ABSTRACT
The concept of Chronic Obstructive Pulmonary Disease (COPD) control has been developed to inform therapeutic decision-making. We explored the validity of a definition of COPD control in a representative population of patients with COPD in the United Kingdom. Electronic medical records and linked COPD questionnaire data from the Optimum Patient Care Research Database were used to characterize control status. Patients were aged ≥40 years, with spirometry-confirmed COPD, current or ex-smokers, and continuous records throughout the study period. Control was evaluated based on COPD stability and patients' (i) clinical features or (ii) COPD Assessment Test (CAT) score over a three-month baseline period and linked to time to first exacerbation. Of 2788 eligible patients, 2511 (90%) had mild/moderate COPD and 277 (10%) had severe/very severe COPD based on Body Mass Index, Obstruction, Dyspnoea, Exacerbations (BODEx) cut-off of 4. Within the mild/moderate cohort, 4.5% of patients were controlled at baseline according to clinical features and 21.5% according to CAT threshold of 10. Within the severe/very severe cohort, no patients were controlled at baseline according to the proposed clinical features and 8.3% were controlled according to CAT threshold of 20. Compared with uncontrolled patients, time to first exacerbation was longer for controlled patients with mild/moderate COPD but not for those with severe/very severe COPD. Lowering the BODEx threshold for severity classification to 2 increased the number of patients achieving control. CAT scores were not good predictors of the risk of future exacerbation. With the proposed definition, very few patients were defined as controlled.
Introduction
Chronic obstructive pulmonary disease (COPD) is a heterogeneous condition requiring therapeutic management to be tailored to the clinical characteristics and disease severity of the individual patient (Citation1–4). Treatment of COPD should be aimed to the control of symptoms and the reduction of exacerbation risk (Citation5); if these objectives are achieved, we could consider that the disease is under control, independently of the degree of impairment in lung function or health status (Citation6).
The new GOLD strategy indicates for the first time that medication step up and step down must be considered in COPD, but no clear criterion for increasing or decreasing intensity of treatment is provided beyond the clinical perception of the healthcare provider (Citation5). The concept of clinical control in COPD has been introduced to guide decisions in treatment based on changes in the condition of patients with COPD (e.g. variation in symptoms and changes in physical activity) that could indicate a need for treatment modification, but do not constitute a change in severity classification (Citation6,7). Closer consideration of such clinical variations may enable better evaluation of patients' current disease state and their potential need for treatment.
The concept of disease control has been extensively developed in asthma resulting in the development of evaluation tools such as the Royal College of Physicians three questions (RCP3Q) (Citation8), the Asthma Control Test (ACT) (Citation9) and Asthma Control Questionnaire (ACQ) (Citation10). Although traditionally less accepted in the context of COPD, Soler-Cataluña et al. (Citation6) proposed a new definition of control in COPD. The definition aims to describe the current clinical status of patients with COPD in terms of its current impact and its recent clinical stability. Impact is defined as a cross-sectional evaluation and can be assessed by questionnaire – by the COPD Assessment Test (CAT) or the Clinical COPD Questionnaire (CCQ) – or by a clinical assessment of the patient's degree of dyspnoea, use of rescue medication, level of physical activity and sputum quantity and colour. In contrast, stability is defined as the temporal evolution of this impact and is a longitudinal, dynamic concept that requires both absence of COPD exacerbations and maintenance of low disease impact. Hence, a patient with COPD is defined as controlled when their condition has both low impact and is stable in this state (Citation7).
This new concept requires validation in terms of its ability to predict outcomes and to provide additional clinical management insights. It is now necessary to understand its potential utility as a tool to guide COPD management in routine care. In addition, validation work is necessary to inform whether the current thresholds within the proposed definition of control are valid, or whether refinements are required. This study explored the association between COPD control status and clinical outcomes and investigated the optimal thresholds for CAT and Body Mass Index, Obstruction, Dyspnoea, Exacerbations (BODEx) severity categories (Citation11) used within these definitions.
Methods
Study design
This was a retrospective observational cohort study designed to validate the concept of control of COPD proposed by Soler-Cataluña et al. (Citation7). For this objective, we have used routine electronic medical records (EMRs) and linked patient questionnaire data from the Optimum Patient Care Research Database (OPCRD) (Citation12).
As a first step, we investigated the distribution of control status within the population of patients with COPD, and used the time to first exacerbation over a 1-year period (stratified by baseline control status) to validate the concept of control.
Finally, as an exploratory analysis, we evaluated different CAT score cut-offs for the prediction of the risk of exacerbations, as well as different thresholds of the BODEx index to classify the severity of the baseline disease, as explained in the Statistical Analysis section.
The OPCRD is a quality-controlled, respiratory-focused database containing anonymous data from general practices throughout the United Kingdom and is approved for clinical research by the Health Research Authority of the UK NHS (REC reference: 15/EM/0150). At the time of the study, the OPCRD contained longitudinal medical record data from 2.9 million patients from more than 576 UK general practices. The anonymized point-of-care records for each patient include demographic information, disease diagnoses (recorded as Read codes), prescribing information, test results and information transcribed from secondary care visits and hospitalizations (Citation12).
Patients
They have to meet all of the following criteria to be eligible: aged 40 years or older with physician-diagnosed COPD (i.e. carrying a COPD Read code), a positive smoking history (current or ex-smokers), confirmatory spirometry with FEV1/FVC < 0.7, linked COPD questionnaire data including the CAT, and at least 15 months of continuous clinical records. Patients who died within two years of the date of baseline control evaluation were excluded.
Measurements
Control was evaluated at baseline as stable disease plus low disease impact. Stable disease was defined as the absence of exacerbations in the three months preceding index date, and low impact was assessed by: (i) patient's recorded CAT score or (ii) clinical evaluation using appropriate database variables and proxies in accordance with published definitions (Citation7). Low-impact variable thresholds were adjusted for BODEx severity (categorized by the median value as mild/moderate: ≤4; severe/very severe: ≥5) (). Baseline comorbidities were identified based on the presence of a Read code ever prior to the index date.
Table 1. Measures used to evaluate baseline control as defined by Soler-Cataluña et al. (Citation10) and the database proxies used in the study.
A COPD exacerbation was defined as an acute use of oral corticosteroids and/or a course of antibiotics for lower respiratory symptoms or within 5 days of an unscheduled hospital admission or emergency department attendance for acute respiratory symptoms in a patient with COPD.
Data were extracted for a ‘current’ period (March 2010—May 2014) to minimise the potential effect of temporal changes in COPD coding practice or management within the study window. The study period comprised a 15-month observation window: a three-month baseline period (months −3–0) to evaluate the patient's baseline clinical stability; an index date (day 0) at which the patient's baseline CAT score was recorded and control status was evaluated and a one-year follow-up period (months 0–12). The CAT is a specific questionnaire that measures the impact of disease in a patient using 8 questions which evaluate cough, expectoration, dyspnoea, chest tightness, patient confidence, limitations in daily activities, quality of sleep and energy. The CAT score ranges from 0 to 40, and the higher the score the worse the health status of the patient (Citation13).
The study protocol was approved by the OPCRD's independent Ethics and Protocol Transparency (ADEPT) Review Committee (ADEPT0115) and registered with the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance (ENCePP; reference: ENCEPP/SDPP/10697).
Statistical analysis
Summary statistics (sample size, mean, standard deviation, range, median and interquartile range) were produced for patients' demographic and clinical characteristics. Categorical comparisons between baseline controlled and uncontrolled groups were made using the chi-square test. Variables measured on the interval or ratio scale were compared with a t-test or, if the distributions were skewed, a Mann-Whitney U test. Receiver operating characteristic (ROC) curves were produced to assess the utility of patients' CAT scores at baseline to predict exacerbation occurrence in the following year. Survival curves obtained from Cox regression were used to analyse the time to first COPD exacerbation, and groups were adjusted for age, gender, smoking status and BODEx score.
Exploratory analyses assessed the utility of CAT score at baseline as a predictor of future COPDexacerbation occurrence and the sensitivity of the BODEx category thresholds used for low-impact disease adjustments.
Statistically significant results were defined as those with p < 0.05. Analyses were performed using STATA software version 14.0 (StataCorp, Texas, USA) and Microsoft Excel 2013 (Microsoft Corp., Redmond, Washington, USA).
Results
Population of study
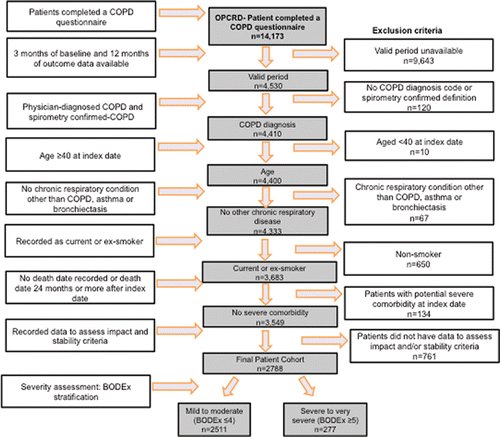
A total of 14,173 patients with linked EMRs and COPD questionnaire data were identified, of which 3,549 patients (25%) fulfilled the inclusion criteria. Of these patients, 2,788 (79%) had complete data on all BODEx severity and control variables and were included in the study (). Using the proposed BODEx threshold of 4 units, 2,511 (90%) patients were classified as having mild/moderate and 277 (10%) as severe/very severe COPD.
Figure 1. Patient selection flow diagram showing identification of the study cohort following application of the study inclusion/exclusion criteria.
Patients with mild/moderate COPD had a mean (SD) age of 71.1 (9.5) years, 58.3% were men, and the mean FEV1 (% predicted) was 62.7%, whereas patients with severe/very severe COPD were aged 72 (8.8) years, 59.6% were male, and mean FEV1 (% predicted) was 35.5%.
Control status
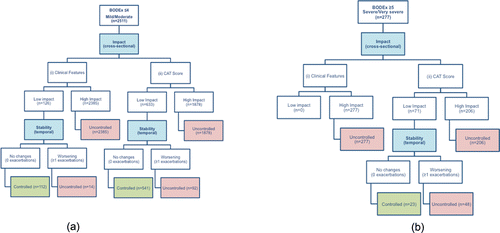
Of the 2511 patients with mild/moderate COPD, 126 (5%) had low impact disease based on their clinical features, of whom 112 (88.8% or 4.5% of the total) were considered controlled at baseline as they did not suffer any exacerbation in the previous 3 months. In contrast, when using a CAT score <10 to define low-impact disease, 541 patients (21.5%) were classified as controlled ().
Figure 2. Evaluation of Control within the study population. (A) Flow diagram showing the number of patients within the mild/moderate COPD cohort with controlled/uncontrolled COPD, split by (i) Clinical-Feature-defined low impact (ii) CAT-defined low impact. (B) Flow diagram showing the number of patients within the severe/very severe COPD cohort with controlled/uncontrolled COPD, split by (i) Clinical-Feature-defined low impact (ii) CAT-defined low impact.
Patients who were controlled according to their clinical features tended to have a lower burden of comorbidities, notably asthma (13.4% vs. 30.0%), higher frequency of current smokers (39.3% vs. 28.5%) and better FEV1 (%) (72.3% vs 62.3%) and CAT scores (10.2 vs 16.7) compared to patients with uncontrolled COPD. Furthermore, controlled patients had lower modified Medical Research Council (mMRC) scores and reported higher levels of physical activity, lower use of rescue medication and had less phlegm than uncontrolled patients.
Similarly, when control was assessed by CAT scores, controlled patients showed better FEV1 (%) and lower mMRC scores, reported higher levels of physical activity and lower use of rescue medication, and had less phlegm than uncontrolled patients (p < 0.001 for all variables). However, the proportion of current smokers and incidence of co-morbid asthma was similar for both controlled and uncontrolled subpopulations ().
Table 2. Baseline demographics and clinical characteristics by COPD Control definition and resultant control status for patients with Mild/Moderate COPD, stratified by clinical features/CAT scores.
Of a total 277 patients with severe/very severe COPD, no patients fulfilled the definition of low impact based on their clinical features and, thus, no patients were defined as controlled. When CAT score was used to define low impact (threshold <20), 71 patients (25.6%) fulfilled the definition of having low impact disease, of whom 23 (32.4% and 8.3% of the total) also had stable disease in the preceding three months and were, therefore, defined as controlled at baseline. As in the mild/moderate COPD cohort, a lower proportion of controlled patients had lower use of rescue medication and lower phlegm. Co-morbid asthma, cardiovascular disease and physical activity levels were lower in the controlled patients, though this was not found to be statistically significant. Further, the degree of dyspnoea was no different between controlled and uncontrolled, though FEV1 (%predicted) was significantly worse (p = 0.015; ).
Table 3. Baseline demographics for patients with Severe/Very Severe COPD, stratified by clinical feature/CAT score assessment of COPD impact and resultant control status.
Follow-up: Exacerbations
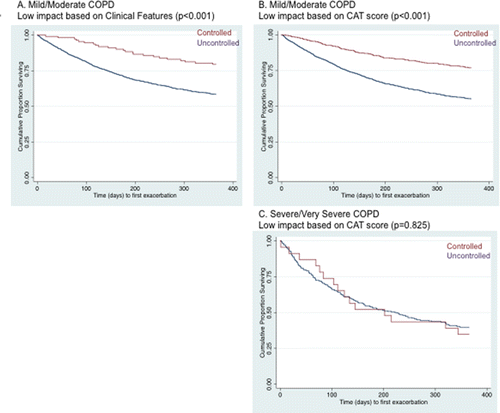
Among patients with mild/moderate COPD, mean time to first exacerbation over the 1-year primary outcome period was significantly longer for patients controlled at baseline, whether evaluated by clinical features or CAT score (, and ). For patients with severe/very severe COPD, no significant differences were found for time to the first exacerbation between both groups (; ).
Table 4. Time to first exacerbation according to control status (by clinical symptom or CAT scores) in patients with different degrees of severity using two different BODEx thresholds (4 and 2 units).
Definition refinements
The relatively low number of patients classified as controlled at baseline prompted a critical analysis of the disaggregated impact variables to identify the key barriers to attaining low impact disease, and subsequent control. In addition, sensitivity analyses were conducted to explore the impact of altering the thresholds for CAT score-defined low/high COPD impact and BODEx categories for COPD severity.
Analysis of clinical variables of impact
A majority of patients with mild/moderate COPD failed to meet the rescue medication (75.4%) and CAT score (74.8%) thresholds to be defined as low impact. In the severe/very severe population, the majority failed to meet the dyspnoea, rescue medication, physical activity and CAT thresholds ().
Table 5. Percentage of patients that failed to meet the threshold for control for each disaggregated component of low impact, split by COPD severity.
CAT score threshold for low impact
Patients with mild/moderate COPD had a mean (SD) CAT score of 16.4 (7.6) and patients with severe/very severe COPD had a mean (SD) CAT score of 26.1 (7.6). The majority of patients (74.8% mild/moderate and 74.4% severe/very severe) failed to meet the proposed CAT score thresholds for low impact.
An ROC analysis suggested baseline CAT score was poorly predictive of exacerbation at one year from index date, with area under the curve (AUC) 95% confidence interval of 0.61 (0.58–0.63) for patients with mild/moderate COPD and 0.43 (0.36–0.50) for patients with severe/very severe COPD.
BODEx severity threshold
The effect of lowering the BODEx threshold for mild/moderate COPD from ≤4 to ≤2 was also examined. Using this new threshold, the proportion of patients classified as controlled at baseline increased from – mild/moderate: 4.5% to 5.8% (clinical features) and 21.5% to 27% (CAT score); severe/very severe: 0% to 2.0% (clinical features) and 8.3% to 25.5% (CAT score) ().
Over the 1-year follow-up, time to first exacerbation remained significantly longer for controlled patients with mild/moderate COPD (p < 0.001; both definitions) and numerically, but failed to reach statistical significance patients with severe/very severe COPD (B). Alteration of the BODEx threshold had little overall impact on mean time to first exacerbation in any of the control–severity subpopulations ().
Discussion
The concept of control in COPD is intended as a tool to help guide physicians in individualized treatment modifications with a view to optimizing outcomes in patients with COPD. Our study showed that, according to the definition of control proposed by Soler-Cataluña et al.. (Citation6,7), only a small percentage of patients within an UK population of COPD managed in routine primary care could be classified as controlled. Even when disease impact was adjusted for disease severity, less than one-third of patients fulfilled the criteria for control, whether defined by clinical features or CAT score. Since this patient population as a whole did not present an increased risk of exacerbation during the follow-up year, our results suggest that the proposed criteria of control are too strict and may be too difficult to reach in real life. In fact, prospective studies should help to identify the best variables and thresholds to differentiate between patients and disease states that imply an increased risk of poor outcomes. Nevertheless, in the mild/moderate patient group, patients who were controlled at baseline according to the proposed definition had significantly longer time to first exacerbation over the following year than uncontrolled patients, validating the concept that identifying uncontrolled patients can be useful to intensify treatment in order to avoid future events. In contrast, in the severe/very severe group, no significant differences were observed in time to first exacerbation between controlled and uncontrolled patients, likely reflecting the low number of patients or the need for different approach in this subpopulation.
The exploratory investigations of the clinical thresholds for disease severity indicated that altering the BODEx categorization of mild/moderate and severe/very severe scores to ≤2 and >2, respectively, increased the number of patients controlled at baseline in both severity groups, but had little overall impact on the predictive value of the concept of control. The exploratory investigations also suggested that the CAT thresholds within the current definition of control may be too strict as more than three-quarters of patients failed to meet the definition of control based on this variable alone. However, the ROC analysis indicated that CAT score could not be further optimized. This is particularly relevant because CAT scores are used in the GOLD strategy to classify patients into different categories of symptoms that require different intensities of treatment (Citation5). Some previous studies have suggested that the threshold of 10 units to separate low intensity and high intensity of symptoms may be too low and not always corresponds to a level of dyspnoea of 2 in the mMRC scale (Citation14).
It is possible that a different threshold of CAT, as a proxy for symptom impact, is required in order to predict outcomes. Previous studies have shown that CAT score can be useful for predicting readmission (Citation15) and that a cut-off of 13.5 is significantly associated with an increased risk of a new exacerbations (Citation16) and 17 with a significant increase in risk of mortality (Citation17). It is important to highlight that baseline CAT scores may vary in different patient populations. Interestingly, in our study, the scores were quite high, with a mean value of 16 in mild/moderate and 26 in severe/very severe patients in contrast to 11 to 13 in COPD patients in Spain (Citation16,18,19). These scores compare with those obtained in an European study that ranged from 14 to 18 across different countries (Citation20). Such differences may be related to the different degrees of severity of the patients investigated or to other characteristics as comorbidities, sex distribution or treatment.
The choice of clinical variables used to define control has been extensively justified in the original publications (Citation6,7). The increased use of rescue medication has been associated with an increased risk of future exacerbations (Citation21) and may be a marker of insufficient bronchodilator therapy. The majority of patients in both severity subgroups did not fulfil the criterion for rescue medication to be considered controlled. From our results, it is clear that a less restrictive threshold of rescue medication should be explored.
From the remaining clinical variables of impact, only daily physical activity and dyspnoea in severe patients had thresholds that were difficult to achieve. Daily physical activity was measured by self-declared walking time, which is subject to bias, but previous studies have demonstrated that patients who report walking less than 30 minutes per day on average tended to be more severe and to have poorer outcomes (Citation22).
In our primary care population, only 10% had a BODEx of 5 or higher, which means that a cut-off of 3 or higher would be more appropriate to separate mild/moderate from severe/very severe. With this new cut-off, the percentage of severe patients increased to 35.8%; however, the number of controlled patients using the clinical variables was still very low, indicating again that these criteria were very restrictive.
Irrespective of the cut-offs used and the evaluation of control based on clinical variables or CAT, mild/moderate patients fulfilling the criteria of control had a prolonged time to the next exacerbation compared with patients uncontrolled at baseline. This finding validates the concept of control and indicates that it is feasible to identify patients at increased risk of poor outcomes by using simple variables that can be collected in routine follow-up visits in clinical practice. In addition, the variables used for the definition of control are susceptible to be modified by treatment of COPD; therefore the concept of control, if prospectively validated can potentially be included in guidelines for management of COPD (Citation23).
Our study has some limitations. The OPCRD is a routine care database enriched by disease-specific questionnaires. It does not contain all the data variables required to directly assess COPD control and so a number of proxy measures were used, e.g. for rescue medication, sputum colour, disease stability (see ). Longitudinal CAT scores were not available; hence, stability was based on the absence of exacerbations in the preceding three months only. Due to the retrospective design of the study, a significant number of identified patients were excluded due to the lack of complete information in the database. The way the exclusion criteria influence the results is unknown, and the results would therefore need to be validated in a prospective study. Future research is required to explore the clinical implications of using control to guide prospective management decisions.
Conclusion
This study explores the relevance and validity of COPD control in a large, routine care patient population and demonstrates a clear association between patients' control status and time to first exacerbation in the following year. It also illustrates the potential clinical value of COPD control status, whether evaluated via an assessment of patients' clinical features or by CAT score, providing confidence in the utility of the tool across a range of clinical/data capture settings. The high proportion of patients failing to meet control based on CAT score alone and lower proportion of patients controlled by clinical features (vs. CAT score) suggests further work is warranted to optimize the sensitivity and specificity of the proposed COPD Control definition.
Declaration of interest
AN and AC are both employees of REG Ltd.
DBP has board membership with Aerocrine, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Meda, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; consultancy agreements with Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Meda, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, and Theravance; grants and unrestricted funding for investigator-initiated studies (conducted through Observational and Pragmatic Research Institute Pte Ltd) from Aerocrine, AKL Ltd, AstraZeneca, Boehringer Ingelheim, British Lung Foundation, Chiesi, Meda, Mundipharma, Napp, Novartis, Pfizer, Respiratory Effectiveness Group, Takeda, Teva Pharmaceuticals, Theravance, UK National Health Service, Zentiva; payment for lectures/speaking engagements from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Meda, Merck, Mundipharma, Novartis, Pfizer, Skyepharma, Takeda, and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; payment for the development of educational materials from Mundipharma and Novartis; payment for travel/accommodation/meeting expenses from Aerocrine, AstraZeneca, Boehringer Ingelheim, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; funding for patient enrolment or completion of research from Chiesi, Novartis, Teva Pharmaceuticals, and Zentiva; stock/stock options from AKL Research and Development Ltd which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd, UK, and 74% of Observational and Pragmatic Research Institute Pte Ltd, Singapore; and is a peer reviewer for grant committees of the Efficacy and Mechanism Evaluation programme, Health Technology Assessment, and Medical Research Council.
MM has received speaker fees from Boehringer Ingelheim, AstraZeneca, Chiesi, GlaxoSmithKline, Menarini, Teva, Grifols and Novartis, and consulting fees from Boehringer Ingelheim, GlaxoSmithKline, Gebro Pharma, CLS Behring, Cipla, MediImmune, Mereo Biopharma, Teva, Novartis and Grifols.
BA reports personal fees and grants from Novartis AG, personal fees from Boehringer Ingelheim, personal fees from GSK, personal fees from AstraZeneca, grants and personal fees from Menarini, outside the submitted work;
JJSC has received speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, Esteve, Ferrer, GSK, Menarini, Novartis, and Pfizer, and consulting fees from AirLiquide, Boehringer Ingelheim, Chiesi, GSK, AstraZeneca, Ferrer and Novartis.
Funding
Data acquisition and the analyses were funded by the Respiratory Effectiveness Group (REG; www.effectivenessevaluation.org). The study was designed and coordinated by REG, in collaboration with an independent advisory group comprising members of the REG COPD Working Group. The study report and the dissemination of the results were approved by the advisory group and were conducted in accordance with REG standards and the ENCePP Code of Conduct. The authors received no funds or honoraria for participation in the study.
References
- Agusti A, MacNee W. The COPD control panel: towards personalised medicine in COPD. Thorax 2013; 68:687–690.
- McDonald VM, Higgins I, Wood LG, Gibson PG. Multidimensional assessment and tailored interventions for COPD: respiratory utopia or common sense? Thorax 2013; 68:691–694.
- Miravitlles M, Soler-Cataluna JJ, Calle M, Soriano JB. Treatment of COPD by clinical phenotypes: putting old evidence into clinical practice. Eur Respir J. 2013; 41:1252–1256.
- Kerkhof M, Freeman D, Jones R, Chisholm A, Price DB. Respiratory effectiveness group. Predicting frequent COPD exacerbations using primary care data. Int J Chron Obstruct Pulmon Dis. 2015; 10:2439–2450.
- Vogelmeier CF, Criner GJ, Martínez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol. 2017; 53:128–149.
- Soler-Cataluña JJ, Alcazar-Navarrete B, Miravitlles M. The concept of control in COPD: a new proposal for optimising therapy. Eur Respir J. 2014; 44:1072–1075.
- Soler-Cataluña JJ, Alcazar B, Miravitlles M. The concept of control of COPD in clinical practice. Int J Chron Obst Pulm Dis. 2014; 9:1397–1405.
- Pinnock H, Price DB, et al. Clinical implications of the Royal College of Physicians three questions in routine asthma care: a real-life validation study. Prim Care Respir J. 2010; 21:288–294.
- Thomas M, Price D, et al. The Asthma Control TestTM (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Prim Care Resp J. 2009; 18:41–49.
- Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999; 14:902–907.
- Soler-Cataluña JJ, Martínez-García MA, Sánchez L, Perpiña M, Román P. Severe exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med. 2009; 103:692–699.
- Optimum Patient Care Research Database (OPCRD). Available at: http://www.optimumpatientcare.org/Html_Docs/OPCRD.html (acc-essed on July 4, 2016).
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009; 34:648–654.
- Karloh M, Fleig Mayer A, Maurici R, Pizzichini MM, Jones PW, Pizzichini E. The COPD Assessment Test: What Do We Know So Far?: A Systematic Review and Meta-Analysis About Clinical Outcomes Prediction and Classification of Patients Into GOLD Stages. Chest. 2016; 149:413–425.
- Jing Z, Chun C, Ning S, Hong Z, Bei H, Wan-Zhen Y. Systemic inflammatory marker CRP was better predictor of readmission for AECOPD than sputum inflammatory markers. Arch Bronconeumol. 2016; 52:138–144.
- Miravitlles M, García-Sidro P, Fernández-Nistal A, Buendía MJ, Espinosa de Los Monteros MJ, Esquinas C, Molina J. The chronic obstructive pulmonary disease assessment test improves the predictive value of previous exacerbations for poor outcomes in COPD. Int J Chron Obstruct Pulmon Dis. 2015; 10:2571–2579.
- Casanova C, Marin JM, Martinez-Gonzalez C, de Lucas-Ramos P, Mir-Viladrich I, Cosio B, et al. COPD history assessment in Spain (CHAIN) cohort. Differential effect of modified medical research council dyspnea, COPD assessment test, and clinical COPD questionnaire for symptoms evaluation within the new GOLD staging and mortality in COPD. Chest. 2015; 148:159–168.
- de Torres JP, Marin JM, Martinez-Gonzalez C, de Lucas-Ramos P, Mir-Viladrich I, Cosio B, et al. COPD history assessment in spain (CHAIN) cohort*. Clinical application of the COPD assessment test: longitudinal data from the COPD History Assessment in Spain (CHAIN) cohort. Chest. 2014; 146:111–122.
- Soler-Cataluña JJ, Sauleda J, Valdés L, Marin P, Agüero R, Pérez M, Miravitlles M. Prevalence and perception of 24-h symptom patterns in patients with stable chronic obstructive pulmonary disease in Spain. Arch Bronconeumol. 2016; 52:308–315.
- Jones PW, Brusselle G, Dal Negro RW, Ferrer M, Kardos P, Levy ML, Perez T, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011; 38:29–35.
- Jenkins CR, Postma DS, Anzueto AR, Make BJ, Peterson S, Eriksson G, Calverley PM. Reliever salbutamol use as a measure of exacerbation risk in chronic obstructive pulmonary disease. BMC Pulm Med. 2015; 15:97.
- Ramon MA, Esquinas C, Barrecheguren M, Pleguezuelos E, Molina J, Quintano JA, et al. Self-reported daily walking time in COPD: relationship with relevant clinical and functional characteristics. Int J Chron Obst Pulm Dis. 2017; 12:1173–1181.
- Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, et al. Spanish COPD guidelines (GesEPOC) 2017. Pharmacological treatment of stable chronic obstructive pulmonary disease. Arch Bronconeumol. 2017; 53:324–335.