ABSTRACT
Human regulatory T cells (Tregs) have been reported to be not significantly different in the peripheral blood of patients with chronic obstructive pulmonary disease (COPD) and healthy controls. Recent research has identified some new markers for Tregs and indicated that Tregs are composed of distinct subpopulations. The aim of the study was to describe the changing patterns of circulating Treg subpopulations in patients with acute exacerbation of COPD (AECOPD) and healthy controls, and to explore their potential roles in AECOPD pathogenesis. Blood samples were obtained from 30 never-smokers with normal lung function and 30 patients with COPD before and after they had an exacerbation. The proportions of Treg subpopulations were evaluated using flow cytometry. In the peripheral blood, decreased proportions of CD4+CD25+CD127low Tregs, CD4+CD25+CD45RA+ Tregs, and CD4+CD25+CD62L+ Tregs and an increased proportion of CD4+CD25+CD45RO+ Tregs were found in patients with stable COPD compared with non-smokers with normal lung function. The patients showed further changes in Treg subpopulations when they had an AECOPD, with an overall decrease in a suppressive subset, indicating that the immune negative regulatory population of Tregs did not play an effective role. Immune homeostasis favored inflammation, and a negative correlation between the circulating tumor necrosis factor-alpha and the proportions of CD4+CD25+CD62L+ cells (r = −0.698, p < 0.05) in patients with AECOPD was found. The imbalance between the suppressive subsets and the proinflammatory subset of Tregs and the decline of Treg subpopulations with immunosuppressive activity may play important roles in AECOPD progression.
Introduction
Chronic obstructive pulmonary disease (COPD) is a common disease that severely threatens human health. The social and economic burden of COPD will rise to the 5th worldwide, and COPD will become the 3rd leading cause of death worldwide by 2020 Citation(1). The prevention, early detection, and treatment of acute exacerbation of COPD (AECOPD) are clinically significant because of its increasing social burden and negative impact on the quality of life, pulmonary function, and disease progression.
Recently, accumulated evidence has indicated that patients with COPD exhibit many of the characteristics of a classical autoimmune response Citation(2,3). T lymphocytes are believed to be the key cells in regulating airway inflammation in COPD Citation(4). Human regulatory T cells (Tregs), a critical subset of T cells that use a variety of mechanisms to suppress the immune response, have gained considerable attention Citation(5–9) and Treg abnormalities have been described in adaptive immunity of COPD. In patients with COPD, Treg proportions fluctuated in bronchoalveolar lavage (BAL) or in different types of pulmonary tissues. However, the frequencies of Tregs in the peripheral blood do not differ significantly among patients with COPD, healthy smokers, and never-smokers Citation(10–13). In most previous studies, Tregs have only been considered as a single population. However, the phenotypic and functional heterogeneity of human Tregs is a major obstacle for understanding their clinical relevance. Dissecting Tregs into subsets, especially defining the suppressive population, may contribute to the striking findings of the studies in the adaptive immunity of COPD Citation(14–16).
The aim of this study was to explore whether there were abnormal distributions in the suppressive phenotype of Tregs in the blood of patients with AECOPD. The findings showed their potential roles in AECOPD and provided new insight into the potential mechanisms underlying acute inflammation and adaptive immunity in AECOPD.
Materials and methods
Study subjects
Patients with COPD were recruited in the First Affiliated Hospital of Guangxi Medical University, China. Never-smokers with normal lung function were also recruited as controls. Written informed consent was obtained from all the volunteers. The study was approved by the local Ethics Review Board at Guangxi Medical University, China (number 2014KY-C-008), and performed according to the Declaration of Helsinki. COPD diagnosis was established according to the definition supplied by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) document Citation(17). Patients with COPD had a forced expiratory volume in 1 second (FEV1)/forced vital capacity ratio <70%, and FEV1 was <80% of the predicted value post bronchodilators. All patients with COPD were clinically stable and had not experienced any exacerbations for ≥3 months preceding inclusion in the study. Participants with neoplasm, interstitial lung disease, autoimmune disease, cardiovascular disease, cerebrovascular disease, diabetes, allergic disease, infection, or other immune-related diseases were excluded. Patients receiving systematic corticosteroids were excluded.
We aimed to explore whether there were abnormal distributions in the subpopulations of Tregs in the blood of COPD patients in exacerbated and stable phases, and whether the frequency of different Treg subsets was correlated with disease severity as defined by FEV1, the inflammatory agent in the blood, oxygen pressure (PO2), and partial pressure of carbon dioxide (PCO2).
The diagnosis of AECOPD was primarily determined by the clinical presentations of the patients complaining of an acute change in symptoms (baseline dyspnea, cough, and/or sputum production) that is beyond normal day-to-day variation Citation(18). Other medical conditions such as pneumonia, congestive heart failure, pneumothorax, pleural effusion, PE, or arrhythmia were ruled out before a patient was considered to have AECOPD. Acute dyspnea of congestive heart failure can be differentiated by the increase in plasma B-type natriuretic peptides associated with other clinical manifestations Citation(1,18,19). Full lung function including gas transfer at the time of exacerbation was obtained. Subject baseline characteristics are summarized in .
Table 1. Baseline characteristics of all subjects.
Spirometry and lung function
The COPD patients did not receive any treatment with inhaled corticosteroids or oral anti-inflammatory drugs for at least 4 weeks prior to the start of the study, and neither regular long-acting β2-agonists nor long-acting anti-cholinergic drugs were allowed within 2 weeks. Short-acting β2-agonists and/or anti-cholinergic drugs were used on demand. All COPD patients had a post bronchodilator FEV1/FVC of less than 70% that were not reversible. Dynamic spirometry (FVC and FEV1) was performed post-bronchodilatation using a Vitalograph spirometer (Vitalograph Ltd., Buckingham, UK), as outlined previously Citation(20). Pulmonary function tests were completed by patients when they were recruited. COPD was confirmed by using a 5th percentile as a lower limit of normal as estimated by the global lung initiative (GLI) predicting equations Citation(21). The quality of spirometry measurement was ensured by looking at the length of expiration Citation(22).
Laboratory assays
Peripheral venous blood samples of all patients with COPD were collected to carry out routine blood tests, blood gas analysis, and C reactive protein (CRP), fibrinogen, interleukin (IL)-8, tumor necrosis factor-alpha (TNF-α), IL-35, and Treg subset analyses. Peripheral venous blood samples (30 mL) from each patient were collected in heparin-coated anticoagulant tubes. Plasma was separated by centrifugation at 1,800 g for 20 minutes and stored at −80°C. All AEOCD patients' peripheral venous blood samples were collected on the first day after hospitalization. The concentrations of TNF-α, IL-8, and IL-35 in the peripheral venous blood samples were measured using a multiplex ELISA system (Lincoplex Systems, St. Charles, MO, USA). Fibrinogen and CRP were measured in peripheral blood samples using a radioimmunoassay (RIA) as per the manufacturer's instructions (RIA Kits, Beijing North Institute of Biological Technology, China).
Cell collection
Peripheral blood samples from each subject were collected in ethylenediaminetetraacetic acid-treated tubes and were processed to measure peripheral blood mononuclear cells (PBMCs) for flow cytometry setup procedures. Blood samples were layered onto Ficoll-Paque Plus tubes (Amersham Biosciences, Amersham, Bucks, UK) and centrifuged (400 g for 20 minutes at 21°C); PBMCs were then harvested. Cells were washed twice in divalent cation-free Hanks balanced salt solution at 300 g for 5 minutes at 4°C. PBMCs were resuspended, and viable counts were obtained.
Flow cytometry of Treg subsets
Flow cytometry freshly obtained human PBMC samples were stained as previously described Citation(23) with anti-hCD4-FITC (e-Bioscience, San Diego, CA, USA), anti-hCD25-PE-Cy5 (BD Biosciences, San Jose, CA, USA), and anti-hCD45RA-APC (BD Biosciences). Anti-hCD45RO-APC, anti-hCD26L-APC, and anti-hCD127-APC (BD Biosciences) antibodies were used for surface marker staining of each subpopulation. We calculated the ratio of CD127low, CD45RA+, CD45RO+, and CD62L+ Tregs from the peripheral blood.
Statistical analysis
The results are presented as the mean ± SEM. The Shapiro–Wilk test was used to evaluate the distribution of variance. For data distributed normally, comparisons between three groups were analyzed by one-way analysis of variance (ANOVA). The Wilcoxon signed-rank test was used to compare data with a non-normal distribution Citation(24). Correlations were assessed by Spearman's rank correlation coefficient. Statistical analysis was performed using SPSS for Windows V.16.0 (Chicago, IL, USA). Here, a value of p < 0.05 was considered significant in all statistical tests.
Results
Demographic characteristics of the study population
A total of 30 COPD patients and 30 healthy controls were included in the study. The characteristics of the population are summarized in . There was no difference between the groups in terms of age and sex. There were significantly more number of smokers or ex-smokers in the COPD group than the health control group. Lung functions of COPD patients were examined at stable (SCOPD) and AECOPD phases. As expected, the lung function was significantly better in the stable phase than that in the exacerbated phase ().
Varied systemic inflammatory media in AECOPD patients
shows the main clinical characteristics of participants. Compared with the healthy control group, blood gas analysis in the COPD group showed whether AECOPD or SCOPD had statistically significant differences, and PaCO2 values were higher in the AECOPD group than those in the SCOPD group; PaO2 values were lower in AECOPD group than those in the SCOPD group.
Figure 1. Laboratory data for patients with AECOPD and healthy controls. Results are expressed as the (mean ± SD). n = 30; *p < 0.05; **p < 0.001.
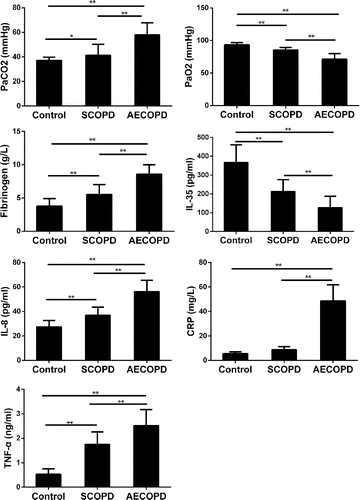
Compared with the healthy control group, IL-8, CRP, fibrinogen, and TNF-α were significantly higher in the AECOPD group. Fibrinogen, IL-8, CRP, and TNF-α in the AECOPD group were higher than those in the SCOPD group, and there was a significant difference.
IL-35, a newly identified inhibitory cytokine, is preferentially expressed by Tregs and is required for their maximum suppressive activity Citation(25). We observed that IL-35 levels were persistently lower in the peripheral blood from COPD patients than the healthy controls.
Varied subpopulation frequencies of circulating Tregs in patients with AECOPD, SCOPD, and healthy controls
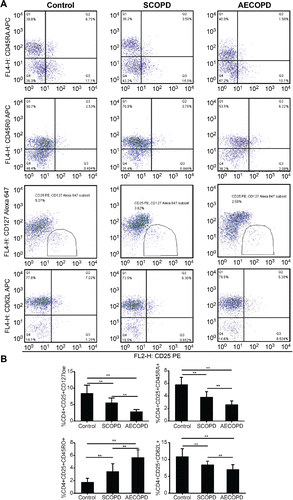
In line with previous studies Citation(10,11), Treg identification using CD4+CD25+Foxp3+ showed no difference in the circulating Treg number in patients with COPD compared with smokers and never-smokers. However, as shown in , patients with SCOPD showed significantly increased proportions of CD4+CD25+CD45RO+ Tregs but decreased proportions of CD4+CD25+CD127low Tregs, CD4+CD25+CD45RA+ Tregs, and CD4+CD25+CD62L+ Tregs. This indicated that the imbalance between immunosuppressive subsets and immunoenhancing subsets in CD4+CD25+ T cells shifted toward immune tolerance in patients with COPD responses. Strikingly, in comparison to the SCOPD group, patients with AECOPD showed the same trend of variation as the proportion of each subset. Varied subpopulation frequencies suggested that the Treg subsets with low immunosuppressive activity and low expression of Foxp3 increased and the Treg subsets with high immunosuppressive activity or/and high expression of Foxp3 decreased in patients with AECOPD responses, which may lead to the development of inflammation and the disorder of acute exacerbation.
Figure 2. Flow cytometry analysis of Treg cell subpopulations in patients with AECOPD. (A) Flow cytometry analysis of CD62L expression in CD4+CD25+ Treg cells. (B) The percentages of each circulating T-regulatory subset among CD4+ T cells in patients with AECOPD, SCOPD, and healthy controls. The results are expressed as the (mean ± SD). n = 30; *p < 0.05; **p < 0.001.
Correlation between the suppressive phenotype of circulating Tregs, systemic inflammatory media, and disease severity
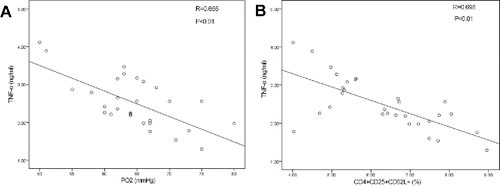
To further explore the role of phenotypes of circulating Tregs in AECOPD, we next analyzed the correlation between suppressive phenotypes of circulating Tregs and systemic inflammatory media; i.e., PaO2, PaCO2, and spirometry. TNF-α was negatively correlated with PaO2 (r = 0.666, p < 0.05) and the proportions of CD4+CD25+CD62L+ cells in patients with AECOPD (r = 0.698, p < 0.05; ).
Figure 3. Correlations between suppressive phenotypes of circulating Tregs in AECOPD patients and systemic inflammatory media. (A) TNF-alpha and the frequency of CD4+CD25+CD62L+ Treg cells. There was a correlation between TNF-alpha and PO2. (B) TNF-alpha and PaO2 in AECOPD patients. There was a correlation between TNF-alpha and CD62. Data were determined by Spearman's rank correlation coefficients.
Discussion
AECOPD is an acute event characterized by worsening of the patient's respiratory symptoms (typically dyspnea, cough, increased sputum volume, and/or sputum purulence) that is beyond normal day-to-day variations and leads to a change in medication Citation(1). On average, each COPD patient suffers from 0.5 to 3.5 episodes of acute exacerbations per year, and AECOPD is a major cause of death with associated large medical expenditures. The prevention, early detection, and treatment of AECOPD are clinically significant because of its increasing social burden and negative impact on quality of life, pulmonary function, and disease progression. Previous studies that identified Tregs as a whole did not find any significant differences in the frequencies of circulating Tregs in patients with COPD. According to an epidemiological study, the cost of AECOPD for an inpatient in the People's Republic of China was 11,598 RMB a year Citation(26). Especially for patients whose hospital stay ends in death, their expenses, which are related to the high cost of life-support, increased remarkably Citation(27). Here, based on this new definition of Treg subsets, we revealed significantly decreased proportions of Treg subpopulations with high immunosuppressive activity and an increased proportion of the Treg subpopulation with low immunosuppressive activity in patients with COPD compared with healthy controls with normal lung function. More importantly, we found an imbalance between these subsets in the inflammatory media in patients with AECOPD. Investigations of these markers in COPD are rare, and to the best of our knowledge, this is the first study addressing CD4+CD25+CD62L+ expression on blood cells from subjects with COPD and AECOPD.
Tregs with a suppressive function play a major role in maintaining self-tolerance. It has been reported that only a minority of human CD4 T cells expressing the highest levels of CD25 (called CD25bright cells) have suppressor activity Citation(28), and in humans, up to 40% of the peripheral-blood CD4 T cells express CD25 to some extent. Therefore, many recent studies have focused on this population Citation(29–32). However, the proportion of cells in the CD25bright population in peripheral blood does not differ Citation(32) significantly among patients with COPD, healthy smokers, and never-smokers Citation(10–13). Recently, some new markers for Tregs have been reported, suggesting that better markers or combinations of markers are, therefore, clearly required to accurately identify Tregs in humans. We screened some markers, in combination with CD4 and CD25, with the aim of finding suppressive activity.
CD127 expression was shown to be inversely associated with FoxP3 and a suppressive function of human CD4+ Tregs in the peripheral blood Citation(23). CD127 expressing cells have been studied in allergic asthma, gastric cancer, and glioma Citation(33–35). As indicated above, CD25bright expression on CD4+ cells usually implies Tregs. The present study showed that a decreased percentage of CD4+CD25+CD127low cells was associated with both COPD patients and those patients who had COPD acute exacerbation (). Although the number of patients in the present study was rather small, the data are consistent with previously published results Citation(20,36). As described previously Citation(37,38), human CD4+Foxp3+ T cells are phenotypically heterogeneous and include the CD45RO+ and CD45RA+ T cell subtypes. CD4+CD25+CD45RA+ Tregs possess a suppressive capacity and continuously express Foxp3 in vitro, and CD4+CD25+CD45RO+ Tregs lack suppressive activity or have limited suppressive activity, but can produce IL-2, IL-17, and interferon (IFN)-γ Citation(14–16,39,40), which are important for T-cell activation and pulmonary inflammation Citation(41,42). Our study showed that an increased percentage of CD4+CD25+CD45RO+ Treg cells and a decreased percentage of CD4+CD25+CD45RA+ Treg cells were associated with both COPD patients and those patients who had COPD acute exacerbation (). One report showed that human memory CD4+CD25− T cells could be efficiently differentiated into Foxp3+ T cells via TCR activation with TGF-β, but this phenomenon was restricted to CD62L+CCR7+ memory CD4+ T cells. Furthermore, the phenomenon demonstrated that such CD4+CD62L+ central memory T cell-derived Foxp3+ T cells were functional in suppressing the proliferation of T effector cells in mice Citation(43). Our study showed that a decreased percentage of the memory CD4+CD25+CD62L+ cells was associated with both COPD patients and those patients who had COPD acute exacerbation.
Based on this new definition of Treg subsets, we revealed significantly decreased proportions of CD4+CD25+CD127low Tregs, CD4+CD25+CD45RA+ Tregs, and CD4+CD25+CD62L+ Tregs and an increased proportion of the CD4+CD25+CD45RO+ Tregs in patients with SCOPD compared with non-smokers with normal lung function. The present data implied that the expression of Treg subsets with suppressive activity in the blood appears to decline, but the expression of Treg subsets that lack suppressive activity in the blood appears to increase in patients with COPD, and those appear to further decline in patients with AECOPD, which suggested that an imbalance between anti-inflammatory and pro-inflammatory subsets may induce prolonged immune activation. More importantly, we found a negative correlation between circulating TNF-α and PaO2 and a stronger negative correlation between the circulating TNF-α and the proportions of CD4+CD25+CD62L+ cells in patients with AECOPD, which further linked the immune imbalance with inflammatory and disease severity in patients with AECOPD. We did not find any correlation between the circulating Treg subsets and airflow limitations or lung function. Therefore, from these findings, we hypothesize that a decrease in the immunosuppressive Treg populations, together with enhanced pro-inflammatory responses, induced AECOPD. This persistent systemic inflammation is believed to be involved in the progressive acute exacerbation characteristic of COPD.
The COPD subjects included in this study were clinically stable with no history of recurrent infectious exacerbations and in no need of regular medications, apart from short-acting bronchodilators on demand. Additionally, our data indicated that there was a negative correlation between the circulating TNF-α and the proportions of CD4+CD25+CD62L+ cells in patients with AECOPD. However, the detailed mechanism of this correlation is still unclear and requires further study. We focused on the characteristics of the frequency of different Treg subsets for AECOPD. We hypothesize that it is related to the intrinsic constraints of the cells and not due to their similarity to the Treg phenotype. A limitation of the study was that we did not investigate the control of response to infection/inflammation by regulating the balance between Th17 and Treg. The balance between Th17 and Treg has been shown to be important for the control of response to inflammation/infection Citation(44). Another limitation of the study was that the types of AECOPD (e.g. viral versus bacterial versus non-infective) were not analyzed separately. In real clinical practice, it was difficult to definitively determine whether an episode of AECOPD was caused by bacteria or virus.
In summary, this study provided further evidence for the role of adaptive immunity in COPD pathogenesis and demonstrated that the imbalance between the subpopulations of Tregs may contribute to the progression of inflammation in patients with AECOPD. Of note, the correlations between the CD4+CD25+CD62L+ cells in the peripheral blood and circulating TNF-α indicated a closer correlation between systemic immune activation and systemic inflammation activation, which might facilitate our understanding of the underlying mechanisms of systemic inflammation and adaptive immunity in AECOPD. The clinical implication of the present study may be that the exacerbation of COPD can be ameliorated by modulating different subpopulations of Tregs to restore immune homeostasis.
Authors' contributions
XY and BH contributed to recruiting the patients, performing all data collection, collecting and processing samples, and writing the manuscript. XZ contributed as primary investigator and was responsible for designing the study and writing the manuscript. WS and WL performed laboratory-based assays. ZH, JD, and JZ recruited the patients and collected clinical data. JB contributed as lead investigator and was responsible for designing the study, analyzing the data, and writing the manuscript. All authors read and approved the final manuscript.
Declaration of interest
None.
Ethical approval
This study is approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University.
Acknowledgments
The authors thank Guorong Liang, Haijuan Tang, ZhixiuRan and Yi Liang (First Affiliated Hospital of Guangxi Medical University) for their support with screening the participating subjects throughout the study.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81260011 and 30860106) and the Guangxi Natural Science Foundation (No. 2013GXNSFAA019136).
References
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–65.
- Bonarius HP, Brandsma CA, Kerstjens HA, Koerts JA, Kerkhof M, Nizankowska-Mogilnicka E, Roozendaal C, Postma DS, Timens W. Antinuclear autoantibodies are more prevalent in COPD in association with low body mass index but not with smoking history. Thorax. 2011;66(2):101–7.
- Feghali-Bostwick CA, Gadgil AS, Otterbein LE, Pilewski JM, Stoner MW, Csizmadia E, Zhang Y, Sciurba FC, Duncan SR. Autoantibodies in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(2):156–63.
- Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax. 2004;59(7):574–80.
- Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17(6):638–42.
- Lan RY, Ansari AA, Lian ZX, Gershwin ME. Regulatory T cells: development, function and role in autoimmunity. Autoimmun Rev. 2005;4(6):351–63.
- Jiang H, Chess L. Regulation of immune responses by T cells. N Engl J Med. 2006;354(11):1166–76.
- Ait-Oufella H, Salomon BL, Potteaux S, Robertson AK, Gourdy P, Zoll J, Merval R, Esposito B, Cohen JL, Fisson S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12(2):178–80.
- Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate co-stimulatory molecules on antigen-presenting cells. Eur J Immunol. 2000;30(6):1538–43.
- Smyth LJ, Starkey C, Vestbo J, Singh D. CD4-regulatory cells in COPD patients. Chest. 2007;132(1):156–63.
- Barcelo B, Pons J, Ferrer JM, Sauleda J, Fuster A, Agusti AG. Phenotypic characterisation of T-lymphocytes in COPD: abnormal CD4+CD25+ regulatory T-lymphocyte response to tobacco smoking. Eur Respir J. 2008;31(3):555–62.
- Baraldo S, Saetta M. To reg or not to reg: that is the question in COPD. Eur Respir J. 2008;31(3):486–8.
- Isajevs S, Taivans I, Strazda G, Kopeika U, Bukovskis M, Gordjusina V, Kratovska A. Decreased FOXP3 expression in small airways of smokers with COPD. Eur Respir J. 2009;33(1):61–7.
- Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911.
- Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106(12):4793–8.
- Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, Valmori D. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci U S A. 2009;106(21):8635–40.
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 Report: GOLD executive summary. Eur Respir J. 2017;49:1700214.
- Cai BQ, Cai SX, Chen RC, Cui LY, Feng YL, Gu YT, Huang SG, Liu RY, Liu GN, Shi HZ, et al. Expert consensus on acute exacerbation of chronic obstructive pulmonary disease in the People's Republic of China. Int J Chron Obstruct Pulmon Dis. 2014;9:381–95.
- Bertoletti L, Quenet S, Mismetti P, Hernandez L, Martin-Villasclaras JJ, Tolosa C, Valdes M, Barron M, Todoli JA, Monreal M. Clinical presentation and outcome of venous thromboembolism in COPD. Eur Respir J. 2012;39(4):862–8.
- Roos-Engstrand E, Ekstrand-Hammarstrom B, Pourazar J, Behndig AF, Bucht A, Blomberg A. Influence of smoking cessation on airway T lymphocyte subsets in COPD. COPD. 2009;6(2):112–20.
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, et al. Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40(6):1324–43.
- Miller MR. Defining airflow obstruction. Eur Respir J. 2015;45(2):560.
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–11.
- Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med. 2016;4(5):91.
- Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450(7169):566–9.
- Chen YH, Yao WZ, Cai BQ, Wang H, Deng XM, Gao HL, Huang JS, Wang XM. Economic analysis in admitted patients with acute exacerbation of chronic obstructive pulmonary disease. Chin Med J (Engl). 2008;121(7):587–91.
- Zhu ML, Cai BQ. Costs of the last hospitalization for patients with acute exacerbation of chronic obstructive pulmonary disease and patients with lung cancer. Zhonghua Jie He He Hu Xi Za Zhi. 2009;32(4):258–61.
- Baecher-Allan C, Brown JA, Freeman GJ, Hafler DA. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167(3):1245–53.
- Sullivan KE, McDonald-McGinn D, Zackai EH. CD4(+) CD25(+) T-cell production in healthy humans and in patients with thymic hypoplasia. Clin Diagn Lab Immunol. 2002;9(5):1129–31.
- Sanchez J, Casano J, Alvarez MA, Roman-Gomez J, Martin C, Martinez F, Gomez P, Serrano J, Herrera C, Torres A. Kinetic of regulatory CD25high and activated CD134+ (OX40) T lymphocytes during acute and chronic graft-versus-host disease after allogeneic bone marrow transplantation. Br J Haematol. 2004;126(5):697–703.
- Makita S, Kanai T, Oshima S, Uraushihara K, Totsuka T, Sawada T, Nakamura T, Koganei K, Fukushima T, Watanabe M. CD4+CD25bright T cells in human intestinal lamina propria as regulatory cells. J Immunol. 2004;173(5):3119–30.
- Cao D, van Vollenhoven R, Klareskog L, Trollmo C, Malmstrom V. CD25brightCD4+ regulatory T cells are enriched in inflamed joints of patients with chronic rheumatic disease. Arthritis Res Ther. 2004;6(4):R335–46.
- Shen LS, Wang J, Shen DF, Yuan XL, Dong P, Li MX, Xue J, Zhang FM, Ge HL, Xu D. CD4(+)CD25(+)CD127(low/–) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin Immunol. 2009;131(1):109–18.
- Ardon H, Verbinnen B, Maes W, Beez T, Van Gool S, De Vleeschouwer S. Technical advancement in regulatory T cell isolation and characterization using CD127 expression in patients with malignant glioma treated with autologous dendritic cell vaccination. J Immunol Methods. 2010;352(1–2):169–73.
- Nguyen KD, Fohner A, Booker JD, Dong C, Krensky AM, Nadeau KC. XCL1 enhances regulatory activities of CD4+CD25(high) CD127(low/–) T cells in human allergic asthma. J Immunol. 2008;181(8):5386–95.
- Roos-Engstrand E, Pourazar J, Behndig AF, Bucht A, Blomberg A. Expansion of CD4+CD25+ helper T cells without regulatory function in smoking and COPD. Respir Res. 2011;12:74.
- Wing K, Ekmark A, Karlsson H, Rudin A, Suri-Payer E. Characterization of human CD25+CD4+ T cells in thymus, cord and adult blood. Immunology. 2002;106(2):190–9.
- Seddiki N, Santner-Nanan B, Tangye SG, Alexander SI, Solomon M, Lee S, Nanan R, Fazekas de Saint Groth B. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006;107(7):2830–8.
- Simonetta F, Lecuroux C, Girault I, Goujard C, Sinet M, Lambotte O, Venet A, Bourgeois C. Early and long-lasting alteration of effector CD45RA(–)Foxp3(high) regulatory T-cell homeostasis during HIV infection. J Infect Dis. 2012;205(10):1510–9.
- Marwaha AK, Crome SQ, Panagiotopoulos C, Berg KB, Qin H, Ouyang Q, Xu L, Priatel JJ, Levings MK, Tan R. Cutting edge: increased IL-17-secreting T cells in children with new-onset type 1 diabetes. J Immunol. 2010;185(7):3814–8.
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8(4):345–50.
- Vanaudenaerde BM, Verleden SE, Vos R, De Vleeschauwer SI, Willems-Widyastuti A, Geenens R, Van Raemdonck DE, Dupont LJ, Verbeken EK, Meyts I. Innate and adaptive interleukin-17-producing lymphocytes in chronic inflammatory lung disorders. Am J Respir Crit Care Med. 2011;183(8):977–86.
- Zhang X, Chang Li X, Xiao X, Sun R, Tian Z, Wei H. CD4(+)CD62L(+) central memory T cells can be converted to Foxp3(+) T cells. PLoS One. 2013;8(10):e77322.
- Sehrawat S, Rouse BT. Interplay of regulatory T cell and Th17 cells during infectious diseases in humans and animals. Front Immunol. 2017;8:341.
