ABSTRACT
Nutritional abnormalities and physical inactivity are highly prevalent in patients with chronic obstructive pulmonary disease (COPD). The aim of this study was to determine the association between nutritional status/body composition and physical performance in patients with COPD.
A cross-sectional observational study was conducted in outpatients with clinically stable, moderate to very severe COPD. In the assessment of nutritional status, we used dual energy X-ray absorptiometry, anthropometry, serum biomarkers, and the Mini-Nutritional Assessment (MNA) questionnaire. Physical performance was measured by the 6-minute walk distance (6MWD), 4-metre gait speed (4MGS), and physical activity. Univariate and multivariate analyses were performed.
In 111 patients (mean age 68 years, 69% men), the mean 6MWD was 376 ± 119 m, 4MGS 0.9 ± 0.2 m/s, and the average daily step count 8,059 ± 4,757. Patients with low exercise capacity (6MWD ≤ 350 m) had a significantly lower lean mass index (LMI) (p < 0.01), fat-free mass index (FFMI) (p < 0.01), bone mineral content (p < 0.01), bone mineral density (p < 0.01), T-score (p < 0.05), MNA score (p < 0.01), and serum albumin and prealbumin levels (p < 0.05). Patients with low physical activity (daily step count ≤ median) had lower LMI, FFMI, MNA score, serum prealbumin (for all comparisons p < 0.05) and vitamin D levels (p < 0.01). However, none of the nutritional variables showed an independent association with low physical performance in the multivariate models. In conclusion, patients with low physical performance have deficient nutritional status, but we could not demonstrate an independent relationship between nutritional parameters and physical performance.
Introduction
Extrapulmonary manifestations of chronic obstructive pulmonary disease (COPD) are increasingly being recognised as important determinants of disease severity and prognosis, and are receiving closer attention Citation(1). One of the extrapulmonary manifestations of COPD is an alteration in nutritional status and body composition, i.e., changes in the muscle mass, fat mass and bone mineral content, which may have a significant impact on physical performance, and vice versa Citation(2,3).
Loss and dysfunction of skeletal muscle mass are observed in 20–40% of patients with COPD, often accompanied by a reduction in body weight Citation(4). Furthermore, skeletal muscle mass wasting, demonstrated by a reduction of lean mass and fat-free mass, as well as low body weight are recognised as predictors of mortality in COPD Citation(5). In addition to body composition abnormalities, physical inactivity is also highly prevalent in patients with COPD, and both are associated with a higher risk of functional limitation, COPD exacerbations, impaired health-related quality of life, anxiety/depression and mortality Citation(3,6–10).
In contrast to severe COPD, in which muscle mass wasting and weight loss are frequent findings, patients with mild-to-moderate COPD are often overweight or obese with coexisting metabolic syndrome Citation(11). In these patients, a higher fat mass is associated with slower walking speed, greater likelihood of functional limitation and increased cardiovascular risk Citation(3,12).
In clinical practice exercise capacity, physical activity and nutritional status are rarely assessed because their determination is time consuming and requires special equipment. Previous studies have mainly investigated either physical performance or nutritional status and their independent association with COPD severity and prognosis Citation(5–7).
The aim of the present study was to investigate the relationship between nutritional status and physical performance in COPD, and to assess the differences in nutritional status and body composition in patients with low and normal physical performance.
Methods
Subjects
A cross-sectional observational study was conducted in outpatients with clinically stable COPD in the Dubrava University Hospital, Zagreb, Croatia, from February 2015 to June 2016. The inclusion criteria were the following: age ≥40 years, current or former smoking ≥10 pack-years, documented COPD history ≥1 year, post-bronchodilator ratio of forced expiratory volume in 1 second (FEV1) to the forced vital capacity (FVC) <0.70, and FEV1 <80% predicted.
The exclusion criteria included concomitant diseases and conditions that might influence nutritional status and/or physical performance, such as unstable cardiac disease (symptomatic ischaemic heart disease, heart failure, and uncontrolled arrhythmias), peripheral artery disease with intermittent claudication, limitation in walking due to neurological or osteomuscular disorder, active malignant disease, COPD exacerbation within 3 months prior to enrolment, asthma, active respiratory disease other than COPD, end-stage renal disease, decompensated liver cirrhosis, uncontrolled diabetes, uncontrolled thyroid pathology, dementia and sustained systemic corticosteroid therapy.
The study was approved by the hospital Ethics Committee of the Dubrava University Hospital, Zagreb, and each participant gave written informed consent.
Study protocol
Two study visits were scheduled within 7 ± 2 days. At the first visit, demographic and clinical data were collected, and pulmonary function tests, nutritional assessment, and physical performance tests were performed.
Demographic data and clinical assessment. Age, sex, smoking history, treatment and duration of COPD were recorded, along with the number of COPD exacerbations in the previous year. Moderate COPD exacerbations were those requiring treatment with antibiotics and/or oral corticosteroids in the outpatient settings, while severe exacerbations required hospitalisations. Co-morbidities were evaluated according to the Charlson index Citation(13). The modified Medical Research Council (mMRC) dyspnoea scale Citation(14) and COPD Assessment Test (CAT) Citation(15) were used for the assessment of symptoms severity. Psychological status was evaluated using the Hospital Anxiety and Depression Scale (HADS) Citation(16), and health-related quality of life was assessed with the EuroQol questionnaire (EQ-5D-5L) Citation(17).
Pulmonary function tests. Pre- and post-bronchodilator spirometry, diffusing capacity of the lung for carbon monoxide, and body plethysmography were performed (Vmax Series Software Version 20–7, VIASYS Healthcare, Inc., SensorMedics Corporation, USA) following the current guidelines for lung function testing Citation(18,19). Arterial blood gases were determined in subjects under resting conditions breathing room air.
Nutritional assessment. Body weight and height were measured, and the body mass index (BMI) was calculated as weight/height squared (kg/m2). The subjects were categorised into 4 groups based on the BMI: underweight (<21 kg/m2), normal weight (≥21, <25 kg/m2), overweight (≥25, <30 kg/m2) and obese (≥30 kg/m2). The choice of a cut-off <21 kg/m2 for underweight was based on previous studies reporting a higher mortality in COPD patients with BMI <21 kg/m2 Citation(20). Additional anthropometric measurements and calculations were performed by the routine methods Citation(21): waist, hip, and calf circumference (CC) (cm), waist-to-hip ratio, mid-upper arm circumference (MUAC) (cm), triceps skin fold (TSF) (measured in mm with the Harpenden Skinfold Caliper, British Indicators, Ltd., England), mid-arm muscle circumference (MAMC) calculated by the equation: MAMC (cm) = MUAC (cm) − 0.314 × TSF (mm), arm muscle area (AMA) calculated by the equation: AMA (cm2) = [MUAC (cm) − 0.314 × TSF (mm)]2/[4 × 3.14]. All anthropometric measurements were performed twice, except for the TSF, which was measured in triplicate, and the mean values were recorded.
Body composition was assessed by the dual energy X-ray absorptiometry (DEXA) total body scan (Lunar PRODIGY Primo, enCORE Software version 11.1, GE Healthcare, 2007). Lean soft tissue mass (kg), fat-free mass (kg), fat mass (kg), fat proportion (%), bone mineral content (BMC) (kg), bone mineral density (BMD) (g/cm2), and the T-score were measured. Additionally, the lean mass index (LMI), the fat-free mass index (FFMI), and the fat mass index (FMI) were calculated by dividing the lean soft tissue mass, fat-free mass, and fat mass, respectively, by height squared and expressed in kg/m2.
For rapid clinical evaluation of nutritional status, we used the Mini-Nutritional Assessment (MNA) questionnaire. Patients were stratified into three groups based on the MNA score: ‘normal nutritional status’ (24–30 points), ‘at risk of malnutrition’ (17–23.5 points), and ‘malnourished’ (<17 points) Citation(22).
Blood samples were collected and analysed for serum albumin, prealbumin, high-sensitivity C-reactive protein (hs-CRP), and 25-hydroxyvitamin D using commercially available assays.
Physical performance assessment. Three different methods were used: 4-metre gait speed (4MGS), 6-minute walk distance (6MWD), and an objective measurement of daily physical activity. The 4MGS was measured as previously described Citation(23), and the better of two measurements was recorded. The 6-minute walk test (6MWT) was performed following American Thoracic Society guidelines Citation(24). During both walk tests, the subjects were allowed to use their usual walking aids, and those with chronic respiratory insufficiency were provided with a portable oxygen source that was carried by the study personnel during the tests.
At the end of the first visit, the patients were supplied with the StepWatch Activity Monitor® (SAM) (Modus Health LLC, Washington, DC, USA), an ankle-worn accelerometer for the assessment of physical activity, which has already been tested in COPD patients Citation(7,25). Patients were instructed to wear the SAM during 7 ± 2 consecutive days except while washing and sleeping, to perform physical activity as usual, and to return the device at the second study visit when the data were downloaded. The days of the study visits, as well as no-wear days (<8 hours of wear time) were excluded from the analysis, and the average daily step count was recorded.
Statistical analysis
Continuous variables were presented as means (standard deviations [SD]), or medians (1st quartile; 3rd quartile) in the case of non-normal distribution. For categorical variables, absolute numbers (percentages) were used. For each measure of physical performance (6MWD, 4MGS, and daily physical activity), the results were divided in the ‘low’ and ‘normal’ groups with cut-offs of 350 m for the 6MWD, 0.8 m/s for the 4MGS and the median for the average daily step count in line with previous studies Citation(23,26,27). Demographic, clinical and nutritional variables were compared between the ‘low’ and ‘normal’ groups. Categorical variables were compared with the Chi square or Fisher's test, while Student's t-test or Mann–Whitney U-test were used for comparison of continuous variables. The association between independent variables and ‘low’ physical performance was assessed using univariate and multivariate stepwise logistic regression analysis. Variables showing a p-value <0.1 in the univariate analysis were included in the multivariate models. Linear relationships between variables were studied using the Pearson or Spearman test. All the tests were conducted at a significance level of α = 0.05. The SPSS version 20 software (IBM Corp., Armonk, NY, USA) was used for all the analyses.
Results
Patients' characteristics and physical performance
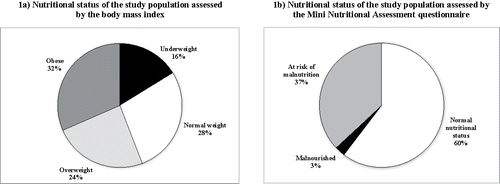
From the 1,297 patients with COPD (J41-44 according to the International Classification of Diseases, ICD-10) attended in the Dubrava University Hospital (Zagreb) during the period of the study, a convenience sample of 112 patients that fulfilled all inclusion criteria were recruited, and 1 was excluded because of non-compliance, leaving 111 patients for analysis. The patients' characteristics are summarised in . The mean age was 67.7 (SD = 7.8) years, and 76 (68.5%) were men. The mean BMI was 27.1 (SD = 5.8) kg/m2, and shows the stratification based on the BMI and MNA scores. Pulmonary function tests showed moderately severe airflow obstruction (mean post-bronchodilator FEV1 48.9% of predicted, SD = 15%), lung hyperinflation, mild-to-moderate decrease in diffusing capacity and mild hypoxemia. The mean CAT score was 17.9 (SD = 6.4) and mMRC dyspnoea grade 2.0 (SD = 1.0). The health-related quality of life of the study population was impaired with an EQ-5D-5L index value of 0.7 (SD = 0.2), and visual analogue scale score of 57.9 (SD = 15.2). Based on the HADS score, 38% of patients had some degree of anxiety and 43% presented clinically significant depression.
Table 1. Characteristics of the study population, patients with low and normal physical performance based on the 6-minute walk distance (6MWD) and daily physical activity (PA).
Figure 1. Categorisation of the study population regarding nutritional status assessed by the body-mass index (a) and the Mini-Nutritional Assessment questionnaire (b).
On average, in the 6MWT patients achieved 376 m (SD = 119, range 78–627 m), and 0.9 m/s (SD = 0.2, range 0.33–1.35 m/s) in the 4MGS. The average daily step count for the study population was 8,059 steps/day (SD = 4,757), but ranged from extremely poor (minimum 220 steps/day) to excellent (maximum 23,342 steps/day).
The correlation between the 6MWD and the 4MGS was very high (r = 0.852, p < 0.001), and therefore only the 6MWD was used as a measure of exercise capacity in subsequent analyses. The correlation between the 6MWD and physical activity was also high (r = 0.681, p < 0.001), while the correlation between physical activity and the 4MGS was moderate (r = 0.451, p < 0.001).
Comparison of patients with low and normal physical performance measured by exercise capacity
Fifty-three (47.7%) patients presented low exercise capacity (6MWD ≤ 350 m) and they were significantly older, had worse lung function, a higher CAT score and mMRC dyspnoea grade (p < 0.01). They had more frequent exacerbations, worse health-related quality of life, as well as a higher score in depression in the HADS (p < 0.01). Patients with low exercise capacity had a slower gait speed and lower daily step counts (p < 0.01) ().
Regarding nutritional status, patients with low exercise capacity had a lower LMI, FFMI, BMC, BMD (p < 0.01), and T-score (p < 0.05) (). In anthropometric measurements they had a significantly smaller CC, MUAC, MAMC and AMA. Additionally, they had a lower score in MNA questionnaire (p < 0.01) as well as lower serum albumin and prealbumin levels (p < 0.05). The remaining nutritional parameters are presented in .
Table 2. Comparison of nutritional status in patients with low and normal physical performance based on the 6-minute walk distance (6MWD) and daily physical activity (PA).
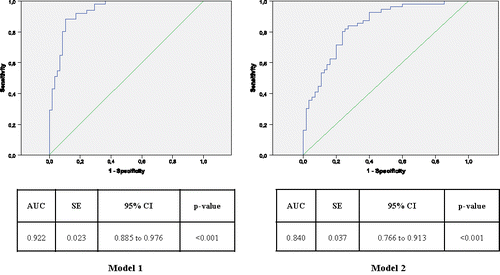
The factors that were independently and significantly related to low exercise capacity in the multivariate analysis were age, previous exacerbations, ratio of residual volume to total lung capacity, transfer coefficient of the lung for carbon monoxide (KCO), and mMRC dyspnoea level (). The multivariate model showed good goodness-of-fit (p-value of 0.608 in the Hosmer–Lemeshow test) and a good predictive power (area under the curve [AUC] = 0.922; p < 0.001) (, Model 1).
Table 3. Univariate and multivariate logistic regression analysis for the association with low exercise capacity defined by a 6-minute walk distance ≤ 350 m.
Figure 2. Receiver operating characteristic analysis of significant variables derived from the 2 multivariate logistic regression models and their capacity to predict low exercise capacity defined by the 6-minute walk distance ≤ 350 m (Model 1) and low physical activity defined by the daily step count ≤ 7,128 steps/day (Model 2). Model 1 includes age, moderate/severe exacerbations in the previous year, ratio of residual volume to total lung capacity, transfer coefficient of the lung for carbon monoxide (KCO), and mMRC dyspnoea level. Model 2 includes age, KCO, and mMRC dyspnoea level. AUC: area under the curve; SE: standard error; CI: confidence interval.
Comparison of patients with low and normal physical performance measured by physical activity
Similar to patients with low exercise capacity, patients with low physical activity (≤7,128 steps/day) were significantly older, had worse lung function, a higher CAT score and mMRC dyspnoea grade, more frequent exacerbations and worse health-related quality of life. They also had a slower gait speed and shorter 6MWD (p < 0.01). There were no significant differences between patients with low and normal physical activity with respect to HADS anxiety and depression scores ().
Regarding nutritional status, patients with low physical activity had a lower LMI, FFMI, along with a smaller CC, MAMC and AMA (p < 0.05). Furthermore, they had a significantly lower MNA score, as well as lower serum prealbumin and vitamin D levels. The comparison of nutritional variables is shown in .
According to the multivariate analysis, low physical activity was significantly related to age, KCO and mMRC dyspnoea level (). This multivariate model was well calibrated (p-value of 0.331 in the Hosmer–Lemeshow test) and showed a good predictive power (AUC = 0.840; p < 0.001) (, Model 2).
Table 4. Univariate and multivariate logistic regression analysis for the association with low physical activity defined by a daily step count ≤ 7,128 steps/day.
Discussion
Our results indicate that patients with low physical performance have deficient nutritional status in terms of reduced skeletal muscle mass and serum prealbumin levels, and in patients with low exercise capacity this was accompanied by a reduction of bone tissue. In the multivariate analysis, low exercise capacity was independently associated with older age, previous exacerbations, lung hyperinflation, decreased gas transfer and higher level of dyspnoea. Similarly, older age, reduced gas transfer and dyspnoea were independently and significantly associated with low physical activity. However, the nutritional factors were not identified as independently associated with physical performance in the multivariate analysis.
In the present study, physical performance was evaluated by three different methods – the 4MGS, the 6MWD and physical activity. We found a very high correlation between the 4MGS and the 6MWD, and therefore used only the 6MWD as a measure of exercise capacity in further analyses. We also found a high correlation between the 6MWD and objectively measured physical activity, which is in line with previous observations Citation(6).
In the assessment of nutritional status, we used several methods including DEXA, which is considered a reference method for the measurement of body composition and is superior to some more available methods such as bioelectric impedance analysis Citation(28). We found significant differences in several DEXA parameters (LMI and FFMI) and anthropometric measurements related to skeletal muscle mass (CC, MUAC, MAMC, and AMA) between patients with low and normal physical performance. These results are in accordance with previously published studies using bioelectric impedance that found a positive correlation between FFMI and the 6MWD Citation(29–31). Fat-free mass consists of skeletal muscles, bones, visceral organs, and water, while lean soft tissue mass represents bone-free fat-free mass, and there is evidence that reduced FFMI and/or LMI in patients with COPD are associated with more severe disease and increased mortality Citation(30,32). Although univariate analysis indicated that reduced FFMI, LMI, CC and AMA are more frequent among patients with low physical performance, none of the nutritional parameters were independently associated with low physical performance in the multivariate models. This suggests that age, air trapping, gas transfer and dyspnoea have a stronger association with physical performance, and changes in the body composition observed in patients with different levels of physical performance are mainly dependent on other factors. In the first multivariate model with low exercise capacity as a dependent variable, moderate/severe exacerbations in the previous year and dyspnoea level showed the strongest relationship with low exercise capacity (OR 4.216 and 7.389, respectively). These results indicate that the reduction in exercise capacity might be expected not only during exacerbations and shortly afterwards Citation(33), but also as a result of frequent exacerbations in the past Citation(34).
The DEXA parameters of bone tissue (BMC, BMD, and T-score) were significantly different between groups with low and normal exercise capacity, but not between groups with low and normal physical activity. This might be due to a higher proportion of women with increased loss of bone tissue in the group with low exercise capacity. However, it is surprising that no differences were found in bone tissue parameters between the groups with normal and low physical activity, especially when significant differences were observed in the serum concentrations of vitamin D, which is very important in bone metabolism. The causal relationship between reduced vitamin D concentrations and a low physical activity level in our study could not be established because of the cross-sectional study design, but the association might be bidirectional. Patients who are more active may have more sun exposure, which is crucial for vitamin D production. On the other hand, vitamin D may improve physical activity through its role in musculoskeletal function, especially in elderly population Citation(35,36). It is of note, however, that the mean serum levels of vitamin D were < 50 nmol/L both in patients with normal and low physical performance, indicating vitamin D deficiency Citation(37), which is consistent with the previously documented high prevalence of vitamin D deficiency in the general elderly population Citation(36).
It is important to note that despite the differences in body composition, the BMI and FMI did not differ between patients with low and normal physical performance, and on average the study population was slightly overweight. Previous studies have stressed the importance of reduced body weight as a predictor of health-care use and mortality in COPD Citation(5,20). However, when defining underweight in COPD different cut-offs were used for the BMI. A definition of BMI <18.5 kg/m2 given by the World Health Organisation is widely accepted for the general population, but studies on COPD frequently use a BMI <20 or <21 kg/m2 to define underweight, since a higher mortality has been observed in COPD patients below these values Citation(20). The cut-off <21 kg/m2 was used in the current study and we identified 18 underweight patients (16.2%) (there would have been only 4 patients if a BMI <18.5 kg/m2 had been applied). Moreover, stratification into different BMI categories revealed that overnutrition was more prevalent than undernutrition in our study population (24.3% of patients were overweight and a further 31.5% were obese), and this concurs with some previous studies that reported a high proportion of overweight/obese COPD patients, especially with mild-to-moderate disease Citation(38).
Serum proteins might give additional information on nutritional status, and we explored serum concentrations of albumin and prealbumin. Since both proteins belong to the negative acute phase reactants, the concentration of which fall during inflammation Citation(39), we also determined serum levels of hs-CRP, a marker of systemic inflammation. A positive association was found between exercise capacity and both albumin and prealbumin, as well as between physical activity and prealbumin, while no relation was found between hs-CRP and physical performance.
Besides the nutritional status in COPD patients, we additionally wanted to investigate their psychological status and its association with physical performance. It is well known that psychological status may affect someone's physical performance, and vice versa. Published data suggest that depressive COPD patients have reduced exercise capacity and physical activity Citation(40), while on the other hand, increased anxiety has been associated with higher levels of physical activity Citation(41). Based on the HADS score, a high proportion of our COPD patients manifested clinically relevant anxiety and depression (38% and 43%, respectively), and patients with low exercise capacity had significantly higher HADS depression scores. However, in our study, patients with low and normal physical activity, as measured by a daily step count, did not differ in the level of anxiety or depression.
Several study limitations need to be addressed. Since only patients that fulfilled our strict inclusion/exclusion criteria were included, they may not be fully representative of all the COPD population. Physical activity was measured as a daily step count and not as a distance walked or energy expenditure; therefore, it does not take into account differences in body height that may translate into a different number of steps for the same distance walked. However, the same activity monitor was already validated in the assessment of physical activity in COPD patients Citation(7,25). In addition, despite previously published data suggesting a trend of lower activity during periods of the year with poor weather and lower temperatures Citation(42), the seasonal variability of physical activity was not considered in the analysis. Finally, parameters of nutritional status and physical performance were not evaluated separately for men and women. Despite having a higher proportion of women in the group with a low 6MWD, female sex did not show significance in the multivariate analysis. Additionally, no association was found between female sex and low physical activity; thus, alterations in the nutritional status of the study population regarding their physical performance cannot be attributed to the difference in gender distribution.
Conclusions
Our results indicate that in addition to having more severe lung disease, a high burden of symptoms, and impaired health-related quality of life, COPD patients with low physical performance frequently have nutritional abnormalities, namely reduced skeletal muscle mass and decreased serum protein levels. However, none of the nutritional parameters showed independent associations with physical performance in our study. This would suggest that changes in the nutritional status and body composition observed with low physical performance are secondary and influenced by other factors. Further prospective studies are needed to more closely examine the relation between nutritional abnormalities and physical inactivity in COPD.
Abbreviations
| AMA | = | arm muscle area |
| AUC | = | area under the curve |
| BMC | = | bone mineral content |
| BMD | = | bone mineral density |
| BMI | = | body mass index |
| CAT | = | COPD Assessment Test |
| CC | = | calf circumference |
| COPD | = | chronic obstructive pulmonary disease |
| DEXA | = | dual energy X-ray absorptiometry |
| EQ-5D-5L | = | EuroQol questionnaire |
| FEV1 | = | forced expiratory volume in 1 second |
| FFMI | = | fat-free mass index |
| FMI | = | fat mass index |
| FVC | = | forced vital capacity |
| HADS | = | Hospital Anxiety and Depression Scale |
| hs-CRP | = | high-sensitivity C-reactive protein |
| KCO | = | transfer coefficient of the lung for carbon monoxide |
| LMI | = | lean mass index |
| MAMC | = | mid-arm muscle circumference |
| 4MGS | = | 4-metre gait speed |
| mMRC | = | modified Medical Research Council |
| MNA | = | Mini-Nutritional Assessment |
| MUAC | = | mid-upper arm circumference |
| 6MWD | = | 6-minute walk distance |
| 6MWT | = | 6-minute walk test |
| SAM | = | StepWatch Activity Monitor® |
| SD | = | standard deviation |
| TSF | = | triceps skin fold |
Declaration of interest
The purchase of four StepWatch Activity Monitors® with dedicated dock station and software was possible thanks to an unrestricted grant from Boehringer Ingelheim Zagreb, Croatia. The sponsor had no involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the manuscript, and nor in the decision to submit the article for publication. Dario Rahelic has received speaking fees from Abbott and Solgar. Zinka Matkovic, Danijel Cvetko, Cristina Esquinas, Marko Zarak, Marc Miravitlles and Neven Tudoric declared no conflict of interest.
References
- Nussbaumer-Ochsner Y, Rabe KF. Systemic manifestations of COPD. Chest. 2011;139(1):165–73.
- Bolton CE, Ionescu AA, Shiels KM, Pettit RJ, Edwards PH, Stone MD, et al. Associated loss of fat-free mass and bone mineral density in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(12):1286–93.
- Eisner MD, Blanc PD, Sidney S, Yelin EH, Lathon PV, Katz PP, et al. Body composition and functional limitation in COPD. Respir Res. 2007;8:7.
- Hopkinson NS, Tennant RC, Dayer MJ, Swallow EB, Hansel TT, Moxham J, et al. A prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary disease. Respir Res. 2007;8:25.
- Vestbo J, Prescott E, Almdal T, Dahl M, Nordestgaard BG, Andersen T, et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am J Respir Crit Care Med. 2006;173(1):79–83.
- Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33(2):262–72.
- Moy ML, Teylan M, Weston NA, Gagnon DR, Garshick E. Daily step count predicts acute exacerbations in a US cohort with COPD. PLoS One. 2013;8(4):e60400.
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61(9):772–8.
- Esteban C, Quintana JM, Aburto M, Moraza J, Egurrola M, Pérez-Izquierdo J, et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J. 2010;36(2):292–300.
- Ramon MA, Esquinas C, Barrecheguren M, Pleguezuelos E, Molina J, Quintano JA, et al. Self-reported daily walking time in COPD: relationship with relevant clinical and functional characteristics. Int J Chron Obst Pulm Dis. 2017;12:1173–81.
- Cebron Lipovec N, Beijers RJ, van den Borst B, Doehner W, Lainscak M, Schols AM. The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: a systematic review. COPD. 2016;13(3):399–406.
- Sternfeld B, Ngo L, Satariano WA, Tager IB. Associations of body composition with physical performance and self-reported functional limitation in elderly men and women. Am J Epidemiol. 2002;156(2):110–21.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–6.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–54.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70.
- Van Hout B, Janssen MF, Feng YS, Kohlmann T, Busschbach J, Golicki D, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value in Health. 2012;15(5):708–15.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38.
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–22.
- Cao C, Wang R, Wang J, Bunjhoo H, Xu Y, Xiong W. Body mass index and mortality in chronic obstructive pulmonary disease: A meta-analysis. PLoS One. 2012;7(8):e43892.
- WHO Expert Committee. Physical status: the use and interpretation of anthropometry: Report of a WHO Expert Committee. Geneva: World Health Organization; 1995.
- Vellas B, Villars H, Abellan G, Soto ME, Rolland Y, Guigoz Y, et al. Overview of the MNA – its history and challenges. J Nutr Health Aging. 2006;10(6):456–63.
- Kon SS, Patel MS, Canavan JL, Clark AL, Jones SE, Nolan CM, et al. Reliability and validity of 4-metre gait speed in COPD. Eur Respir J. 2013;42(2):333–40.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117.
- Moy ML, Danilack VA, Weston NA, Garshick E. Daily step counts in a US cohort with COPD. Respir Med. 2012;106(7):962–9.
- Karpman C, DePew ZS, LeBrasseur NK, Novotny PJ, Benzo RP. Determinants of gait speed in COPD. Chest. 2014;146(1):104–10.
- Moy ML, Teylan M, Danilack VA, Gagnon DR, Garshick E. An index of daily step count and systemic inflammation predicts clinical outcomes in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(2):149–57.
- Steiner MC, Barton RL, Singh SJ, Morgan MDL. Bedside methods versus dual energy X‐ray absorptiometry for body composition measurement in COPD. Eur Respir J. 2002:19(4):626–31.
- Ischaki E, Papatheodorou G, Gaki E, Papa I, Koulouris N, Loukides S. Body mass and fat-free mass indices in COPD: relation with variables expressing disease severity. Chest. 2007;132(1):164–9.
- Luo Y, Zhou L, Li Y, Guo S, Li X, Zheng J, et al. Fat-free mass index for evaluating the nutritional status and disease severity in COPD. Respir Care. 2016;61(5):680–8.
- Sabino PG, Silva BM, Brunetto AF. Nutritional status is related to fat-free mass, exercise capacity and inspiratory strength in severe chronic obstructive pulmonary disease patients. Clinics (Sao Paulo). 2010;65(6):599–605.
- Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82(1):53–9.
- Alahmari AD, Kowlessar BS, Patel AR, Mackay AJ, Allinson JP, Wedzicha JA, et al. Physical activity and exercise capacity in patients with moderate COPD exacerbations. Eur Respir J. 2016;48(2):340–9.
- Ramon MA, Gimeno-Santos E, Ferrer J, Balcells E, Rodríguez E, de Batlle J, et al. Hospital admissions and exercise capacity decline in patients with COPD. Eur Respir J. 2014;43(4):1018–27.
- Wicherts IS, van Schoor NM, Boeke AJ, Visser M, Deeg DJ, Smit J, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92(6):2058–65.
- Bischoff-Ferrari HA, Dietrich T, Orav EJ, Hu FB, Zhang Y, Karlson EW, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged ≥ 60 y. Am J Clin Nutr. 2004;80(3):752–8.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–30.
- Cecere LM, Littman AJ, Slatore CG, Udris EM, Bryson CL, Boyko EJ, et al. Obesity and COPD: associated symptoms, health-related quality of life, and medication use. COPD. 2011;8(4):275–84.
- Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–54.
- Martinez Rivera C, Costan Galicia J, Alcázar Navarrete B, Garcia-Polo C, Ruiz Iturriaga LA, Herrejón A, et al. Factors associated with depression in COPD: a multicenter study. Lung. 2016;194(3):335–43.
- Nguyen HQ, Fan VS, Herting J, Lee J, Fu M, Chen Z, et al. Patients with COPD with higher levels of anxiety are more physically active. Chest. 2013;144(1):145–51.
- Sewell L, Singh SJ, Williams JE, Morgan MD. Seasonal variations affect physical activity and pulmonary rehabilitation outcomes. J Cardiopulm Rehabil Prev. 2010;30(5):329–33.
