ABSTRACT
This study evaluated the bioequivalence, safety, and immunogenicity of a new liquid formulation of human plasma–derived alpha1-proteinase inhibitor, Liquid Alpha1-PI, compared with the Lyophilized Alpha1-PI formulation (Prolastin®-C), for augmentation therapy in patients with alpha1-antitrypsin deficiency (AATD). In this double-blind, randomized, 20-week crossover study, 32 subjects with AATD were randomized to receive 8 weekly infusions of 60 mg/kg of Liquid Alpha1-PI or Lyophilized Alpha1-PI. Serial blood samples were drawn for 7 days after the last dose followed by 8 weeks of the alternative treatment. The primary endpoint was bioequivalence at steady state, as measured by area under the concentration versus time curve from 0 to 7 days (AUC0–7 days) postdose using an antigenic content assay. Bioequivalence was defined as 90% confidence interval (CI) for the ratio of the geometric least squares (LS) mean of AUC0–7 days for both products within the limits of 0.80 and 1.25. Safety and immunogenicity were assessed. Mean alpha1-PI concentration versus time curves for both formulations were superimposable. Mean AUC0–7 days was 20 320 versus 19 838 mg × h/dl for Liquid Alpha1-PI and Lyophilized Alpha1-PI, respectively. The LS mean ratio of AUC0–7 days (90% CI) for Liquid Alpha1-PI versus Lyophilized Alpha1-PI was 1.05 (1.03–1.08), indicating bioequivalence. Liquid Alpha1-PI was well tolerated and adverse events were consistent with Lyophilized Alpha1-PI. Immunogenicity to either product was not detected. In conclusion, Liquid Alpha1-PI is bioequivalent to Lyophilized Alpha1-PI, with a similar safety profile. The liquid formulation would eliminate the need for reconstitution and shorten preparation time for patients receiving augmentation therapy for AATD.
Introduction
Alpha1-antitrypsin deficiency (AATD) is an autosomal codominant genetic disorder characterized by low serum levels of alpha1-protease inhibitor (alpha1-PI; historically called alpha1-antitrypsin). AATD manifests clinically in adults as chronic obstructive pulmonary disease (COPD) and liver cirrhosis Citation(1–3). Evidence-based guidelines recommend augmentation therapy with alpha1-PI in subjects with AATD (excluding PiMZ phenotype) with evidence of COPD to bolster the protective protease inhibitory shield and slow emphysema progression Citation(4).
Augmentation therapy is available in the United States as an approved therapy for AATD since 1987. The first lyophilized formulation of alpha1-PI, Prolastin®, was further modified with additional purification, filtration, and production steps with viral clearance capacity and rebranded as Prolastin®-C (Grifols Therapeutics Inc., Research Triangle Park, NC, USA) Citation(5). Liquid Alpha1-PI is a new alanine-stabilized, liquid formulation of human plasma–derived alpha1-PI, developed as an alternate dosage form of Lyophilized Alpha1-PI that does not require reconstitution during its preparation for use; thus, the preparation time is shortened. This study evaluated the bioequivalence, safety, and immunogenicity of Liquid Alpha1-PI relative to Lyophilized Alpha1-PI in subjects with AATD.
Material and Methods
Subject selection
Eligible subjects had a diagnosis of congenital AATD and were ≥18 and ≤70 years of age, with documented allelic combination of ZZ, SZ, Z(null), (null)(null), S(null), or other “at-risk” alleles, and a serum alpha1-PI concentration <11 µM. Postbronchodilator forced expiratory volume in 1 second (FEV1) was required to be ≥30% and <80% of predicted and FEV1/forced vital capacity <70% (Global Initiative for Chronic Obstructive Lung Disease stage II or III) Citation(6). Prior or current alpha1-PI augmentation therapy was permitted, provided the treatment was discontinued at the baseline visit (week 1).
Key exclusion criteria included a moderate or severe pulmonary exacerbation during the 4 weeks before baseline; history of lung or liver transplant; lung surgery during the previous 2 years; confirmed liver cirrhosis; severe concomitant disease (e.g., congestive heart failure, clinically significant pulmonary fibrosis, malignant disease [with the exception of skin cancers other than melanoma], history of acute hypersensitivity pneumonitis reaction, or current chronic hypersensitivity pneumonitis); known previous infection or clinical signs and symptoms consistent with current hepatitis A virus, hepatitis B virus, hepatitis C virus, or human immunodeficiency virus infection; smoking within the past 6 months (or positive urine cotinine test); participated in another investigational drug study within 1 month prior to the week 1 (baseline) visit; use of systemic or aerosolized antibiotics for exacerbation within 4 weeks of study start; and use of steroids above a stable dose equivalent to 5 mg/day prednisone within 4 weeks of study start (permissible stable doses could be maintained at the same dose throughout the study); known selective or severe immunoglobulin A deficiency.
The study was conducted in compliance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. In addition, all local regulatory requirements were followed. The protocol was approved by the Institutional Review Board or Ethics Committee at each center (www.ClinicalTrials.gov NCT02282527). All subjects signed an informed consent statement.
Objectives
The pharmacokinetic primary objective was to demonstrate the bioequivalence of 60 mg/kg of Liquid Alpha1-PI compared with 60 mg/kg of Lyophilized Alpha1-PI, both at approximate steady state after eight weekly infusions, as measured by area under the concentration versus time curve from 0 to 7 days (AUC0–7 days) of alpha1-PI using an antigenic content assay. In addition, AUC0–7 days was assessed using a functional activity assay and an adjusted AUC0–7 days was calculated by subtracting estimated endogenous levels of alpha1-PI. Safety objectives included evaluation and comparison of adverse events (AE), COPD exacerbations, and immunogenicity of the two investigational treatments when administered for eight weeks.
Study design
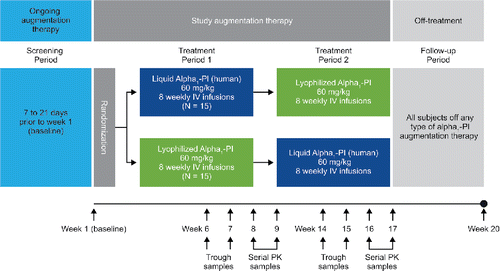
This study had a multicenter, randomized, double-blind crossover design (). The screening and baseline period included chemistry and hematology labs, inclusion/exclusion criteria check, virus safety testing, physical examination, pulmonary function testing, and reporting of genotyping and phenotyping results for potentially eligible subjects whose AATD genotype had not previously been documented. Eligible subjects were randomized 1:1 by computer-generated schedule to one of two treatment sequences to either receive weekly infusions of 60 mg/kg Liquid Alpha1-PI (supplied in 20-ml glass vials as a sterile, stable solution containing approximately 1 g of functionally active alpha1-PI per vial, delivered at a rate of 0.08 ml/kg/min) for 8 weeks or weekly infusions of 60 mg/kg Lyophilized Alpha1-PI (supplied as a lyophilized powder in 50-ml glass vials that was reconstituted with 20-ml sterile water; infused dose contained approximately 1 g of active alpha1-PI per vial) for 8 weeks followed by weekly infusions of the alternate treatment for another 8 weeks, according to the crossover study design (). The off-treatment follow-up period (weeks 17 to 20) was to provide safety information off treatment, collect pharmacokinetic samples, and provide a measure of endogenous concentration (Cweek20) of alpha1-PI.
Figure 1. Schematic of the study design. Liquid Alpha1-PI: human plasma–derived alpha1-proteinase inhibitor; IV: intravenous; PI: proteinase inhibitor; PK: pharmacokinetic.
Following preparation by an unblinded pharmacist, prepared intravenous infusion bags for investigational treatments were indistinguishable to blinded study staff and subjects, and were marked only with sufficient identification for correct administration and were covered with nontransparent blinding covers.
Data collection
Serum concentration of alpha1-PI was measured in trough (preinfusion) and serial blood samples from all subjects for the purpose of pharmacokinetic analysis (). Trough samples during the treatment periods were drawn prior to infusions at weeks 6–9 and weeks 14–17. For AUC determination, a total of 12 serial blood samples were collected, starting at the end of the treatment period (week 8 or 16) and extending over 7 days after the previous treatment period infusion (week 9 or 17). Blood samples were collected immediately prior to the infusion, immediately after a saline flush when the infusion was completed, and at the following times from completion of the infusion: 0.25, 0.5, 1, 2, 4, 8 hours, and then days 1 (±4 hours), 2 (±4 hours), 5 (±1 day), and 7 (±1 day).
A blood sample for alpha1-PI concentration was also drawn at the week 20 visit, at the end of the 4-week off-treatment follow-up period, for the purpose of calculating the adjusted AUC0–7 days values.
AEs/treatment-emergent AEs, serious adverse events (SAEs), and adverse drug reactions were recorded. Treatment-emergent AEs were defined as any AE during the study that began on or after the date and time of first dose of investigational product. A suspected adverse drug reaction included any AEs with investigator's causality assessment of “definitive,” “probable,” “possible,” or “doubtful/unlikely”. In addition, vital signs and COPD exacerbations were recorded Citation(7). As COPD exacerbations are part of the natural history of AATD, they were not reported as AEs unless they met SAE criteria. Additionally, immunogenicity assessment and parvovirus B19 safety testing were carried out (at least at baseline and at the end of each treatment period).
Laboratory assessments and statistical analyses
Blood concentration of alpha1-PI was assessed by two assays: antigenic content assay and functional activity (potency) assay. Antigenic content assay measures both functionally active and inactive forms of the alpha1-PI protein and was the primary assay. The concentration of alpha1-PI was measured by immunonephelometry using a standard control that is traceable to the international reference standard CRM 470. Functional activity assay measures only the concentration of protein capable of inhibiting neutrophil elastase and was supportive to the primary assay. To quantify alpha1-PI inhibitory activity, a chromogenic elastase substrate was exposed to a patient sample and residual elastase activity was measured using a standard control. Both assays were validated according to current regulatory guidelines Citation(5).
The primary pharmacokinetic endpoint was measured by the AUC0–7 days postdose (i.e., at steady state) using the antigenic content assay. Bioequivalence was defined as 90% confidence interval (CI) for the geometric least squares mean (LSM) ratio of AUC0–7 days for the two products falling between the limit of 0.80 and 1.25 Citation(8). Natural log-transformed AUC0–7 days values were analyzed by the mixed-effect model, including treatment, treatment sequence, and study period as fixed effects and subject (nested within sequence) as a random effect.
Exploratory analysis of the geometric LSM ratio of AUC0–7 days adjusted by subtraction of Cweek20 (adjusted AUC0–7 days) and the geometric LSM ratio of maximum concentration (Cmax) were also performed for both antigenic content assay and functional activity assay. Time to reach Cmax, time to descend to half of Cmax (t½), rate of clearance, and mean trough concentration at steady state were also descriptively summarized.
Pharmacokinetic data from a previous study comparing Prolastin®-C with Prolastin® indicated that the standard deviation in log scale for AUC0–7 days is 0.174 Citation(5). Assuming a standard deviation of 0.174, a sample size of 12 subjects (6 subjects per treatment sequence) was needed to demonstrate bioequivalence with 90% power. Considering additional assessment of safety and immunogenicity, a sample size of 30 subjects (15 subjects per treatment sequence) was planned for the study.
Results
Subjects
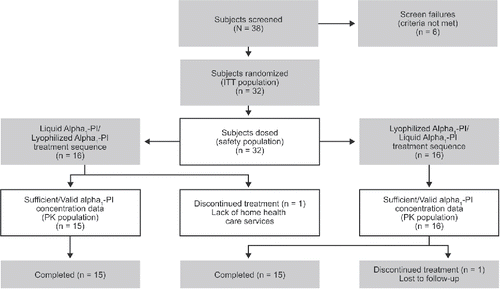
Subjects were enrolled at six investigative centers in the United States. Of the 38 subjects screened, six did not meet the entry criteria (four spirometric, one specific allelic combination, and one informed consent) and 32 were randomized and dosed (intent-to-treat and safety populations; ). One subject in the Liquid Alpha1-PI/Lyophilized Alpha1-PI treatment sequence discontinued after week 1 owing to lack of home health care service and was excluded from the pharmacokinetic analysis. One patient in the Lyophilized Alpha1-PI/Liquid Alpha1-PI treatment sequence was lost to follow-up after completing both treatment periods and was included in the pharmacokinetic analysis. Thus, 31 subjects were included in the pharmacokinetic analysis.
Figure 2. Subject disposition – CONSORT diagram. Liquid Alpha1-PI: human plasma–derived alpha1-proteinase inhibitor; ITT: intent-to-treat; PI: proteinase inhibitor; PK: pharmacokinetic.
Demographics and baseline characteristics were similar across the two treatment sequences (). All subjects were white (genotype PI*ZZ, n = 31; PI*SZ, n = 1). Alpha1-PI blood levels at time of diagnosis ranged from 2.7 µM to 9.2 µM. Four patients were naïve to alpha1-PI augmentation therapy, whereas 28 had received prior therapy with at least one drug (Prolastin®-C, 20; Prolastin®, 5; Zemaira®, 3; Glassia®, 1). Baseline postbronchodilator FEV1 percentage predicted values ranged from 30.0% to 79.0%.
Table 1. Demographics and AATD history.
Pharmacokinetic parameters
Mean alpha1-PI concentrations versus time curves at steady state after 8 weeks of weekly 60 mg/kg intravenous doses of Liquid Alpha1-PI were essentially indistinguishable from those following a weekly 60 mg/kg intravenous dose of Lyophilized Alpha1-PI using the antigenic content assay () or the functional activity assay (). Mean AUC0–7 days was 20 320 versus 19 838 mg × h/dl for Liquid Alpha1-PI and Lyophilized Alpha1-PI, respectively () and the geometric LSM ratio for AUC0–7 days with Liquid Alpha1-PI versus Lyophilized Alpha1-PI was 1.05 with 90% CI of 1.03 to 1.08 ().
Figure 3. Mean alpha1-PI concentration (± SD) using the (A) antigenic content assay and (B) functional activity assay at week 8 (steady state). Liquid Alpha1-PI: human plasma–derived alpha1-proteinase inhibitor; PI = proteinase inhibitor; SD: standard deviation.
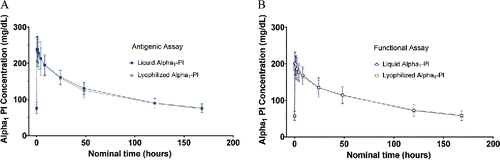
Table 2. Summary of pharmacokinetic parameters.
Figure 4. Geometric least squares mean ratio (90% confidence interval) for Liquid Alpha1-PI versus Lyophilized Alpha1-PI (antigenic content assay). AUC0–7 days: area under the concentration versus time curve from 0 to 7 days; Cmax: maximum concentration; Liquid Alpha1-PI: human plasma–derived alpha1-proteinase inhibitor.
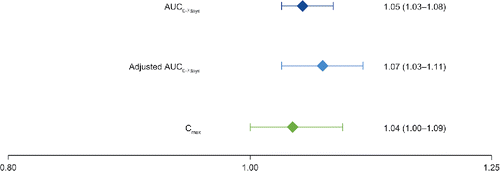
Mean alpha1-PI concentration at week 20 (4 weeks after the last study drug infusion) was 29.9 mg/dl using the antigenic content assay (). The geometric LSM ratio for the adjusted AUC0–7 days for Liquid Alpha1-PI versus Lyophilized Alpha1-PI was 1.07 with 90% CI of 1.03 to 1.11, also indicating bioequivalence between the two products (). The geometric LSM ratio of Liquid Alpha1-PI/Lyophilized Alpha1-PI for Cmax also falls within the range of bioequivalence ().
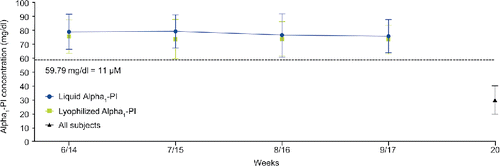
Figure 5. Mean (± SD) steady-state trough Alpha1-PI concentrations (antigenic content). Liquid Alpha1-PI: human plasma–derived alpha1-proteinase inhibitor; PI: proteinase inhibitor; SD: standard deviation.
All of the alpha1-PI concentration–based pharmacokinetic parameters were larger with the antigenic content assay compared with the functional assay, but both showed comparable similarity between the two study treatments (). From exploratory statistical analyses of functional assay data, the 90% CI corresponding to geometric LSM ratios for AUC0–7 days (1.007–1.072), adjusted AUC0–7 days (0.998–1.089), and Cmax (0.997–1.075) were also bound within the predefined range to indicate bioequivalence. With the antigenic content assay, once Cmax was achieved (≈250 mg/dl) after approximately 40 minutes, alpha1-PI concentrations declined in a multi-exponential manner (mean clearance ≈ 0.0040 dl/kg/h, t½ ≈ 160 hours). The intersubject variability (% coefficient of variation) in AUC0–7 days, adjusted AUC0–7 days, Cmax, t½, and clearance of alpha1 PI was ≤26.7% for each treatment using either assay, indicating general consistency in the distribution and elimination of alpha1-PI between subjects and treatments.
Steady state for trough alpha1-PI concentrations using the antigenic content assay was reached by the start of the sixth weekly infusion at week 6/14 (). The mean trough of alpha1-PI over the 4-week period at steady state was above the historical target of 11 µM in all subjects of this study at each time point, with the exception of one subject that measured 10.62 µM at week 8 (while receiving Lyophilized Alpha1-PI). Steady-state trough alpha1-PI concentrations using the functional assay were similar between treatments.
Safety
Overall, 32 subjects received at least one dose of Liquid Alpha1-PI and 31 subjects received at least one dose of Lyophilized Alpha1-PI. Mean duration of exposure was 7.92 and 7.90 weeks with Liquid Alpha1-PI and Lyophilized Alpha1-PI, respectively. The mean number of infusions per subject was 7.9 for both treatments, which represented overall 252 infusions with Liquid Alpha1-PI and 245 infusions with Lyophilized Alpha1-PI.
No deaths occurred during the study and no subject experienced an SAE while receiving Liquid Alpha1-PI. One subject had an SAE (moderate infective exacerbation of COPD) during the follow-up period after receiving Lyophilized Alpha1-PI that was not considered to be related to investigational product. No subjects withdrew from the study owing to an AE.
Treatment-emergent AEs were experienced by 59% of subjects when treated with Liquid Alpha1-PI and 42% of subjects when treated with Lyophilized Alpha1-PI (). Overall, the AE profile for both study treatments showed no consistent treatment-related pattern for any particular type of event and none occurred in more than two subjects per treatment (). All treatment-emergent AEs in the study were considered by the investigator to be of mild or moderate intensity, except one case of severe back pain (worsened back pain due to motorcycle riding) with Lyophilized Alpha1-PI. Adverse drug reactions suspected by the investigator to be related to treatment included fatigue, diarrhea, contusion, decrease in platelet count, and insomnia during infusions with Liquid Alpha1-PI and abnormal hepatic function and dizziness with Lyophilized Alpha1-PI. No AEs with either treatment were considered by the investigator as definitely related to treatment.
Table 3. Summary of safety.
Exacerbations of COPD occurred in 18 subjects, with a total of 23 events. The distribution of occurrences was similar across treatments (). Of these, four COPD exacerbations occurred during the follow-up period: three after Liquid Alpha1-PI treatment and one after Lyophilized Alpha1-PI treatment. All COPD exacerbations were graded as mild or moderate, though one was reported as an SAE (discussed above) owing to hospitalization. No treatment-emergent COPD exacerbation resulted in discontinuation or was considered to be related to the study treatment.
No changes from baseline of clinical concern were noted at any time point for any laboratory measurement, vital sign, physical exam, or pulmonary function test parameter.
Discussion
This trial compared pharmacokinetic parameters and the safety profile of Liquid Alpha1-PI with Lyophilized Alpha1-PI in subjects with AATD. The trial achieved the primary pharmacokinetic objective by demonstrating bioequivalence of the AUC0–7 days at steady state for the two treatments following weekly infusions over 8 weeks. Safety outcomes and other pharmacokinetic parameters were also comparable between the two treatments. Importantly, the 90% CI for the geometric LSM ratio, Liquid Alpha1-PI/Lyophilized Alpha1-PI, of the AUC0–7 days fell within the range that demonstrated that a weekly infusion of 60 mg/kg Liquid Alpha1-PI produces an AUC of serum alpha1-PI that is bioequivalent to that produced by a weekly infusion of 60 mg/kg Lyophilized Alpha1-PI. This is supported by the mean alpha1-PI concentration versus time curves of the two treatments that were essentially superimposable. Bioequivalence was also confirmed by AUC0–7 days adjusted for the week 20 alpha1-PI concentrations (representative of endogenous alpha1-PI) and Cmax based on both antigenic content assay and functional activity assay.
The mean Cweek20 (29.9 mg/dl) is consistent with the known endogenous alpha1-PI concentrations in AATD patients with the PI*ZZ genotype (10–30 mg/dl) Citation(9,10), and with baseline alpha1-PI levels of four naïve AATD patients in this study (mean concentration of 24 mg/dl) as well as four patients from the SPARK study (mean ≈ 30 mg/dl) Citation(11). Trough concentrations determined over a 4-week period in each treatment period indicate that an approximate steady-state condition was achieved by the start of the sixth weekly infusion for both Liquid Alpha1-PI and Lyophilized Alpha1-PI. This confirms that the pharmacokinetic parameters of alpha1-PI after 8 weeks of treatment in this study were determined under an approximately steady-state condition for both Liquid Alpha1-PI and Lyophilized Alpha1-PI. Furthermore, trough values of alpha1-PI were above the historical “protective” level of 11 µM, as has been previously reported with weekly doses of Lyophilized Alpha1-PI in the ChAMP and SPARK studies Citation(5,11).
This study used two assays to measure alpha1-PI levels. Concentrations measured by the functional activity assay were slightly lower than those using the antigenic content assay, and so pharmacokinetic parameter results were slightly different depending on the assay used. However, Liquid Alpha1-PI and Lyophilized Alpha1-PI were comparable across pharmacokinetic parameters whether using the functional activity assay or the antigenic content assay.
In addition, the key pharmacokinetic parameters in this study are similar to those observed in the ChAMP study and the SPARK study—two pharmacokinetic studies that also evaluated the pharmacokinetics of Lyophilized Alpha1-PI Citation(5,11). The mean AUC0–7 days values (antigenic content) for Liquid Alpha1-PI and Lyophilized Alpha1-PI were 20 320 and 19 838 mg × h/dL, respectively, which are consistent with those for Lyophilized Alpha1-PI observed in ChAMP (19 010 mg × h/dL Citation(5)) and SPARK (20 360 mg × h/dL) Citation(11)). The mean Cmax values for Liquid Alpha1-PI and Lyophilized Alpha1-PI were 254 and 249 mg/dL, respectively, for the antigenic content assay, which are consistent with those for Lyophilized Alpha1-PI observed in the two previous studies: 233 mg/dL Citation(5) and 247 mg/dL Citation(11). In addition, the results obtained with Lyophilized Alpha1-PI in this study based on the functional activity assay are also consistent with those reported in ChAMP Citation(5) for Lyophilized Alpha1-PI (eg, Cmax of 208 mg/dL in this study vs Cmax of 180 mg/dL in ChAMP).
The AE profile of Liquid Alpha1-PI was consistent with Lyophilized Alpha1-PI and showed no consistent treatment-related pattern for any particular type of event. Most AEs were infrequent and few were reported in more than 1 subject. All AEs were mild or moderate in intensity, with the exception of one SAE considered unrelated to study treatment that occurred during the off-treatment follow-up period after Lyophilized Alpha1-PI treatment. Overall, the frequencies of AEs and COPD exacerbations were similar between treatments. However, because this study was not powered nor long enough to assess the effect of the treatments on exacerbation rates, no conclusions can be made regarding efficacy.
Immunogenicity was not detected in any subject in the trial. There were no instances of Parvovirus seroconversions or any clinical indication of seroconversions against any other blood-borne agent.
In the clinic, practical differences between Liquid Alpha1-PI and Lyophilized Alpha1-PI should be considered. First, the Liquid Alpha1-PI does not require reconstitution, whereas the Lyophilized Alpha1-PI is a lyophilized powder that requires sterile water; thus, the use of Liquid Alpha1-PI may save preparation time over Lyophilized Alpha1-PI. Second, the two versions provide the same concentrated formula and volume per dose. Third, the Liquid Alpha1-PI requires refrigeration for long-term storage (up to the expiration date) but can be kept at room temperature for up to 1 month, whereas Lyophilized Alpha1-PI, in the lyophilized form, can be stored at room temperature for the period indicated by the expiration date. Once reconstituted, Lyophilized Alpha1-PI should be administered within 3 hours. Similarly, Liquid Alpha1-PI should be used within 3 hours of withdrawing it from the vial and adding it to the empty intravenous bag for administration.
Conclusions
Liquid Alpha1-PI 60 mg/kg/wk was demonstrated as bioequivalent to Lyophilized Alpha1-PI 60 mg/kg/wk with a comparable tolerability profile. Liquid Alpha1-PI treatment was well tolerated with no reported SAEs. Liquid Alpha1-PI may provide enhanced convenience to patients receiving augmentation therapy for AATD.
Declaration of Interest
AF Barker: Clinical research funding provided by Grifols Therapeutics Inc.
MA Campos: Research grants from the Alpha-1 Foundation and CSL Behring; participated in clinical trials sponsored by Baxalta, CSL Behring, and Grifols Therapeutics Inc.; participated in advisory boards for CSL Behring and Grifols Therapeutics Inc.
ML Brantly: Research grants from Grifols Therapeutics Inc.; owner of GeneAidyx.
JM Stocks: Research grants from Grifols Therapeutics Inc. and Kamada.
RA Sandhaus: Research grants from CSL Behring and Grifols Therapeutics Inc.; trains speakers on their speaker bureau for Grifols Therapeutics Inc.; advisory boards for Shire (Baxalta).
D Lee: Speaker for Boehringer Ingleheim, Genentech, Grifols Therapeutics Inc., and Otsuka; participated in clinical trials sponsored by Grifols Therapeutics Inc.
K Steinmann, J Lin and S Sorrells: employees and stockholders of Grifols Therapeutics Inc.
Authors' Contributions
AFB, MAC, MLB, JMS, RAS, DL, KS, JL, and SS contributed to the study design and implementation and provided meaningful input to this manuscript. JL provided statistical analyses for this study.
Acknowledgments
The authors would like to thank the study coordinators for their dedication and care of the study participants, and the participants of this study for giving of their valuable time. Writing and editorial assistance was provided to the authors by David Macari, PhD, and Susan Sutch, PharmD, CMPP, on behalf of Evidence Scientific Solutions, Philadelphia, Pennsylvania, USA, and funded by Grifols Inc., Research Triangle Park, North Carolina, USA.
Funding
Grifols Therapeutics Inc. (Research Triangle Park, NC, USA) funded this study.
References
- Mulgrew AT, Taggart CC, McElvaney NG. Alpha-1-antitrypsin deficiency: current concepts. Lung 2007;185(4):191–201. Epub 2007/06/15. https://doi.org/10.1007/s00408-007-9009-y PMID:17562108.
- Köhnlein T, Welte T. Alpha-1 antitrypsin deficiency: pathogenesis, clinical presentation, diagnosis, and treatment. Am J Med. 2008;121(1):3–9. Epub 2008/01/12. https://doi.org/10.1016/j.amjmed.2007.07.025 PMID:18187064
- Fregonese L, Stolk J. Hereditary alpha-1-antitrypsin deficiency and its clinical consequences. Orphanet J Rare Dis. 2008;3:16. https://doi.org/10.1186/1750-1172-3-16 PMID:18565211
- Sandhaus RA, Turino G, Brantly ML, Campos M, Cross CE, Goodman K, et al. The diagnosis and management of alpha-1 antitrypsin deficiency in the adult. Chronic Obstr Pulm Dis (Miami). 2016;3(3):668–82. Available from: https://journal.copdfoundation.org/jcopdf/id/1115/The-Diagnosis-and-Management-of-Alpha-1-Antitrypsin-Deficiency-in-the-Adult.https://doi.org/10.15326/jcopdf.3.3.2015.0182
- Stocks JM, Brantly ML, Wang-Smith L, Campos MA, Chapman KR, Kueppers F, et al. Pharmacokinetic comparability of Prolastin®-C to Prolastin® in alpha1-antitrypsin deficiency: a randomized study. BMC Clin Pharmacol. 2010;10:13. https://doi.org/10.1186/1472-6904-10-13 PMID:20920295
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2016. Available from: http://www.goldcopd.org/.
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31(2):416–69. Epub 2008/02/02. https://doi.org/10.1183/09031936.00099306 PMID:18238951
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Guidance for industry – statistical approaches to establishing bioequivalence. Rockville (MD): Center for Drug Evaluation and Research, Food and Drug Administration, US Department of Health and Human Services; 2001. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm070244.pdf.
- Balduyck M, Odou MF, Zerimech F, Porchet N, Lafitte JJ, Maitre B. Diagnosis of alpha-1 antitrypsin deficiency: modalities, indications and diagnosis strategy. Rev Mal Respir. 2014;31(8):729–45. Epub 2014/11/14. https://doi.org/10.1016/j.rmr.2014.06.001 PMID:25391508
- American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society statement: standards for the diagnosis and management of individuals with alpha-1 antitrypsin deficiency. Am J Respir Crit Care Med. 2003;168(7):818–900. Epub 2003/10/03. https://doi.org/10.1164/rccm.168.7.818 PMID:14522813
- Campos MA, Kueppers F, Stocks JM, Strange C, Chen J, Griffin R, et al. Safety and pharmacokinetics of 120 mg/kg versus 60 mg/kg weekly intravenous infusions of alpha-1 proteinase inhibitor in alpha-1 antitrypsin deficiency: a multicenter, randomized, double-blind, crossover study (SPARK). COPD. 2013;10(6):687–95. Epub 2013/07/19. https://doi.org/10.3109/15412555.2013.800852 PMID:23862647
