200/µL and very likely if they were above 400/µL, leaving a grey area in between these values.
To date, no prospective study has assessed the efficacy of ICS using BEC as a biomarker. The data available was retrospectively collected from clinical trials. In the study comparing the efficacy of vilanterol vs. vilanterol plus fluticasone furoate, there was a significant reduction in the exacerbation rate with the addition of fluticasone only in patients with BEC >2% – the effect was more pronounced with increasing values Citation(25). Similarly, comparison of formoterol vs. formoterol plus extrafine beclomethasone diproprionate in patients with severe COPD with exacerbations showed the greatest efficacy of ICS in patients with BEC >279.8 cells/µL Citation(26).
ICS withdrawal studies also showed similar patterns. A post-hoc analysis of the WISDOM trial showed increased risk of exacerbations in patients with BEC ≥300 cells/µL and a history of 2 or more exacerbations but not in patients with a history of only one exacerbation in the previous year Citation(27, 28).
The most recent data are from the FLAME trial. This trial showed that LABA/LAMA is at least as effective or superior to LABA/ICS in reducing the exacerbation rate for any given BEC. Nevertheless, also in this trial, the efficacy of ICS in the prevention of exacerbations increased in patients with higher BEC, showing the best performance (albeit not better than LABA/LAMA) in the group with BEC ≥300 cells/µL Citation(29). This is consistent with a higher efficacy of ICS in the reduction of exacerbations in patients with traits of eosinophilic inflammation.
In summary, studies that reported relationships between BEC and the effect of ICS on the reduction of exacerbations showed that ICSs in addition to (dual) bronchodilator therapy are likely effective in exacerbating patients with high BEC, who should therefore be prescribed ICS. On the other hand, ICSs seem ineffective in exacerbating patients with low BEC (i.e. <2% or <150 cells/µL) in whom alternative approaches should be considered Citation(30). If we are to use BEC as a biomarker for ICS discontinuation, the existing studies suggest that a high cut-off value (≥300 cells/µL) should be used to detect patients with possible asthma traits and increased risk of exacerbations and a fall in lung function after discontinuation.
Proposal for discontinuation of ICS in COPD patients switched from D to B
Several studies have demonstrated that ICS can be safely withdrawn at least in some patients on concurrent bronchodilator therapy Citation(17–20). Importantly, in none of the studies did withdrawal of ICS increase the risk of moderate or severe exacerbations or severe deterioration of the disease. The INSTEAD and WISDOM trials showed that ICS can be discontinued in non-exacerbating or exacerbating COPD patients with moderate or severe obstruction without relevant adverse effects Citation(17, 20). Although some authors considered the results of the WISDOM trial to be controversial, it is highly likely that the results are valid at least for the non- and low-exacerbating COPD populations. In fact, this encompasses patients who will be reclassified in GOLD 2017 from group D to B and in whom ICS will no longer be the recommended therapy. Thus, a trial on withdrawal of ICS should be conducted in this group of COPD patients.
Nevertheless, when the decision about ICS discontinuation is being considered, there is always looming concern that the patient is not exacerbating because of ICS. Indeed, there is growing evidence that ICSs are beneficial in COPD patients with patterns of eosinophilic inflammation. These patients can be identified by a high BEC and/or an ACO phenotype Citation(8, 31).
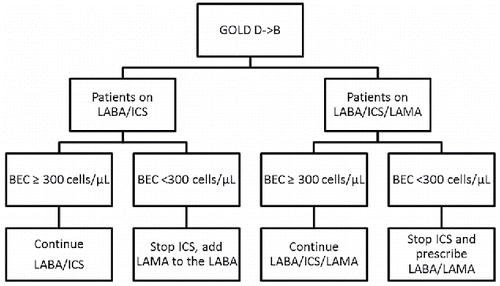
Therefore, we propose that in patients who are re-classified from group D to B in the GOLD 2017 revision and do not have co-existing asthma, BEC should be determined and ICSs be discontinued in all patients with BEC <300 cells/µL (). As dual bronchodilator therapy is more effective than LABA/ICS in reducing the risk of exacerbation Citation(21), it should be initiated upon ICS discontinuation. The proposed strategy incorporates the evidence about the feasibility of safe ICS withdrawal in non-exacerbating COPD patients and growing evidence about the phenotypes of COPD patients that respond to ICS. This raises the safety bar even beyond the level demonstrated in the INSTEAD and WISDOM trials and should reduce reluctance of practicing clinicians to change therapy for the sake of reducing the future risk of ICS side effects.
Conclusion
A sizable proportion of patients will be re-classified from group D to B following the new GOLD strategy; for them, ICS will no longer be the recommended therapy. According to several studies, ICS withdrawal is feasible and safe in this group of patients. We propose using BEC as a biomarker to assist in the decision of ICS discontinuation and to even reduce the risk of worse outcomes following ICS withdrawal.
Declaration of interests
Marc Miravitlles has received speaker fees from Boehringer Ingelheim, AstraZeneca, Chiesi, GlaxoSmithKline, Menarini, Teva, Grifols and Novartis, and consulting fees from Boehringer Ingelheim, GlaxoSmithKline, Gebro Pharma, CLS Behring, Cipla, MediImmune, Mereo Biopharma, Teva, Novartis and Grifols. Matevz Harlander has received speaker fees from Novartis, Boehringer Ingelheim, Teva and Berlin-Chemic Menarini, and consulting fees from Boehringer Ingelheim and Novartis. Matjaz Turel has received speaker fees from GlaxoSmithKline, Novartis, Boehringer Ingelheim, Teva, AstraZeneca, Berlin-Chemie Menarini and Chiesi, and consulting fees from GlaxoSmithKline, Novartis, Boehringer Ingelheim and Teva. Miriam Barrecheguren has no conflict of interest in relation to this article.
References
- Barrecheguren M, Monteagudo M, Ferrer J, Borrell E, Llor C, Esquinas C, et al. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir Med 2016;111:47–53.
- Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2013;187:347–365.
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol 2017;53:128–149.
- Tudoric N, Koblizek V, Miravitlles M, Valipour A, Milenkovic B, Barczyk A, et al. GOLD 2017 on the way to phenotypic approach? Analysis from the Phenotypes of COPD in Central and Eastern Europe (POPE) Cohort. Eur Respir J 2017;49:1602518.
- Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, et al. Spanish COPD guidelines (GesEPOC) 2017. Pharmacological treatment of stable chronic obstructive pulmonary disease. Arch Bronconeumol 2017;53:324–335.
- Koblizek V, Chlumsky J, Zindr V, Neumannova K, Zatloukal J, Zak J, et al. Chronic obstructive pulmonary disease: official diagnosis and treatment guidelines of the Czech Pneumological and Phthisiological society; a novel phenotypic approach to COPD with patient-oriented care. Biomed Pap 2013;157:189–201.
- Kankaanranta H, Harju T, Kilpeläinen M, Mazur W, Lehto JT, Katajisto M, et al. Diagnosis and pharmacotherapy of stable chronic obstructive pulmonary disease: the finnish guidelines. Basic Clin Pharmacol Toxicol 2015;116:291–307.
- Miravitlles M, Alvarez-Gutierrez FJ, Calle M, Casanova C, Cosio BG, López-Viña A, et al. Algorithm for identification of asthma–COPD overlap: consensus between the Spanish COPD and asthma guidelines. Eur Respir J 2017;1(49):1700068.
- Miravitlles M, Vogelmeier C, Roche N, Halpin D, Cardoso J, Chuchalin AG, et al. A review of national guidelines for management of COPD in Europe. Eur Respir J 2016;47:625–637.
- Ernst P, Saad N, Suissa S. Inhaled corticosteroids in COPD: the clinical evidence. Eur Respir J 2015;45:525–537.
- Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2014;10:CD010115.
- Loke YK, Cavallazzi R, Singh S. Risk of fractures with inhaled corticosteroids in COPD: systematic review and meta-analysis of randomised controlled trials and observational studies. Thorax 2011;66:699–708.
- Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am J Med 2010;123:1001–1006.
- van der Valk P, Monninkhof E, van der Palen J, Zielhuis G, van Herwaarden C. Effect of discontinuation of inhaled corticosteroids in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:1358–1363.
- Choudhury AB, Dawson CM, Kilvington HE, Eldridge S, James W-Y, Wedzicha JA, et al. Withdrawal of inhaled corticosteroids in people with COPD in primary care: a randomised controlled trial. Respir Res 2007;8:93.
- Wouters EFM, Postma DS, Fokkens B, Hop WCJ, Prins J, Kuipers AF, et al. COSMIC (COPD and Seretide: a Multi-Center Intervention and Characterization) Study Group t. Withdrawal of fluticasone propionate from combined salmeterol/fluticasone treatment in patients with COPD causes immediate and sustained disease deterioration: a randomised controlled trial. Thorax 2005;60:480–487.
- Rossi A, van der Molen T, Olmo R, del Papi A, Wehbe L, Quinn M, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J 2014;44:1548–1556.
- Rossi A, Guerriero M, Corrado A, Group OS. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res 2014;15:77.
- Vogelmeier C, Worth H, Buhl R, Criée CP, Lossi NS, Mailänder C, Kardos P. “Real-life” inhaled corticosteroid withdrawal in COPD: a subgroup analysis of DACCORD. Int J Chron Obstruct Pulmon Dis 2017 Feb 1;12:487–494.
- Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med 2014;371:1285–1294.
- Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, et al. FLAME investigators. Indacaterol–glycopyrronium versus salmeterol–fluticasone for COPD. N Engl J Med. 2016;374:2222–2234.
- Leigh R, Pizzichini MM, Morris MM, Maltais F, Hargreave FE, Pizzichini E. Stable COPD: predicting benefit from high dose inhaled corticosteroid treatment. Eur Respir J 2006;27:964–971.
- Brightling CE, McKenna S, Hargadon B, Birring S, Green R, Siva R, et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 2005;60:193–198.
- Negewo NA, McDonald VM, Baines KJ, Wark PA, Simpson JL, Jones PW, et al. Peripheral blood eosinophils: a surrogate marker for airway eosinophilia in stable COPD. Int J Chron Obstruct Pulmon Dis 2016;11:1495–1504.
- Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015;3:435–442.
- Siddiqui SH, Guasconi A, Vestbo J, Jones P, Agusti A, Paggiaro P, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2015;192:523–525.
- Watz H, Tetzlaff K, Wouters EFM, Kirsten A, Magnussen H, Rodriguez-Roisin R, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med 2016;4:390–398.
- Calverley PMA, Tetzlaff K, Vogelmeier C, Fabbri LM, Magnussen H, Wouters EFM, et al. Eosinophilia, frequent exacerbations, and steroid response in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2017; rccm.201612-2525LE.
- Roche N, Chapman KR, Vogelmeier CF, Herth FJF, Thach C, Fogel R, et al. Blood eosinophils and response to maintenance COPD treatment: data from the FLAME trial. Am J Respir Crit Care Med 2017; rccm.201701-0193OC.
- Santos S, Marin A, Serra-Batlles J, de la Rosa D, Solanes I, Pomares X, et al. Treatment of patients with COPD and recurrent exacerbations: the role of infection and inflammation. Int J Chron Obst Pulm Dis 2016;11:515–525.
- Barrecheguren M, Esquinas C, Miravitlles M. The asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): opportunities and challenges. Curr Opin Pulm Med 2015;21:74–79.