ABSTRACT
The interactions between obesity and chronic obstructive pulmonary disease (COPD) are being increasingly explored. In part, this is due to the globally increasing prevalence rates of obesity. The prevalence of obesity in COPD patients is variable, and it seems that obesity is more common in COPD patients compared with subjects who do not have COPD. However, further studies are encouraged in this area due to observed inconsistencies in the current data. In this review, we focus on the knowledge of the effects of obesity on dyspnea, pulmonary function, exercise capacity and exacerbation risk. Reduction of dyspnea is one of the main therapy targets in COPD care. There is still no consensus as to whether obesity has a negative or even a positive effect on dyspnea in COPD patients. It is hypothesized that obese COPD patients might benefit from favourable respiratory mechanics (less lung hyperinflation). However, despite less hyperinflation, obesity seems to have a negative influence on exercise capacity measured with weight-bearing tests. This negative influence is not seen with weight-supported exercise such as cycling. With respect to severe exacerbations, obesity seems to be associated with better survival.
In summary, it is concluded that due to differences in study methodology and cohort selection, there are still too many knowledge gaps to develop guidelines for clinical practice. Further exploration is needed to get conclusive answers.
Introduction
Chronic obstructive pulmonary disease (COPD) and obesity are two major health problems. According to the World Health Organization (WHO), the prevalence of obesity (body mass index (BMI) ≥30 kg/m2) has doubled since 1980, reaching 600 billion in 2014 (Citation1). The prevalence of obesity among COPD patients is variable (Citation2); however, obesity seems to be more common in global initiative for chronic obstructive lung disease (GOLD) stages I–II and less prevalent in GOLD IV (Citation3). While the prevalence of both conditions are projected to increase in the near future (Citation4,5), clear guidelines on a clinical approach to obese COPD patients are lacking.
Combining obesity with COPD leads to an interesting paradox. While on one hand, obesity seems to be associated with increased morbidity (Citation6), overweight and obese COPD patients tend to have lower mortality rates compared to their normal weight counterparts (Citation7). Some physiologic and metabolic explanations may contribute to this phenomenon, but the exact mechanism remains unclear (Citation8). With emerging data, more insight is gained regarding the complex interaction between these conditions. However, there are important knowledge gaps and unrevealed issues (Citation9,10), which limits our understanding. Currently, these gaps prevent conclusive guidelines for daily practice regarding the management of obese COPD patients (Citation11).
The aim of this paper is to give a brief overview of the prevalence of obesity in COPD and its consequences on certain clinically important domains. The differences between COPD patients with obesity and those with normal weight concerning dyspnea, main pulmonary function parameters, exercise capacity and exacerbation risk will be reviewed. Limitations of available data regarding these issues will be outlined and knowledge gaps that need further exploration will be discussed.
Methods
For the purpose of this narrative review, we carried out a search in PubMed covering all papers published until November 2016. The following search terms were used:
For articles covering COPD:
| 1. | (“Lung Diseases, Obstructive”[Mesh:NoExp] OR “Pulmonary Disease, Chronic Obstructive”[Mesh] OR “Pulmonary Emphysema”[Mesh] OR COPD[tiab] OR Chronic Obstructive Pulmonary Disease[tiab] OR Chronic Obstructive Lung Disease[tiab] OR Chronic Airflow Obstructions[tiab] OR Chronic Airflow Obstruction[tiab]) | ||||
For articles covering obesity:
| 2. | (“Obesity”[Mesh:NoExp] OR “Obesity, Morbid”[Mesh] OR “Obesity, Abdominal”[Mesh] OR “Body Weight Changes”[Mesh] OR “Overweight”[Mesh:NoExp] OR Obesity[tiab] OR Obese[tiab] OR Overweight[tiab]) | ||||
A total of 1,424 papers in English or Dutch were found with the search terms in PubMed. The titles and abstracts of the papers of the last 10 years were screened by the first author. Then, the articles were read in full text by 2 authors and selected on relevance to the various topics of our overview such as prevalence of obesity in COPD, impact of obesity on dyspnea, pulmonary function and exercise capacity. If for a particular topic only a few studies could be selected, then papers published more than 10 years ago were also screened and included. Finally, the selected papers were evaluated in detail and discussed with the other co-authors.
Is obesity more or less common in COPD?
The prevalence of obesity in COPD has been studied in several countries (Citation12). Only two studies included both COPD and non-COPD subjects and could therefore directly compare the prevalence rates of obesity between COPD and non-COPD subjects (Citation13,14). Vozoris et al. used self-reported data of 95,707 individuals participating in the Canadian National Health Survey to compare the prevalence of obesity among COPD (N = 3,470) and non-COPD subjects (Citation14). Obesity was significantly more prevalent in COPD compared to non-COPD (24.6% vs. 17.1%, respectively, p < 0.0001). In contrast, the other study that compared the prevalence of obesity both in COPD and non-COPD subjects indicates that obesity is less prevalent in COPD compared to non-COPD (Citation13). In this study, interviews and spirometry were performed in 5,314 individuals (759 COPD patients) in five Latin American cities. The prevalence of obesity in COPD was 23% compared to 31% in non-COPD subjects (p < 0.001). Both studies indicate similar occurrence rates of obesity in COPD (23–25%); however, the studies disagree on whether obesity is less or more common in COPD compared to non-COPD.
Two Dutch studies evaluated the prevalence in a group of only COPD patients (Citation3,15). Steuten et al. (Citation3) studied 317 COPD patients in a primary care population. Pulmonary function, using a handheld spirometer, and anthropometrical measurements were performed. The prevalence of obesity in this population appeared to be 18%. Furthermore, obesity was more common in GOLD stages I and II (16–24%) and least prevalent in GOLD stage IV (6%). In another study conducted in the Netherlands, Vanfleteren recruited 213 clinically stable COPD patients (GOLD II–IV) from a pulmonary rehabilitation program (Citation15). In this single-centre prospective study, 23% of COPD patients were obese. In comparison, the estimated prevalence of obesity among the general adult population in the Netherlands during the time of this study was 12.7% (Citation16). Hence, it appears that obesity was more common in COPD patients than in the general Dutch population although no direct comparison was made.
While earlier mentioned data are consistent in prevalence rates of obesity in COPD (18–25%), there are also data indicating higher prevalence rates. Recently, Lambert et al. analysed data from a multicentre cohort study in the United States (COPDGene) including 3,631 patients with spirometry confirmed COPD. Within this cohort, 35% of patients were classified as obese. Eisner reported an even higher prevalence of obesity in COPD in Northern California (Citation17). The COPD cohort (N = 355) was prospectively analysed; spirometry and anthropometrical measurements were performed. In this study, obesity was highly prevalent (54%) among the COPD patients. Furthermore, the prevalence of obesity was considerably higher in this study compared to the general population of the same state (20–24%). Finally, Koniski et al. reported that 29.6% of 996 COPD patients were obese in a cross-sectional survey from 10 countries in the Middle East region (Citation18). This study did not perform spirometry to confirm diagnosis and relied on self-reported data.
What can we conclude?
In summary, available data regarding the prevalence of obesity in COPD patients are variable, ranging from 18% to 54%. There are only a few studies with a direct comparison of COPD patients and non-COPD subjects in regard to the prevalence rates. These studies disagree on whether obesity is more or less prevalent in COPD compared to non-COPD (Citation13,14). However, on including studies that have made an indirect comparison, it seems that the prevalence of obesity is higher in COPD patients compared to non-COPD subjects. Nevertheless, this issue still needs further exploration.
Future considerations
Some factors might explain the variability in results between the studies. First, there seems to be a link between the degree of airflow limitation and the prevalence of obesity. Obesity seems to be less common in severe COPD (Citation3). Thus, ideally studies should include large cohorts of COPD patients with all levels of airflow obstruction (GOLD I–IV). Second, gender may play a role in the prevalence of obesity. Several studies indicate that obesity is more prevalent in women (Citation13,14,19). It is noteworthy that the proportion of females was higher in the study with the highest prevalence of obesity in COPD patients (Citation17). Third, it is relevant how information is retrieved regarding the diagnosis of COPD and BMI. Studies with a relatively large sample size have included patients with self-reported diagnoses and BMI to define COPD and obesity (Citation14,18). Utilization of self-reported data is a popular methodology in studies; however, it is proven to be less reliable (Citation20–24). Using physician-based diagnosis and pulmonary function tests to confirm the diagnosis is important. Although it is more difficult to execute, especially in larger cohorts, it gives a more reliable view than self-reported data. Finally, genetic and socio-demographic differences play a role when looking at prevalence rates. Future studies need to consider all these aspects in order to provide a realistic view of the occurrence rates of obesity in COPD ().
Table 1. Knowledge gaps and considerations for future studies.
For clinical practice, it is also interesting to know whether there is a causal link between obesity and COPD. To date, our understanding is limited regarding this issue, mainly due to the cross-sectional design of available studies. Therefore, it remains unclear whether COPD is a risk factor for developing obesity due to a sedentary lifestyle or if obesity is a risk factor for developing COPD due to their combined pathophysiology of inflammation. Longitudinal studies are required in the future to provide more insight into this issue.
Do obese COPD patients experience more or less dyspnea?
Dyspnea is one of the predominant complaints of patients with COPD (Citation25). Obesity itself is also associated with dyspnea (Citation26–30). As a consequence, it might be assumed that obese COPD patients experience more dyspnea than COPD patients with a normal weight. However, studies exploring this issue show conflicting results (Citation6,31–37). Some studies indicate that obesity does not affect dyspnea in COPD, whereas others suggest a negative role for obesity. The evidence for both findings will be discussed below.
Obese COPD patients have similar levels of dyspnea compared to COPD patients with a normal weight
Ora et al. conducted a prospective study investigating the relationship between dyspnea and obesity during incremental cycle exercise in COPD patients (Citation37). This study compared 18 obese (mean BMI ± SD, 35 ± 4 kg/m2) and 18 normal weight (mean BMI ± SD, 22 ± 2 kg/m2) patients with COPD. The groups were matched for forced expiratory volume in 1 second (mean FEV1 49% predicted) and diffusing capacity of the lungs for carbon monoxide (mean DLCO, % pred. >70% in both groups). The groups were also well matched for age, smoking history (pack-years) and gender. The Baseline dyspnea index and Borg dyspnea scale were used to assess dyspnea. Obese patients did not experience more dyspnea at rest or during exercise compared to patients with a normal weight. In fact, at certain ventilation levels during cycling, dyspnea intensity on the Borg scale was even lower in the obese group (), though the differences remained non-significant. When comparing the lung volumes, the obese group had significantly less static and dynamic hyperinflation (). The phenomena of less hyperinflation, thus breathing at relatively lower lung volumes, leads to a mechanical advantage in obese patients. This advantage might be partly responsible for observing less dyspnea in obese COPD patients.
Figure 1. Obese (OB) subjects with chronic obstructive pulmonary disease (COPD) (solid squares) had a rightward shifted dyspnea/ventilation (VE) slope in comparison with normal weight (NW) subjects with COPD (open squares). At an iso-VE of 25 L/minutes (vertical line with arrow), dyspnea intensity was 1.2 ± 1.1 versus 2.4 ± 1.6 Borg units in OB versus NW (p < 0.01). Reproduced from Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA and O'Donnell DE. Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am J Respir Crit Care Med. 2009;180(10):964–71. Reprinted with permission of the American Thoracic Society. Copyright © 2017.
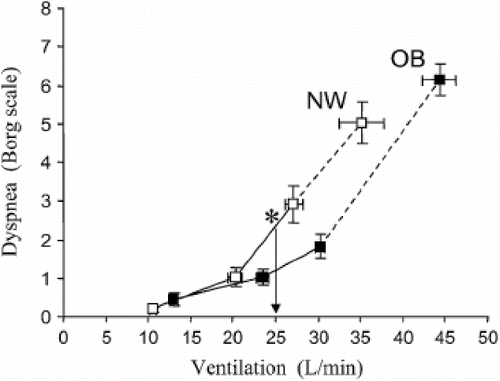
Figure 2. (A) Static lung volumes measured by body plethysmography at rest. Expiratory reserve volume (ERV) and functional residual capacity (FRC) (ERV + RV) were significantly (p < 0.05) lower in the obese (OB) group. (B) Lung volumes are shown from rest to peak exercise in OB patients COPD (closed squares) and in normal weight (NW) patients with COPD (open squares). In the OB compared with the NW group, end-expiratory lung volume (EELV) (standardized as a % of predicted TLC) was consistently lower (*p < 0.01) at rest and throughout exercise; the OB group reached an EELV at peak exercise that was similar to that of the NW group at the pre-exercise resting level. IC = inspiratory capacity; IRV = inspiratory reserve volume; VT = tidal volume (shaded area); RV = residual volume. Values are means ± SE. Reproduced from Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA and O'Donnell DE. Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am J Respir Crit Care Med. 2009;180(10):964–71. Reprinted with permission of the American Thoracic Society. Copyright © 2017.
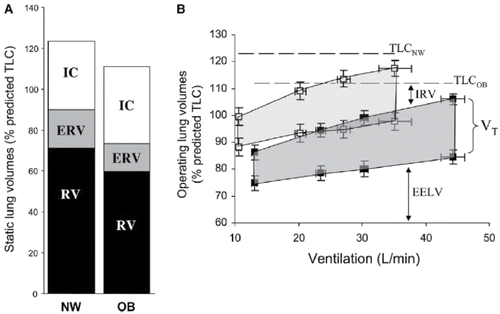
Ora et al. reconfirmed the results in a more recent study, including 12 obese and 12 age- and FEV1-matched COPD patients with a normal weight (mean FEV1 pred. ±60% in both groups) (Citation35). This study was a well-designed prospective study and both groups had comparable DLCO, gender distribution and smoking history. Dyspnea at rest, using the Medical Research Council dyspnea scale (MRC), was comparable in obese and normal weight COPD patients (mean MRC 2.7 and 2.4, respectively). A larger retrospective analysis by Laviolette et al. (Citation36), comparing 64 obese COPD patients with 84 patients with a normal weight, confirmed the results of Ora et al. Ratings of dyspnea (Borg scale) remained similar between the groups at different ventilation levels during exercise. Despite a larger number of subjects, this study had some limitations compared to the studies performed by Ora et al. First, in this study, only male subjects were included. Moreover, the DLCO was significantly better in the obese group, 19.2 ± 6.6 mL/minutes/mmHg (59 ± 27% pred.) compared with the normal weight group 15.4 ± 5.1 mL/minutes/mmHg (46 ± 22% pred.); p = 0.01 (Citation36). This suggests that the obese group may have benefited from less severe emphysema, and thus resulting in relatively low dyspnea scores compared to the group with a normal weight.
Two more recent studies have been conducted, one performed during activities of daily life by Vaes et al. (N = 13 obese; 31 normal weight) (Citation33) and the other during exercise by Rodriguez et al. (N = 108 obese; 143 non-obese) (Citation32). Both studies indicate that obesity does not affect dyspnea in COPD. However, the interpretation of the results is more difficult because the groups were not well matched for important confounders. In the study by Vaes et al., gender distribution in the groups was uneven (obese: 85% male; normal weight: 45%; p < 0.05). Furthermore, relevant information regarding smoking history, DLCO and static lung volumes were lacking. In the study performed by Rodriguez, the obese group had significantly less airflow obstruction (mean FEV1% pred. 58% vs. 49%) and better DLCO (mean % pred. 72% vs. 63%) compared to the non-obese group (Citation32). Furthermore, no real comparison can be made between obese and normal weight patients because the authors divided the patients in two groups: obese and non-obese, with the non-obese group having a mean BMI 26.2 kg/m2 that can be categorized as overweight.
Obese COPD patients experience more dyspnea compared to COPD patients with a normal weight
In contrast to the studies mentioned previously, there are also data indicating more dyspnea in obese COPD patients compared to COPD patients with a normal weight. The most recent and also the largest study discussing this issue was performed by Lambert et al. (Citation6). In this retrospective analysis of a large cohort, including 3,631 COPD patients (normal and overweight: N = 2,383 and obese: N = 1,248), the association between obesity and COPD outcomes was assessed. There were no significant differences in age and smoking history between the groups. Only the mean FEV1% pred. was higher in the obese group (obese: 53% pred. normal and overweight: 49% pred.; p-value for trend <0.001). The odds of having an mMRC ≥2 increased significantly with obesity, to a 4-fold increase in patients having class III obesity (p-value for trend <0.001). Of note, static lung volumes and DLCO were not obtained in this study. Furthermore, the non-obese group included both normal and overweight COPD patients.
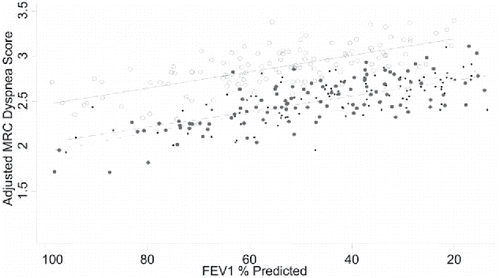
The results from Lambert et al. are supported by earlier reports. Cecere et al. performed data analysis on 364 veterans with COPD and categorized patients by BMI (Citation34). Obese and overweight patients had less airflow obstruction (mean FEV1% pred. obese: 55.4 ± 19.9%; overweight: 50.0 ± 20.4%) than normal weight patients (mean FEV1% pred. 44.2 ± 19.4%; p < 0.001). The authors used the MRC dyspnea scale to measure the intensity of dyspnea in daily life. Despite having less severe airflow obstruction, obese COPD patients reported increased dyspnea. Obese patients were almost 5 times as likely as normal weight COPD patients to experience moderate or severe dyspnea (adjusted OR of MRC score ≥2 = 4.91). Obese COPD patients had significantly higher MRC scores throughout any given FEV1 (). Garcia-Rio et al. also compared dyspnea intensity, but in a smaller group of 97 normal weight, 172 overweight and 113 obese COPD patients (Citation31). Obese patients had more severe dyspnea compared with normal weight patients on the mMRC scale (1.92 vs. 1.49; p < 0.01). However, it must be mentioned that the groups were not matched for lung function or age. Compared with the normal weight group, the obese group consisted of older patients (mean age 66 ± 9 years vs. 61 ± 9 years; p < 0.01). Also, the obese group had a history of significantly more pack-years compared with the normal weight group. Furthermore, FEV1 was slightly lower in the obese group compared with the normal weight group (mean FEV1% pred. 79% vs. 85%).
Figure 3. The adjusted predicted MRC score is given for each subject. For every given severity of airflow obstruction, obese patients are more dyspneic than normal weight patients. Regression lines for each BMI category also shown. (Normal weight: black dots; Overweight: gravy dots; Obese: white dots). Reproduced from Cecere L, Littman A. Obesity and COPD: associated symptoms, health-related quality of life and medication use. COPD 2011;8(4):275–284. Reprinted with permission of Taylor & Francis Ltd, http://www.tandfonline.com. Copyright © 2017.
What can we conclude?
In conclusion, there is no consensus on the role of obesity on dyspnea in COPD patients. Current data suggests two options:
| 1. | Obese patients, contrary to expectation, do not experience more dyspnea. An explanation for this finding could be that obesity may be beneficial regarding dyspnea due to advantages in respiratory mechanics (less hyperinflation). See the section about static lung volumes. | ||||
| 2. | Obese patients experience more dyspnea. An explanation could be that bearing more weight in daily life results in more dyspnea due to the increased work of breathing. | ||||
Future considerations
The exact role of obesity on dyspnea in COPD still needs to be revealed. It has been hypothesized that a lack of significant increase in dyspnea in obese patients compared to patients with a normal weight may be due to the fact that most studies use weight-supported exercise (cycling). This could possibly diminish the negative effects of weight on symptom perception. However, this hypothesis is contradicted by a recent study comparing weight-bearing (walking) and weight-supported (cycle) exercise (Citation38). Despite significant differences in physiological responses between the two test modalities, dyspnea intensity was comparable between walking and cycling at any given power output.
Several aspects need to be considered in future studies. One of the main aspects, making it difficult to compare dyspnea between different weight classes, is the heterogeneity of COPD. A true comparison of the net effect of obesity on dyspnea is only possible when cohorts are matched in pathophysiologic phenotypes. This means that not only the level of airflow obstruction (FEV1) but also the severity of emphysema need to be comparable in cohorts. Ideally, CT scans should be performed as a measure of emphysema, but measuring DLCO is also acceptable in large cohorts. Although in most of the studies presented above, obese and non-obese groups had comparable degrees of airflow obstruction, it remains unclear whether they also had similar degrees of emphysema. The larger studies lack measurements of static lung volumes and DLCO (Citation6,31,34). Studies that have obtained DLCO are usually small (Citation35,37) or compared groups with significantly different DLCO (Citation32,36). The study performed by Ora et al. is one of the few where DLCO was matched in both groups (Citation37). DLCO in the group with a normal weight was 78 ± 28% predicted and in the obese group 72 ± 15% predicted. However, DLCO/total lung capacity (TLC) was higher in obese subjects compared with the normal weight group. It could have been relevant if the authors had compared the DLCO/VA between the two groups as a measure of emphysema (Citation39). It must be mentioned that some studies suggest that obesity itself leads to higher DLCO, probably due to an increase in lung blood volume (Citation10). However, this remains controversial and not all studies support this.
Body composition and adipose tissue distribution should also be taken into account when evaluating the role of obesity on dyspnea. The fat-free mass index (FFMI) seems to be an important determinant of COPD outcomes such as mortality and disease severity (Citation2). Furthermore, FFMI may provide additional information beyond BMI regarding dyspnea and must be considered in future studies assessing the role of weight on dyspnea (Citation40). Thus, other measures of body composition (FFMI, central vs. peripheral obesity) need to be evaluated and, more importantly, be equally distributed when comparing cohorts.
Since several other factors such as age, gender and comorbidities also play a role in symptom perception, it is more difficult to deal with these confounders when a study is not performed in prospection with tools like matching (Citation31). Finally, since dyspnea is a subjective symptom, it is also important to keep in mind that we cannot simply compare different scoring scales with each other interchangeably (Citation41). For example, the Borg scale measures the intensity of dyspnea, while the MRC (or mMRC) gives us insight into the limitations of a patient as a result of dyspnea.
Future prospective studies with larger cohorts need to consider the limitations of available data. Obese and normal weight cohorts need proper randomization, minimizing confounders and distributing different phenotypes of COPD equally between the groups ().
Static lung volumes differ in obese and non-obese COPD patients, but why?
While COPD is characterized by expiratory airflow obstruction, hyperinflation is also often present. Lung hyperinflation in COPD is a result of an increase in lung compliance due to emphysema and effects of expiratory airflow limitation (Citation42). Several definitions for static lung hyperinflation are in use (Citation42–44). However, there is currently no consensus on which to use as a standard. Commonly, hyperinflation is defined by an elevation of the resting functional residual capacity (FRC) above normal (Citation44). Furthermore, an increase of TLC is also considered to be a marker of hyperinflation. However, elevated FRC and residual volume (RV) are also often present in the setting of preserved TLC (Citation42). Several ratios like RV/TLC, FRC/TLC and IC/TLC are also used to define hyperinflation. The IC/TLC ratio appears to be a good predictor for mortality in patients with COPD (Citation42).
While static lung volumes are often altered in COPD, obesity itself appears to affect static lung volumes as well. The most important effect of increasing BMI is a significant reduction in FRC and expiratory reserve volume (ERV) (Citation29,45–48). As a consequence of obesity, intra-abdominal pressure increases, leading to an increased intra-thoracic pressure. This mechanism is exaggerated particularly when subjects are in supine position and results in decreased FRC and ERV (Citation47). Furthermore, obesity leads to reduced lung compliance, which seems to be exponentially related to BMI (Citation49). Reduced lung compliance is demonstrated in other studies as well and may be the result of a combination of factors: increased pulmonary blood volume, closure of dependent airways, leading to small areas of atelectasis or increased alveolar surface tension due to the reduction in FRC (Citation50). Whether obesity also leads to reduced chest wall compliance is less clear due to variable results in studies (Citation50). However, in general, it is presumed that as a consequence of obesity both lung and chest wall compliance are altered (Citation51,52). Despite these negative effects, ventilation seems to remain normally distributed in obesity when FRC values are not extremely altered (FRC % pred. >65%) (Citation53). This might be caused by a putative advantage of an increase in lung elastic recoil correlated with increasing BMI.
Eventually, elevated intra-thoracic pressure and the stiffening of the total respiratory system (combination of reduced lung and chest wall compliance) lead to a reduction in FRC and ERV in obesity. Jenkins and Moxham demonstrated that the FRC and ERV are significantly reduced even in mild obesity (Citation54). The relationship between increasing BMI and decreasing static lung volumes is exponential, thus the dramatic changes in FRC and ERV are flattened out with morbid obesity (Citation45). Furthermore, TLC appears to be reduced only with more severe obesity (Citation45,47).
Although BMI is the most used and easiest way to define weight, some suggest that there are better markers to predict pulmonary function. Ochs-Balcom et al. investigated the correlation between various adiposity and body fat distribution markers with forced vital capacity (FVC) (Citation55). In this large cohort of 2,153 individuals from a general population, abdominal adiposity was a better predictor of pulmonary function FVC than weight or BMI. However, when looking in more detail with MRI, it seems that the topography of adipose tissue is less important than the cumulative effect of increased chest wall fat (Citation48).
Static lung volumes in obese COPD patients
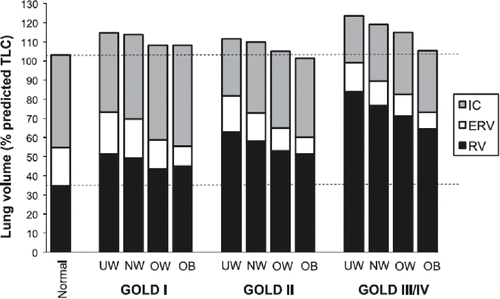
The consequences of increasing BMI have also been documented in the presence of COPD. In a large cohort of 2,265 COPD patients, O'Donnell studied the impact of BMI on static lung volumes (Citation56). This study showed similar effects of increasing BMI on lung volumes as with the previously mentioned data in subjects without COPD. The 654 obese COPD patients showed significantly lower FRC values compared with COPD patients with a normal weight (mean FRC % pred. 124 ± 32 vs. 147 ± 35, respectively; p < 0.05). FRC is a multicomponent value, consisting of ERV and RV. In the data presented by O'Donnell, RV decreased exponentially with increasing BMI. However, ERV contributed the most to the decrement of FRC. Mean ERV % predicted dropped dramatically from 91% in normal weight to 54% in the obese group (p < 0.05). These effects of BMI on static lung volumes were visible across all GOLD stages (). Data from O'Donnell showed that all ratios for lung hyperinflation were in favour of obesity in COPD patients. For example, increasing BMI resulted in significant and linear increase in the IC/TLC ratio (IC/TLC % in obese: 37 ± 10; in normal weight: 30 ± 8; p < 0.05).
Figure 4. Post-bronchodilator lung volume components are shown divided by GOLD stage and BMI. UW = underweight; NW = normal weight; OW = overweight; OB = obese; RV = residual volume; ERV = expiratory reserve volume; IC = inspiratory capacity. Reproduced from O'Donnell DE, Deesomchok A, Lam YM, et al. Effects of BMI on static lung volumes in patients with airway obstruction. Chest. 2011;140 (Citation2):461–8. Reprinted with permission of Elsevier. Copyright © 2017.
There are other studies as well showing relatively lower static lung volumes in obese COPD patients compared with normal weight COPD patients (Citation35–37,Citation57). Recently published data by Aiello et al. showed a IC/TLC ratio (%) of 36 ± 8 in obese COPD compared to 28 ± 9 in COPD patients with a normal weight (p < 0.01) (Citation57). Furthermore, the FRC (% pred.) decreased significantly from 161 ± 34% in NW to 137 ± 40% in obese (p < 0.05).
What can we conclude?
In conclusion, static lung volumes of obese COPD patients are altered. Increasing BMI results in relatively less hyperinflation. Since hyperinflation is a marker of mortality and symptoms, increasing BMI seems to give several advantages in COPD patients. In part, this effect on hyperinflation may explain the better survival of obese COPD patients (obesity paradox).
Future considerations
Several questions remain. First, is increasing adipose tissue the cause of less hyperinflation or do other factors like fat free mass index and systemic inflammation contribute to this phenomenon? Second, which group of COPD patients benefits the most from extra weight (emphysema vs. obstructive)? Finally, at what cut-off point do the disadvantages of extra weight counterbalance the advantages of less hyperinflation regarding survival and symptoms? When considering survival, a recent meta-analysis indicates that in COPD the lowest risk for mortality is observed with a BMI of 30 kg/m2 (Citation58). The survival advantage seems to diminish with BMI >32 kg/m2; however, the authors also state that there is limited evidence to demonstrate a relationship between obesity and mortality and therefore further studies are needed to elucidate this relationship. Future studies need to focus on these questions in order to implement tailored treatment strategies for each individual patient ().
Exercise testing: Does a combination of obesity and COPD lead to reduced exercise capacity?
COPD patients report that they are more inactive in daily life compared to healthy individuals (Citation59). This is also confirmed with quantitative measurements of walking time and movement intensity measured with an activity monitor (Citation60). The 6-minute walk-test (6MWT) or a cycle test is widely used to assess exercise capacity of COPD patients in the diagnostic work up. Whether obesity influences the exercise capacity of COPD patients, measured with either 6MWT or cycle test, will be discussed in this section.
There is accumulating data indicating a negative effect of obesity on exercise capacity in the daily life of COPD patients (Citation61). This is also demonstrated by studies that used the 6MWT as a tool to assess exercise capacity. Recently, Maatman et al. performed a retrospective analysis on data from 108 obese COPD patients vs. 108 age- and FEV1-matched COPD patients with a normal weight to assess the effects of obesity on exercise testing (Citation62). In this study, the walking distance during 6MWT differed significantly between obese (398 ± 107 m) and normal weight patients (446 ± 109 m, p < 0.05). The negative association between BMI and lower functional capacity measured by the 6MWT was also demonstrated in several other studies (Citation6,Citation63–66).
Obesity does not only affect the walking distance measured by the 6MWT but is also associated with exercise induced desaturation (EID). This was demonstrated by a study that included 2,050 COPD patients (mean age: 63.3 ± 7.1 years; FEV1% pred.: 48.7 ± 15.7%) (Citation67). EID, defined by a fall of saturation ≤88% during the 6MWT, occurred in 21% of the patients. One of the determinants of EID was a BMI ≥30 kg/m2 (adjusted OR: 1.57; 95% CI: 1.15–2.14). Moreover, pre-walking saturation ≤93%, emphysema on CT scan and FEV1 ≤44% predicted were stronger determinants of EID.
While the above-mentioned studies clearly indicate that obesity has a negative impact on exercise capacity evaluated with 6MWT, it is also interesting that this finding does not seem to apply for weight-supported tests (cycling). In a study performed by Ora et al., obese COPD patients did not experience greater exercise limitation during cycle exercise compared to normal weight COPD patients with similar FEV1 (both groups 49% pred.) (Citation37). This finding is supported by more recent studies comparing the 6MWT with cycling tests () (Citation62,64). Obesity leads to several physiological changes during exercise that can contribute to reduced exercise capacity. Among these changes is an increased metabolic demand of moving increased weight and the increased work of breathing due to extra weight on the chest wall (Citation68). These changes may be more exaggerated by weight-bearing exercise, thus resulting in worse performance in these tests compared with weight-supported tests.
Figure 5. Main outcomes of cycle ergometry and 6-minute walk-test (6MWD) in normal weight and obese chronic obstructive pulmonary disease (COPD) patients. Reproduced from Maatman RC, Spruit MA, van Melick PP, et al. Effects of obesity on weight-bearing versus weight-supported exercise testing in patients with COPD. Respirology. 2016; 21(3):483–8. Reprinted with permission of John Wiley and Sons. Copyright © 2017.
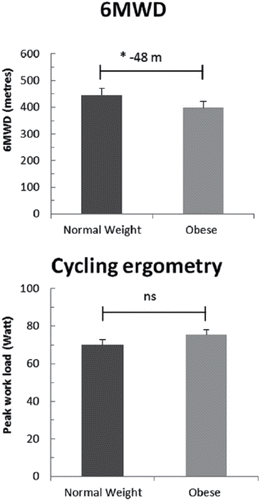
What can we conclude?
In summary, despite a beneficial effect on pulmonary function (less hyperinflation), obesity seems to have a negative influence on exercise capacity in COPD, resulting in less walking distance and EID while walking. This is exposed in studies measuring exercise capacity with weight-bearing tests (activity monitors and the 6MWT). Obesity does not seem to influence outcomes on weight-supported exercise such as cycling.
Future considerations
These results give an interesting insight into the effects of obesity and COPD on functional capacity, but also raise some questions that need to be explored. The 6MWT is a simple test to perform in daily practice and is widely used to assess exercise capacity, but should this test be also applied on obese COPD patients when we only want to measure the effects of COPD on functional status? How can the decreased walking distance by obese patients be interpreted when we evaluate prognosis and disease progression, especially considering the beneficial role of obesity on mortality? Furthermore, since exercise is one of the cornerstones in the non-pharmacologic management of COPD, should obese COPD patients be advised to use different exercise modalities (more weight-supporting exercise)? These questions need to be addressed in translational studies that give guidance for daily practice.
COPD exacerbations: Is obesity a risk or protective factor?
In a large retrospective patient chart review of 313,233 patients admitted to the hospital with a COPD exacerbation in Spain, the association of weight with both mortality and the risk of re-admission within 30 days was assessed (Citation69). Compared to patients with a normal weight, obese patients showed a lower in-hospital mortality risk (OR 0.52; 95% CI 0.49–0.55) and lower early re-admittance risk for the same diagnosis (COPD) (OR 0.87; 95% CI 0.85–0.92). Compared to normal weight patients, the risk of re-admission after discharge was 13% lower in obese patients and 29% higher if malnutrition was documented in the medical chart. Unfortunately, the diagnoses were all based on ICD-9 coding; therefore, weight, height and thus BMI could not be given. Higher BMI, particularly overweight to mild obesity, also proved to be protective for survival in a retrospective study with a median follow-up of 3.26 years after hospitalization due to acute exacerbation of COPD (Citation70). Furthermore, obesity was also associated with decreased mortality after a severe exacerbation of COPD (HR 0.76; 95% CI 0.70–0.82) in a large cohort of Veterans Affairs (Citation71).
While the data presented indicate a beneficial role for obesity regarding survival after COPD exacerbation and a lower risk of 30-day re-admission, it still remains debatable whether obesity affects the risk of exacerbation itself. Several studies indicate that obesity plays neither a beneficial nor a disadvantageous role on exacerbation risk in COPD (Citation18,34). However, interestingly a recent study including 3,631 COPD patients showed increased odds of self-reported severe exacerbation in the past year for obese patients (Citation6). Furthermore, the odds of severe exacerbation increased with increasing obesity class (p = 0.005). The latter finding is supported by Danish data, indicating an association between genetically determined high BMI, defined through the BMI allele score, with an increased risk of recurrent exacerbations (Citation72). Contrary to expectations, a measured high BMI (using weight and height) was not associated with exacerbations of COPD in this study, thus making it difficult to translate these results for clinical practice.
What can we conclude?
Obesity is associated with better survival and a lower risk of early re-admission after hospitalization due to COPD exacerbation in some studies. However, there are also indications that obese COPD patients experience more severe exacerbations. The exact role of obesity on the risk of COPD exacerbation remains disputable.
Future considerations
Our current view is based on variable data from retrospective analyses, self-reported variables, diagnosis based on ICD coding and at best basic spirometry without additional information on static lung volumes or DLCO. Prospective studies with clear definitions for obesity, COPD and exacerbation with proper follow-up are indispensable in the future in order to enhance our understanding of this issue ().
Conclusions
COPD and obesity are both major health problems. While the global prevalence of obesity is rapidly increasing, prevalence rates of obesity among COPD patients are variable (ranging from 18% to 54%). Obesity seems to be more prevalent in COPD patients compared to those without COPD; however, not all data are consistent with this finding. Study methodology, cohort selection, genetic and socio-demographic differences are confounding factors that contribute to the variability in prevalence rates. Future studies have to focus on minimizing confounders, in order to give a realistic view of the prevalence of obesity in COPD. Furthermore, to date, it is not clear whether COPD plays a role in the development of obesity or vice versa. Longitudinal studies with a long period of follow-up may identify possible causal links in the future.
Dyspnea is one of the main symptoms in COPD; however, there is no consensus on how dyspnea is influenced by obesity in COPD patients. It is hypothesized that obese COPD patients might benefit from favourable respiratory mechanics (less lung hyperinflation) and thus experience relatively less dyspnea. However, data are also emerging indicating worse outcomes, including dyspnea, in obese COPD patients. Several factors, including the heterogeneity of COPD, looking beyond BMI and matching study populations in terms of confounding factors, need to be considered when studying this issue. Future studies with larger cohorts in a prospective setting may lead to definite conclusions regarding this subject.
When comparing pulmonary function, COPD patients tend to have a reduction of FRC (especially the ERV) with increasing BMI. This leads to relatively lower levels of lung hyperinflation and could contribute to better survival (obesity paradox) and less symptoms. Other details, including correlations with other components of obesity (fat distribution, inflammation, etc.), remain unclear.
Data indicate that obese COPD patients have deteriorated functional capacity measured by the 6MWT and activity monitors. Obesity also seems to be one of the determinants of EID during these tests. However, weight-supported testing (cycling) does not seem to be negatively influenced by obesity. These results need further exploration in translational studies that give guidance on how to apply these findings for daily practice in the management of COPD patients in different weight categories.
Finally, obesity seems to be associated with lower mortality and lower risk of early re-admission after hospitalization with COPD exacerbation. However, whether obesity reduces the risk of exacerbations remains unclear.
By reviewing the prevalence of obesity in COPD patients and the impact of obesity on dyspnea, pulmonary function, exercise capacity and COPD exacerbations, we can conclude that there are still important knowledge gaps and unrevealed issues. Despite emerging data, our understanding of the complex interaction between obesity and COPD is still limited. To date, conclusive guidelines for daily practice regarding the management of obese COPD patients are lacking. More research in this area is encouraged in order to enhance our knowledge and develop guidelines for the management of obese COPD patients.
Declaration of interest
This work was supported by an unrestricted grant from GlaxoSmithKline. The funding agency had no involvement in study design, data collection, data analysis, data interpretation or writing of the report.
References
- World Health Organization; Fact sheet obesity and overweight; 2016; accessed on November 2016; Available at: http://www.who.int/mediacentre/factsheets/fs311/en/.
- Rutten EPA, Calverley PM, Casaburi R, Agusti A, Bakke P, Celli B, Coxson HO, Crim C, Lomas DA, Macnee W, et al. Changes in body composition in patients with chronic obstructive pulmonary disease: do they influence patient-related outcomes? Ann Nutr Metab. 2013;63(3):239–47.
- Steuten LMG, Creutzberg EC, Vrijhoef HJM, Wouters EF. COPD as a multicomponent disease: inventory of dyspnoea, underweight, obesity and fat free mass depletion in primary care. Prim Care Respir J. 2006;15(2):84–91.
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet (London, England). 2011;378(9793):815–25.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). From the global strategy for the diagnosis, management and prevention of COPD; 2016: 1–94. http://goldcopd.org/.
- Lambert AA, Putcha N, Drummond MB, Boriek AM, Hanania NA, Kim V, Kinney GL, McDonald MLN, Brigham EP, Wise RA, et al. Obesity is associated with increased morbidity in moderate to severe COPD. Chest. 2017;151(1):68–77.
- Cao C, Wang R, Wang J, Bunjhoo H, Xu Y, Xiong W. Body mass index and mortality in chronic obstructive pulmonary disease: a meta-analysis. PLoS One. 2012;7(8):92–99:e43892.
- Blum A, Simsolo C, Sirchan R, Haiek S. “Obesity paradox” in chronic obstructive pulmonary disease. Isr Med Assoc J. 2011;13(11):672–5.
- Franssen FME, O'Donnell DE, Goossens GH, Blaak EE, Schols AMWJ. Obesity and the lung: 5. Obesity and COPD. Thorax. 2008;63(12):1110–7.
- Donnell DEO, Ciavaglia CE, Neder JA. When obesity and COPD collide: physiological and clinical consequences. Am J Respir Crit Care Med. 2014:1–35.
- McDonald VM, Gibson PG, Scott HA, Baines PJ, Hensley MJ, Pretto JJ, Wood LG. Should we treat obesity in COPD? The effects of diet and resistance exercise training. Respirology. 2016;21(5):875–82.
- Hanson C, Rutten EP, Wouters EFM, Rennard S. Influence of diet and obesity on COPD development and outcomes. Int J Chron Obstruct Pulmon Dis. 2014;9:723–33.
- Montes de Oca M, Tálamo C, Perez-Padilla R, Jardim JRB, Muiño A, Lopez MV, Valdivia G, Pertuzé J, Moreno D, Halbert RJ, et al. Chronic obstructive pulmonary disease and body mass index in five Latin America cities: the PLATINO study. Respir Med. 2008;102(5):642–50.
- Vozoris NT, O'Donnell DE. Prevalence, risk factors, activity limitation and health care utilization of an obese, population-based sample with chronic obstructive pulmonary disease. Can Respir J. 2012;19(3):e18–e24.
- Vanfleteren LEGW, Spruit MA, Groenen M, Gaffron S, Van Empel VPM, Bruijnzeel PLB, Rutten EPA, Roodt JOT, Wouters EMF, Franssen FME. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–35.
- Brink CL van den. How many people are overweight? In: Volksgezond Toekomst Verkenning, Natl Kompas Volksgezond. Bilthoven: RIVM; 2016.
- Eisner MD, Blanc PD, Sidney S, Yelin EH, Lathon PV, Katz PP, Tolstykh I, Ackerson L, Iribarren C. Body composition and functional limitation in COPD. Respir Res. 2007;8:7.
- Koniski ML, Salhi H, Lahlou A, Rashid N, El Hasnaoui A. Distribution of body mass index among subjects with COPD in the Middle East and North Africa region: data from the BREATHE study. Int J Chron Obstruct Pulmon Dis. 2015;10(1):1685–94.
- Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–61.
- Jones RCM, Dickson-Spillmann M, Mather MJC, Marks D, Shackell BS. Accuracy of diagnostic registers and management of chronic obstructive pulmonary disease: the Devon primary care audit. Respir Res. 2008;9:62.
- Walters JA, Haydn Walters E, Nelson M, Robinson A, Scott J, Turner P, Wood-Baker R. Factors associated with misdiagnosis of COPD in primary care. Prim Care Respir J. 2011;20(4):396–402.
- Short ME, Goetzel RZ, Pei X, Tabrizi MJ, Ozminkowski RJ, Gibson TB, Dejoy DM, Wilson MJ. How accurate are self-reports? Analysis of self-reported health care utilization and absence when compared with administrative data. J Occup Environ Med. 2009;51(7):786–96.
- Austin EJ, Deary IJ, Gibson GJ, McGregor MJ, Dent JB. Individual response spread in self-report scales: personality correlations and consequences. Pers Individ Dif. 1998;24(3):421–38.
- Fan X. An exploratory study about inaccuracy and invalidity in adolescent self-report surveys. Field Methods. 2006;18(3):223–44.
- McDonald VM, Higgins I, Simpson JL, Gibson PG. The importance of clinical management problems in older people with COPD and asthma: do patients and physicians agree? Prim Care Respir J. 2011;20(4):389–95.
- Rabec C, de Lucas Ramos P, Veale D. Respiratory complications of obesity. Arch Bronconeumol. 2011;47(5):252–61.
- Wei Y, Wu H. Candidates for bariatric surgery: morbidly obese patients with pulmonary dysfunction. J Obes. 2012;2012:878371.
- Babb TG, Ranasinghe KG, Comeau LA, Semon TL, Schwartz B. Dyspnea on exertion in obese women: association with an increased oxygen cost of breathing. Am J Respir Crit Care Med. 2008;178(2):116–23.
- Zutler M, Singer JP, Omachi TA, Eisner M, Iribarren C, Katz P, Blanc PD. Relationship of obesity with respiratory symptoms and decreased functional capacity in adults without established COPD. Prim Care Respir J. 2012;21(2):194–201.
- Sin DD, Jones RL, Man SFP. Obesity is a risk factor for dyspnea but not for airflow obstruction. Arch Intern Med. 2002;162(13):1477–81.
- García-Rio F, Soriano JB, Miravitlles M, Muñoz L, Duran-Tauleria E, Sánchez G, Sobradillo V, Ancochea J. Impact of obesity on the clinical profile of a population-based sample with chronic obstructive pulmonary disease. PLoS One. 2014;9(8):e105220.
- Rodríguez DA, Garcia-Aymerich J, Valera JL, Sauleda J, Togores B, Galdiz JB, Gea J, Orozco-Levi M, Ferrer A, Gomez FP, et al. Determinants of exercise capacity in obese and non-obese COPD patients. Respir Med. 2014;108(5):745–51.
- Vaes AW, Franssen FME, Meijer K, Cuijpers MWJ, Wouters EFM, Rutten EPA, Spruit MA. Effects of body mass index on task-related oxygen uptake and dyspnea during activities of daily life in COPD. PLoS One. 2012;7(7):e41078.
- Cecere L, Littman A. Obesity and COPD: associated symptoms, health-related quality of life, and medication use. COPD 2011;8(4):275–84.
- Ora J, Laveneziana P, Wadell K, Preston M, Webb KA, O'Donnell DE. Effect of obesity on respiratory mechanics during rest and exercise in COPD. J Appl Physiol. 2011;111(1):10–9.
- Laviolette L, Sava F, O'Donnell DE, Webb KA, Hamilton AL, Kesten S, Maltais F. Effect of obesity on constant workrate exercise in hyperinflated men with COPD. BMC Pulm Med. 2010;10:33.
- Ora J, Laveneziana P, Ofir D, Deesomchok A, Webb KA, O'Donnell DE. Combined effects of obesity and chronic obstructive pulmonary disease on dyspnea and exercise tolerance. Am J Respir Crit Care Med. 2009;180(10):964–71.
- Ciavaglia CE, Guenette JA, Ora J, Webb KA, Neder JA, O'Donnell DE. Does exercise test modality influence dyspnoea perception in obese patients with COPD? Eur Respir J. 2014;43(6):1621–30.
- Laszlo G. Exercise tolerance and body mass in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(9):1210.
- Ischaki E, Papatheodorou G, Gaki E, Papa I, Koulouris N, Loukides S. Body mass and fat-free mass indices in COPD: relation with variables expressing disease severity. Chest. 2007;132(1):164–9.
- Perez T, Burgel PR, Paillasseur JL, Caillaud D, Deslée G, Chanez P, Roche N. Modified Medical Research Council scale vs Baseline Dyspnea Index to evaluate dyspnea in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10(1):1663–72.
- O'Donnell DE, Laveneziana P. Physiology and consequences of lung hyperinflation in COPD. Eur Respir Rev. 2006;15(100):61–7.
- Gibson GJ. Pulmonary hyperinflation a clinical overview. Eur Respir J. 1996;9(12):2640–9.
- Ferguson GT. Why does the lung hyperinflate? Proc Am Thorac Soc. 2006;3(2):176–9.
- Jones RL, Nzekwu MMU. The effects of body mass index on lung volumes. Chest. 2006;130(3):827–33.
- Steele RM, Finucane FM, Griffin SJ, Wareham NJ, Ekelund U. Obesity is associated with altered lung function independently of physical activity and fitness. Obesity (Silver Spring). 2009;17(3):578–84.
- Steier J, Lunt A, Hart N, Polkey MI, Moxham J. Observational study of the effect of obesity on lung volumes. Thorax. 2014;69(8):752–9.
- Babb TG, Wyrick BL, DeLorey DS, Chase PJ, Feng MY. Fat distribution and end-expiratory lung volume in lean and obese men and women. Chest. 2008;134(4):704–11.
- Pelosi P, Croci M, Ravagnan I, Tredici S, Pedoto A, Lissoni A, Gattinoni L. The effects of body mass on lung volumes, respiratory mechanics, and gas exchange during general anesthesia. Anesth Analg. 1998;87(3):654–60.
- Salome CM, King GG, Berend N. Physiology of obesity and effects on lung function. J Appl Physiol. 2010;108(1):206–11.
- Naimark A, Cherniack RM. Compliance of the respiratory system and its components in health and obesity. J Appl Physiol. 1960;15:377–82.
- Pelosi P, Croci M, Ravagnan I, Vicardi P, Gattinoni L. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest. 1996;109(1):144–51.
- Pellegrino R, Gobbi A, Antonelli A, Torchio R, Gulotta C, Pellegrino GM, Dellacà R, Hyatt RE, Brusasco V. Ventilation heterogeneity in obesity. J Appl Physiol. 2014;116:1175–81.
- Jenkins SC, Moxham J. The effects of mild obesity on lung function. Respir Med. 1991;85(4):309–11.
- Ochs-Balcom HM, Grant BJB, Muti P, Sempos CT, Freudenheim JL, Trevisan M, Cassano PA, Iacoviello L, Schünemann HJ. Pulmonary function and abdominal adiposity in the general population. Chest. 2006;129(4):853–62.
- O'Donnell DE, Deesomchok A, Lam YM, Guenette JA, Amornputtisathaporn N, Forkert L, Webb KA. Effects of BMI on static lung volumes in patients with airway obstruction. Chest. 2011;140(2):461–8.
- Aiello M, Teopompi E, Tzani P, Ramponi S, Gioia MR, Marangio E, Chetta A. Maximal exercise in obese patients with COPD: the role of fat free mass. BMC Pulm Med. 2014;14(1):96.
- Guo Y, Zhang T, Wang Z, Yu F, Xu Q, Guo W, Wu C, He J. Body mass index and mortality in chronic obstructive pulmonary disease. Medicine (Baltimore). 2016;95(28):e4225.
- Andersson M, Stridsman C, Rönmark E, Lindberg A, Emtner M. Physical activity and fatigue in chronic obstructive pulmonary disease–A population based study. Respir Med. 2015;109(8):1048–57.
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–7.
- Sievi NA, Senn O, Brack T, Brutsche MH, Frey M, Irani S, Leuppi JD, Thurnheer R, Franzen D, Kohler M, et al. Impact of comorbidities on physical activity in COPD. Respirology. 2015;20(3):413–8.
- Maatman RC, Spruit MA, van Melick PP, Peeters JPI, Rutten EPA, Vanfleteren LEGW, Wouters EFM, Franssen FME. Effects of obesity on weight-bearing versus weight-supported exercise testing in patients with COPD. Respirology. 2016;21(3):483–8.
- Ramachandran K, McCusker C, Connors M, Zuwallack R, Lahiri B. The influence of obesity on pulmonary rehabilitation outcomes in patients with COPD. Chron Respir Dis. 2008;5(4):205–9.
- Sava F, Laviolette L, Bernard S, Breton M-J, Bourbeau J, Maltais F. The impact of obesity on walking and cycling performance and response to pulmonary rehabilitation in COPD. BMC Pulm Med. 2010;10(1):55.
- Bautista J, Ehsan M, Normandin E, Zuwallack R, Lahiri B. Physiologic responses during the six minute walk test in obese and non-obese COPD patients. Respir Med. 2011;105(8):1189–94.
- Joppa P, Tkacova R, Franssen FME, Hanson C, Rennard SI, Silverman EK, McDonald MLN, Calverley PMA, Tal-Singer R, Spruit MA, et al. Sarcopenic obesity, functional outcomes, and systemic inflammation in patients with chronic obstructive pulmonary disease. J Am Med Dir Assoc. 2016;17(8):712–8.
- Andrianopoulos V, Celli BR, Franssen FME, Pinto-Plata VM, Calverley PMA, Vanfleteren LEGW, Vogiatzis I, Vestbo J, Agusti A, Bakke PA, et al. Determinants of exercise-induced oxygen desaturation including pulmonary emphysema in COPD: results from the ECLIPSE study. Respir Med. 2016;119:87–95.
- Babb TG. Obesity: challenges to ventilatory control during exercise–a brief review. Respir Physiol Neurobiol. 2013;189(2):364–70.
- Zapatero A, Barba R, Ruiz J, Losa JE, Plaza S, Canora J, Marco J. Malnutrition and obesity: influence in mortality and readmissions in chronic obstructive pulmonary disease patients. J Hum Nutr Diet. 2013;26 Suppl 1:16–22.
- Lainscak M, von Haehling S, Doehner W, Sarc I, Jeric T, Ziherl K, Kosnik M, Anker SD, Suskovic S. Body mass index and prognosis in patients hospitalized with acute exacerbation of chronic obstructive pulmonary disease. J Cachexia Sarcopenia Muscle. 2011;2(2):81–6.
- McGhan R, Radcliff T, Fish R, Sutherland ER, Welsh C, Make B. Predictors of rehospitalization and death after a severe exacerbation of COPD. Chest. 2007;132(6):1748–55.
- Çolak Y, Afzal S, Lange P, Nordestgaard BG. High body mass index and risk of exacerbations and pneumonias in individuals with chronic obstructive pulmonary disease: observational and genetic risk estimates from the Copenhagen General Population Study. Int J Epidemiol. 2016;45(5):1551–9.
