ABSTRACT
More data are needed regarding the radiology, co-morbidities and natural history of smoking-related interstitial fibrosis (SRIF), a common pathological finding, mainly described heretofore in association with lung cancer, where respiratory bronchiolitis (RB) usually co-exists.
We prospectively acquired high resolution CT scan data (edge-enhancing lung reconstructions) to detect any radiologic interstitial lung abnormality (ILA) in individuals who ultimately underwent surgical lobectomy for lung cancer (n = 20), for radiologic/pathologic correlation. We also re-examined other smoking-related benign histologic cases: chronic obstructive pulmonary disease (COPD lung explants, n = 20), alpha 1-antitrypsin deficiency (A1AT, explanted lungs n = 20), combined pulmonary fibrosis and emphysema (CPFE, n = 8) and idiopathic pulmonary fibrosis (IPF, n = 10). Finally, we pooled our data with all peer-reviewed published data describing histologic SRIF of known ILA status.
SRIF was observed in 40% of cancer lobectomies, mean (±SD) age 65.8 ± 8.7 years, none of whom had ILA. SRIF was observed in other smoking-related benign diseases (COPD 35%, A1AT 20%, CPFE 25%, and IPF 10%). 71.4% of benign SRIF cases had no RB (nearly all ex-smokers) versus 0% of cancer-associated SRIF cases (P = 1.7 × 10−3). Pooled data showed that those SRIF subjects without ILA were 15.05 years older than those with ILA (95% confidence interval 8.99 to 21.11, P = 2.5 × 10−5) and more likely to be former smokers (P = 7.2 × 10−3).
SRIF is frequently found without lung cancer, and mostly without RB in former smokers. SRIF is less likely to have ILA in older subjects and with smoking cessation, which could represent RB+/−SRIF regression.
Introduction
Interstitial fibrosis associated with emphysema, also called smoking-related interstitial fibrosis (SRIF), is a pathological finding, distinct from combined pulmonary fibrosis and emphysema (CPFE), observed in the non-neoplastic parenchyma of lobectomies for cancer (Citation1–3), though little is known regarding its radiological and clinical findings. SRIF heretofore is characterised by a distinct type of hyalinised interstitial fibrosis that is associated with emphysema and respiratory bronchiolitis (RB). The affected alveolar septa are thickened by deposition of dense, eosinophilic ropey-appearing collagen often with admixed hyperplastic smooth muscle bundles; the septal thickening is usually quite marked and easily visible at low magnification with a predominance in the subpleural parenchyma where it is associated with emphysema, but it can also be found in non-emphysematous deeper tissue. In contrast to historic descriptions of emphysema, it is now accepted that some microscopic fibrosis commonly accompanies emphysema (Citation4).
Previous reports of SRIF have retrospectively reported various radiologic pulmonary findings, and have tended to focus on lung parenchyma in lung cancer lobectomies (Citation1,3,5), while others have omitted pathologic confirmation in radiologic assumptions regarding SRIF (Citation6). Experts have found a lack of specificity of smoking status for idiopathic interstitial pneumonia findings that could include SRIF (Citation7). In the current study, we asked if SRIF is discernible on cross-sectional imaging, by prospectively acquiring high-resolution chest CT detail of any interstitial lung abnormality (ILA) in subjects being worked up for suspected, and subsequently resected lung cancer. Secondly, we aimed to study the prevalence of SRIF in non-oncologic cohorts and its dependence on RB, by evaluating explanted emphysematous lungs from either chronic obstructive pulmonary disease (COPD) or alpha 1-antitrypsin deficiency (A1AT) patients, along with cases of CPFE syndrome and smokers with idiopathic pulmonary fibrosis (IPF). Finally, we looked for factors that might influence the presence or absence of radiologic ILA in histologic SRIF by pooling the present study's prospective cohort data with all comparable publicly-available SRIF imaging data, to gain indirect insights into SRIF evolution.
Material and methods
The St. Vincent's University Hospital research ethics committee approved the study, conducted as per amended Declaration of Helsinki. For the prospective pathologic–radiologic correlation arm, written, informed consent was obtained from 20 consecutive patients with suspected resectable stage I or II lung cancer attending St. Vincent's hospital. Eligibility Inclusion criteria included a history of current/former smoking and clinical suspicion for resectable early stage lung cancer based on computed tomography (CT) scan. Exclusion criteria included a prior diagnosis of an interstitial lung disease (ILD) other than RB-associated ILD, or potentially unresectable lung cancer. CT imaging was captured using a 64-slice single-source CT system (Siemens Sensation 64, Siemens Medical Solutions, Forcheim, Germany), and data acquired from apex to lung base at peak inspiration. For the duration of subject accrual, the radiology department implemented a protocol of edge-enhancing lung reconstructions on all lung mass protocol CTs to facilitate optimal assessment for ILA. Axial CT slices (1 mm) were analysed using lung windows with a width of 1,500 Hounsfield units and centre of −700 Hounsfield units, conducted on interactive image processing software (Syngo InSpace4D, Siemens Medical Solutions, Forcheim, Germany).
All CT scans were scored for pre-specified radiological patterns of parenchymal abnormality, including (centrilobular) nodules, reticulation, ground glass opacification, honeycombing, traction bronchiectasis, and emphysema (centrilobular, panlobular, paraseptal), by an experienced thoracic radiologist who was unaware of the SRIF histologic status, using established scoring systems (Citation8–11). The distribution of any identifiable abnormalities was localised by lung lobe. The relative low attenuation volume (LAV) method using CT densitometry provided right, left, and total emphysema scores, using a threshold value of <−950 Hounsfield units to measure the percentage of low-attenuation volume (LAV%) indicative of emphysema quantity. Where available, the most recent follow-up chest CT scan available was also reviewed to assess for progressive disease.
At surgery, the surgeon placed one and two sutures at the apical and basal surfaces of the resected lobe respectively, to facilitate resected lobar orientation. The resected cancer lobectomy specimens underwent extensive sampling in subpleural areas away from the cancerous nodule/mass in 15 consecutive blocks of tissue (see map, ). Subsequent to blinded analysis of CT scans by the radiologists for the presence of radiologic ILA, unblinded reassessment of CT imaging was undertaken availing of pathologic SRIF status and localisation information.
For the second arm of the study, further histologic cohorts were retrospectively assessed for pathologic SRIF, including 20 explanted COPD lungs (“COPD Transplant”) from both the Irish National Lung Transplant programme (n = 5) and from the Transplant Unit at Bichat Hospital France (n = 15). Another cohort amounted to 20 explanted A1AT lungs (“A1AT Transplant”) from the Irish National transplant programme (n = 5), and from the Transplant Unit at Bichat Hospital France (n = 15). Inclusion criteria for all of these histologic cohorts was the availability of histologic slides of lung from uninvolved explant/resection regions, and the stipulated smoking-related lung diseases in subjects of known smoking status. Subject biospecimens were not excluded for any other reason if eligible. For all transplant cases, clinical data were gathered but radiology was not reviewed. In addition, CPFE cases with histology available for review were retrieved from a previous published cohort at St. Vincent's hospital, as well as smokers with IPF from the same cohort (Citation12). In all, smoking-related pathological changes were analysed on hematoxylin and eosin stained slides and recorded features are as per and .
Figure 2. Illustrative examples of cancer lobectomy sections showing pre-specified histologic patterns as indicated, including various subtypes of fibrosis patterns. See also .

Interstitial fibrosis was defined as cellular fibrotic expansion on the alveolar wall (), hyaline fibrosis as acellular collagen deposition (), smoking-related interstitial fibrosis as interstitial fibrosis associated with emphysema [as per Katzenstein's proposed criteria (Citation1) and , , ], and peribronchial fibrosis (PBF) as fibrosis around bronchioles (). The diagnosis of SRIF was made using Katzenstein's proposed criteria and usual interstitial pneumonia (UIP) was diagnosed according to consensus guidelines (Citation1,13).
For the third arm of the study, the present prospective data were pooled with all publicly-available peer-reviewed data for all subjects with histologic SRIF and for whom individual CT thorax ILA status was known [n = 8 from the current prospective lung cancer cohort, n = 9 from Yousem (Citation14), n = 7 from Reddy et al (Citation15), and n = 9 from Katzenstein et al (Citation1)], n = 33 subjects overall.
Statistical analysis
For the prospective lung cancer cohort, differences in parametric and non-parametric baseline variables were assessed using student's t-test and Fisher's exact test (two-tailed) respectively. Radiologic scores among SRIF (cases) and non-SRIF (controls) were assessed by a two-tailed independent samples Mann Whitney U test. Correlation coefficients were used to compare the histologic SRIF extent (SRIF score; marked out of 15, i.e. the 15 histologic sections examined per resected lobe) and corresponding total lung radiologic emphysema extent (% emphysema at a cutoff of −950 Hounsfield units). A value of P < 0.05 was considered significant.
Results
Clinical features of the prospective lung cancer lobectomy cohort (n = 20), all of Caucasian ancestry, are outlined in . Histologic SRIF was observed in 8/20 subjects (40%). There was no significant demographic difference among SRIF and non-SRIF subjects with respect to gender, age, pathologic tumour stage, COPD status, or spirometry values. SRIF subjects were more likely to be current smokers than of other smoking status when compared to non-SRIF subjects (p = 0.02, ). One non-smoker subject was mistakenly enrolled as a former smoker, and included in the dataset (no SRIF present). All SRIF cases were accompanied by adenocarcinoma. The average age observed in the present prospective SRIF cohort (65.9 years) was similar to that of the Katzenstein cohort (65.1 years) (Citation1), and 20.4 years older than the mean age (45.4 years) observed in the Yousem SRIF cohort (95% confidence interval 11.36 to 29.44; P < 5 × 10−4) (Citation14). Follow up was available in 16 of the 20 lung cancer cases over a mean of 44.9 months [31–67] and two had local recurrence. Two were lost to follow-up. Three subjects are deceased (one SRIF, two non-SRIF). All the 13 others (five with SRIF, eight non-SRIF) are clinically stable. Nineteen subjects had follow-up scans available over a mean interval of 30.5 ± 16.3 months and in no subject was there subsequent radiologic evidence of ILA.
Table 1. Clinical data from prospective lung cancer lobectomy cohort.
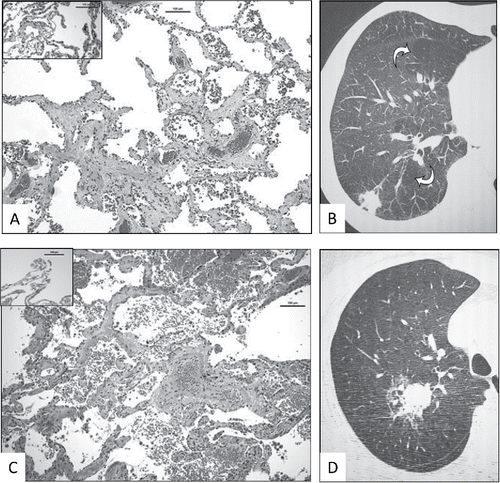
The principal aim of the study was to detect radiologic correlates for SRIF. Careful review did not identify any fibrosis, reticulation, honeycombing or airway traction in the SRIF and non-SRIF lobes, even retrospectively in affected lobes with heavier burden of histologic SRIF. Two examples of lobes with significant SRIF burden and the corresponding CT images are shown in (with corresponding histopathological finding). There was no significant difference in quantitatively-assessed emphysema scores among SRIF and non-SRIF subjects (data not shown). SRIF could be observed histologically in cases with a low emphysema score.
Figure 3. Examples of radiologic–pathologic correlation in prospective lung cancer cohort subjects with heavy burden of SRIF. A. Right upper lobectomy of subject X with histological evidence of SRIF (insert shows normal lung away from SRIF and cancer). Scale as indicated. B. Corresponding magnified view of high resolution CT thorax of right upper lobe, showing centrilobular emphysema (arrows) and no visible interstitial lung abnormality. C. Right upper lobectomy sample of subject Y with histological evidence of SRIF (insert shows normal lung away from SRIF and cancer). Scale as indicated. D. Corresponding magnified view of high resolution CT thorax of right upper lobe, showing tumour, emphysema and no discernible interstitial lung abnormality.
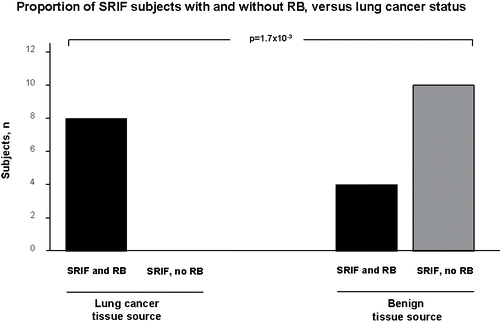
In the second arm of the study, the histopathologic prevalence of SRIF in diverse non-malignant clinical backgrounds was assessed, with emphasis on background co-pathologies (, ). Where relevant demographic information was available, it is presented alongside comparable data from the present study's prospective cancer cohort. All the cohorts had at least some individuals with SRIF, ranging from 10% prevalence in IPF subjects (focal SRIF) to 35% in the COPD transplant cohort, and in 24.1% of all benign cases, providing further evidence that lung cancer is not required for the development of SRIF. Interestingly, for the authors’ combined cohorts (n = 78), 12.8% had SRIF without RB (nearly all these were ex-smokers), representing 71.4% of the benign tissue SRIF cases, while those SRIF subjects with lung adenocarcinoma had significantly greater prevalence of co-existent SRIF and RB (presumably related to a greater current smoker proportion in the cancer cohort), suggesting that RB is not required for the development of SRIF, at least in subjects with a benign background lung pathology (, , P = 1.7 × 10−3).
Table 2. Demographics of comparison cohorts.
Table 3. Histologic findings among the comparison cohorts.
Table 4. Relationship of respiratory bronchiolitis to SRIF as a function of background lung histology in the present authors’ cohorts.
Figure 4. In all SRIF subjects identified in the current authors’ cohorts, the proportion of cases with and without histologic evidence of respiratory bronchiolitis (RB) are shown, parsed by source tissue lung cancer status. The background histologies of the tissue sources are outlined in .
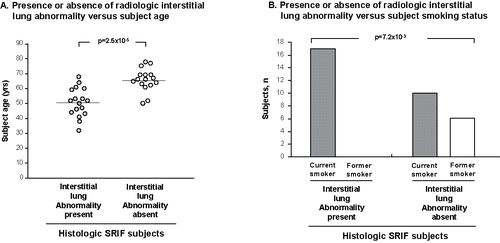
Finally, the prospectively acquired radiologic data on SRIF subjects from the current study were pooled with previous datasets from the literature of all SRIF subjects with known individual ILA status from radiologic CT scan data, to explore the surprising absence of ILA in the current prospective cohort. Interestingly, the pooled data (n = 33) revealed that where radiologic ILA was absent, the SRIF subjects were 15.05 years older (95% confidence interval 8.99 to 21.11, P = 2.5 × 10−5) and were significantly more likely to have ceased to smokexbrk (P = 7.2 × 10−3) than SRIF subjects with ILA present, supporting a hypothesis that SRIF (and/or co-existent RB) regresses radiologically with advancing age and removal of the stimulus of smoke (). When the Yousem dataset (which is biased towards ILA being present) is excluded from the pooled analysis, mean age in SRIF/no ILA (n = 16) is 65.8 ± 1.9 and mean age in SRIF/ILA (n = 8) is 56.8 years ± 1.7, p = 1.9 × 10−3, a similar result to that observed in the total pooled dataset.
Figure 5. Analysis of pooled data of SRIF subjects (authors’ cohorts and other described cohorts in the published literature) with known status regarding radiologic interstitial lung abnormality (ILA), see text. A. Influence of subject age on radiologic ILA. Each dot represents an individual subject. Mean age represented by horizontal line. B. Histogram showing the effect of smoking status on radiologic ILA in the same SRIF subjects as in panel A.
Discussion
The recently described histologic entity of SRIF is surprisingly frequent in lobectomy specimens of smokers with lung cancer, but lacks a less invasive biomarker that would obviate surgical diagnosis, and has a poorly understood natural history. Based on earlier reports that variably related radiologic findings to the diagnosis of SRIF, the present study asked: Is histologic SRIF detectable by high resolution CT scanning? Through prospective acquisition of HRCT thorax images at the time of clinically-indicated lung cancer pre-operative assessment, combined with intralobar pathologic–radiologic correlation, the data fail to demonstrate any evidence of a radiologic ILA attributable to SRIF in the present study of 20 older lung cancer subjects. The study also assessed the potential for SRIF to arise in subjects without cancer or RB, through retrospective examination of archived histologic specimens and found evidence of SRIF without RB in a significant minority of subjects with no cancer. Finally, the current study compared the rate of radiologic ILA seen in the present prospective SRIF-affected lung cancer cohort versus all comparable radiologic data from published series where SRIF was detected (irrespective of background lung disease status) and observed for the first time that SRIF-affected individuals have less evidence of ILA with advancing age and with cessation of smoking, which suggests that the putative radiologic correlate of SRIF and/or associated RB regresses upon cessation of smoking and with the passage of time. The observation remains significant after removal of a SRIF cohort that was biased towards the presence of ILA (Citation14).
We observed SRIF prospectively in the background lung parenchyma of 40% of lungs from smokers with lung cancer (mainly adenocarcinoma). This is comparable to other recently reported studies of SRIF in lung cancer (Citation1,3). SRIF appearances have been described before outside of the lung cancer setting and the present data add to those observations, by finding SRIF in explanted lung for emphysema secondary to COPD and A1AT, as well as focal SRIF areas in histological samples of CPFE and IPF smokers. Interestingly, SRIF was not observed in young smokers, and this could relate to young age with shorter exposure to noxious agents. The lower prevalence of RB in the transplant cohorts (versus cancer cohort) might intuitively be related to fewer active smokers in the transplant cohorts. It is also possible, based on the current data, that RB is not required for the existence of SRIF appearances, in contrast with the description of others (Citation1,14) and conflicting with a recent editorial in which it was proposed that the histological pattern of SRIF should be renamed as “respiratory bronchiolitis with fibrosis” (RBF) (Citation16).
SRIF was upper lobe-predominant in the current study, as previously seen (Citation1), but in the current benign explanted series, all lobes were affected, possibly explained by the predominance of upper lobe cancers among smokers (Citation17). Various histological nomenclature has been applied to SRIF including “respiratory bronchiolitis-associated ILD with fibrosis,” “airspace enlargement with fibrosis,” and “clinically occult fibrosis” (Citation14,18,19). While SRIF seems to be common in smokers, clinical symptoms and radiologic findings are uncommon (Citation1,14,18). In this study, we have shown that the occurrence of histological SRIF is proportionate to the burden of smoking and age but is radiologically occult in our older cohort. In addition, we saw some SRIF associated with quantitatively low emphysema, which interestingly is consistent with previous reports (Citation5,20). In the present dataset, SRIF was significantly associated with current smoker status. A concordant direction of effect for current smoking status versus SRIF status is observed in another published SRIF cohort, which was not statistically significant (Citation1). In another published SRIF cohort with benign lung biospecimens, 8 of the 9 subjects were stated to be current smokers (Citation14).
The pooled data of the present and former radiologic-pathologic correlation studies of SRIF show evidence for radiologic SRIF regression over time with smoking cessation. Potential explanations for the discrepancy in ILD observed by Yousem (all had radiologic ILA) versus the current study include that study's selection bias for radiologic ILD and their subjects’ significantly younger age (Citation14). As previously observed (Citation1), over our median follow-up of 50.3 months, no SRIF case progressed to interstitial lung disease. RB-ILD is known to be capable of regression over time with smoking cessation, and the present data may merely reflect radiologic RB regression in what has been called radiologic SRIF (Citation21). Though less intuitive, in support of potential regression of pathologic SRIF with time is the novel observation that RB is not required for the observation of SRIF, such that at least some previously reported ILA in younger SRIF could be due to SRIF itself and not associated RB, but longitudinal studies would best address the issue of SRIF regression. In support of the current study's finding in an older, lung cancer population of less prevalent ILA in those with SRIF/emphysema (all SRIF subjects had at least mild emphysema) is a very large study performed in similarly older current/former smokers (none of whom had lung cancer) in whom high-resolution lung CT scans were obtained, showing less prevalent ILA in those with emphysema (and potentially therefore some with SRIF) compared to those without emphysema (and in whom SRIF would therefore be less likely to be present) (Citation20).
Limitations to the current study include the heterogeneous data sources for different subject groups including different types of surgical lung resection, the more limited availability of clinical/radiologic/physiologic data from the retrospective cohorts versus the prospective cohort, and the potential for lead-time/selection biases in the interpretation of subject age versus ILA in SRIF subjects in the absence of more long-term prospective enrolment and follow-up. The current data cannot definitively address an alternative hypothesis more relevant to the younger Yousem et al cohort, that in non-malignant settings of SRIF, age has no relationship to ILA, notwithstanding the supportive data alluded to in the preceding paragraph.
Conclusions
The present study shows that in benign diseased lung of smokers, SRIF is frequently present, with significantly less co-existent RB versus malignant cases, and for the first time a prospective radiologic–pathologic correlation study of SRIF shows an absence of radiologic ILA in the older SRIF cohort described herein, suggestive of radiologic RB+/−SRIF regression in older subjects who are ex-smokers. The current evidence lends further support to the prevailing impression that SRIF is a relatively benign albeit common condition. Future work should explore the molecular biologic basis of the potential fibrosis regression in SRIF as a means of gaining insight into molecular pathways of pulmonary fibrosis regression, in the hunt for druggable targets in smoking-related ILD states such as IPF.
Abbreviation
| A1AT | = | alpha 1-antitrypsin deficiency |
| COPD | = | chronic obstructive pulmonary disease |
| CPFE | = | combined pulmonary fibrosis and emphysema |
| CT | = | computerised tomography |
| HRCT | = | high resolution computerised tomography |
| ILA | = | radiologic interstitial lung abnormalities |
| IPF | = | idiopathic pulmonary fibrosis |
| SRIF | = | Smoking-related interstitial fibrosis |
Declaration of interest
The authors have no conflict of interest in relation to this study.
References
- Katzenstein AL, Mukhopadhyay S, Zanardi C, Dexter E. Clinically occult interstitial fibrosis in smokers: classification and significance of a surprisingly common finding in lobectomy specimens. Hum Pathol. 2010;41(3):316–325. doi: 10.1016/j.humpath.2009.09.003. PubMed PMID: 20004953.
- Jankowich MD, Rounds SI. Combined pulmonary fibrosis and emphysema syndrome: a review. Chest. 2012;141(1):222–231. doi: 10.1378/chest.11-1062.
- Primiani A, Dias-Santagata D, Iafrate AJ, Kradin RL. Pulmonary adenocarcinoma mutation profile in smokers with smoking-related interstitial fibrosis. Int J Chron Obstruct Pulmon Dis. 2014;9:525–531. doi: 10.2147/COPD.S61932.
- Wright JL, Tazelaar HD, Churg A. Fibrosis with emphysema. Histopathology. 2011;58(4):517–524. doi: 10.1111/j.1365-2559.2010.03648.x.
- Butler MW, Fabre A, Dodd JD. Smokers with interstitial lung abnormalities. N Engl J Med. 2011;364(25):2465; author reply 6. doi: 10.1056/NEJMc1104014#SA1.
- English C, Churg A, Lam S, Bilawich AM. Respiratory bronchiolitis with fibrosis: prevalence and progression. Ann Am Thorac Soc. 2014;11(10):1665–1666. doi: 10.1513/AnnalsATS.201410-474LE.
- Flaherty KR, Fell C, Aubry MC, et al. Smoking-related idiopathic interstitial pneumonia. Eur Respir J. 2014;44(3):594–602. doi: 10.1183/09031936.00166813.
- Johkoh T, Sakai F, Noma S, et al. Honeycombing on CT; its definition, pathologic correlation, and future direction of its diagnosis. Eur J Radiol. 2014;83(1):27–31. doi: 10.1016/j.ejrad.2013.05.012.
- Ando K, Sekiya M, Tobino K, Takahashi K. Relationship between quantitative CT metrics and pulmonary function in combined pulmonary fibrosis and emphysema. Lung. 2013;191(6):585–591. doi: 10.1007/s00408-013-9513-1.
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697–722. doi: 10.1148/radiol.2462070712.
- Litmanovich D, Boiselle PM, Bankier AA. CT of pulmonary emphysema – current status, challenges, and future directions. Eur Radiol. 2009;19(3):537–551. doi: 10.1007/s00330-008-1186-4.
- Mitchell PD, Das JP, Murphy DJ, et al. Idiopathic pulmonary fibrosis with emphysema: evidence of synergy among emphysema and idiopathic pulmonary fibrosis in smokers. Respir Care. 2015;60(2):259–268. doi: 10.4187/respcare.03389.
- American Thoracic S, European Respiratory S. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. doi: 10.1164/ajrccm.165.2.ats01.
- Yousem SA. Respiratory bronchiolitis-associated interstitial lung disease with fibrosis is a lesion distinct from fibrotic nonspecific interstitial pneumonia: a proposal. Mod Pathol. 2006;19(11):1474–1479. doi: 10.1038/modpathol.3800671.
- Reddy TL, Mayo J, Churg A. Respiratory bronchiolitis with fibrosis. High-resolution computed tomography findings and correlation with pathology. Ann Am Thorac Soc. 2013;10(6):590–601. doi: 10.1513/AnnalsATS.201304-088OC.
- Churg A, Hall R, Bilawich A. Respiratory bronchiolitis with fibrosis-interstitial lung disease: a new form of smoking-induced interstitial lung disease. Arch Pathol Lab Med. 2015;139(4):437–440. doi: 10.5858/arpa.2014-0248-ED.
- Byers TE, Vena JE, Rzepka TF. Predilection of lung cancer for the upper lobes: an epidemiologic inquiry. J Natl Cancer Inst. 1984;72(6):1271–1275.
- Fraig M, Shreesha U, Savici D, Katzenstein AL. Respiratory bronchiolitis: a clinicopathologic study in current smokers, ex-smokers, and never-smokers. Am J Surg Pathol. 2002;26(5):647–653.
- Kawabata Y, Hoshi E, Murai K, et al. Smoking-related changes in the background lung of specimens resected for lung cancer: a semiquantitative study with correlation to postoperative course. Histopathology. 2008;53(6):707–714.
- Washko GR, Hunninghake GM, Fernandez IE, et al. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364(10):897–906. doi: 10.1056/NEJMoa1007285.
- Wells AU, Nicholson AG, Hansell DM, du Bois RM. Respiratory bronchiolitis-associated interstitial lung disease. Semin Respir Crit Care Med. 2003;24(5):585–594. doi: 10.1055/s-2004-815606.

