ABSTRACT
Sputum and blood eosinophils are proposed as candidate biomarkers for the identification of chronic obstructive pulmonary disease (COPD) patients at risk for exacerbation and treatment response. In this study, we evaluated the associations of eosinophils with the presence of emphysema in COPD patients. Induced sputum and blood eosinophil measurements were performed in consecutive COPD patients. Patients underwent lung function testing and high resolution computed tomography (HRCT) of the chest and the presence of emphysema was quantified. Patients with emphysematous lesions in ≥15% of the pulmonary parenchyma were considered having significant emphysema. Ninety-eight patients were included in the study. Patients with significant emphysema had lower blood eosinophil counts compared to patients without emphysema [median (IQR) 34.6 (0.0, 63.0) vs. 169.0 (110.0, 260.0) cells/µL, p < 0.001]; similar results were observed for the percentage (%) of blood eosinophils, but no difference was observed for sputum eosinophils. The differences were evident in frequent and non-frequent exacerbators and irrespective of the use of inhaled corticosteroids (ICS). Patients with significant emphysema in HRCT present lower levels of blood eosinophils and these differences were present irrespective of the frequent exacerbator history or the use of ICS. Blood eosinophils may not represent a clinically relevant biomarker in the presence of emphysema.
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by persistent airflow limitation that is usually progressive and associated with chronic inflammation of the airways and the lung Citation(1). The distinct anatomic change which can be recognized in the lung parenchyma of COPD patients, is emphysema Citation(2). High resolution computed tomography (HRCT) offers the opportunity to study the pathologic processes involved in structural changes within the lung and to investigate the severity, extension, and distribution of emphysema Citation(3, 4).
Although COPD is a disease with predominant neutrophilic inflammation Citation(5), a proportion of COPD patients have elevated eosinophils in their airways Citation(6–8). These patients with sputum eosinophilia seem to have a greater response to oral Citation(6) and inhaled corticosteroids (ICS) Citation(9). However, since the identification of airway eosinophilia using induced sputum in clinical practice is hard, blood eosinophils has been proposed as a more convenient surrogate Citation(10). Increased blood eosinophil count has been proposed as a biomarker to predict response as expressed by exacerbation prevention by LABA/ICS vs. LABA Citation(11–13); however, there is no single established cut-off for blood eosinophils, as studies have used different percentages and cut-offs. Moreover, in the comparison of a LABA/LAMA vs. a LABA/ICS in the FLAME study there was no difference in exacerbation prevention between patients with ≥2% and <2% blood eosinophils Citation(14), whereas the withdrawal of ICS from a triple (LAMA + LABA + ICS) combination in the WISDOM trial led to an increase in exacerbations only in patients with ≥4% or ≥300 cells/µL Citation(15). Finally, blood eosinophils in the ECLIPSE cohort were not a stable biomarker, as in 49% of patients it fluctuated above and below 2% Citation(16), whereas in a population-based analysis in the Copenhagen General Population Study increased blood eosinophil levels >340 cells/µL were a better predictor of future exacerbations than the 2% cut-off Citation(17). Given the contradictory results in the literature, more studies are needed to identify the characteristics of patients with blood eosinophilia and its association with the frequent exacerbator phenotype in COPD.
The aim of the present study was to evaluate the differences in sputum and blood eosinophils in patients with and without significant emphysema in HRCT and to test whether eosinophil concentrations differ between frequent and non-frequent exacerbators in these subgroups of COPD patients.
Methods
Study design
In the present study, we have included consecutive patients with COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations Citation(1) who were treated in the outpatient clinic of the 1st and 2nd University Respiratory Medicine Departments of the National and Kapodistrian University of Athens. Exclusion criteria included the presence of significant respiratory disease other than COPD (asthma was meticulously excluded by two experienced clinicians, who excluded all patients with any personal or family history of asthma and/or atopy), the presence of any malignant disease, and inability or unwillingness of the patient to collaborate with the study investigators. The study followed the tenets of the Declaration of Helsinki and was approved by our institutional review board (Number of Approval 3747/16-2-15). Written informed consent was obtained from all participants.
Patient demographics were recorded, including age, gender, BMI (expressed in kg/m2), smoking habit, and comorbidities. All patients underwent physical examination, pulse oximetry, and pulmonary function tests (PFTs) and sputum induction. Patients' treatment including treatment with ICS was also recorded. Peripheral blood samples were collected from all patients and total blood count test was performed, including white blood cell and blood eosinophil counts. Patients who experienced two or more acute exacerbations of COPD AECOPD during the previous year were considered as frequent exacerbators.
COPD severity
PFTs were performed using a commercially available system (Master Screen, Erich Jaeger GmbH, Wuerzburg, Germany) Forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and forced expiratory flow in the middle 50% of FVC (FEF25–75) were recorded. Post-bronchodilator values (i.e. 30 minutes after the administration of 400 mg salbutamol with a spacer) were used for the evaluation of COPD severity, according to GOLD recommendations Citation(1). Diffusing capacity for carbon monoxide (DLCO) and diffusing capacity for carbon monoxide adjusted for alveolar volume (DLCO/VA) were assessed by means of the single breath method with the patient in the sitting position Citation(18). Lung function measurements were expressed as percentages of predicted values.
HRCT
All patients underwent HRCT using either a Somaton HiQ or a Somaton Plus scanner (Siemens, Erlanger, Germany). Scans were performed with 1–1.5 mm section thickness and a 1–2 second scanning time during breath holding at end inspiration. Films were read by a radiologist with expertise in HRCT, who was blinded to the status of the patients and the rest of the measurements. The degree of emphysema was calculated using a visual emphysema score as previously described Citation(19). Briefly, emphysema was identified as areas of hypovascular low attenuation and was graded with a five-point scale based on the percentage of lung involved: 0: no emphysema; Citation(1) up to 25% of the lung parenchyma involved; Citation(2) between 26% and 50% of lung parenchyma involved; Citation(3) between 51% and 75% of the lung parenchyma involved; and Citation(4) between 76% and 100% of lung parenchyma involved. Grades of the axial images of each lung were added and divided by the number of images evaluated to yield emphysema scores that ranged from 0 to 4 Citation(19). The cut off point for the characterization of the emphysematous phenotype was the presence of emphysematous lesions ≥15% of the pulmonary parenchyma (i.e. score ≥0.6) Citation(20).
Sputum induction
Sputum induction was performed as previously described Citation(21, 22). Sputum supernatant was removed with centrifugation and total cell count was evaluated with a haemocytometer after Trypan Blue stain. Slides were prepared by cytospin (Shandon, Runcorn, UK) and were stained with May-Grunwald and Giemsa for differential cell counts. A blind observer performed counting of a minimum 500 inflammatory cells in each sample. Sputum samples were considered acceptable if they had a volume of at least 2 ml after final expectoration and the percentage of squamous cells on the prepared slides was lower than 10%. Total cell count was expressed as the number of cells × 106/ml and sputum inflammatory cells as percentage (%) of the non-squamous cells. Sputum supernatants were stored at −70°C until measurement.
Statistical analysis
Categorical variables are presented as n (%), whereas numerical variables are presented as mean ± SD or median (interquartile ranges). Normality of distributions was checked using Kolmogorov–Smirnov test. Comparisons between groups were performed using chi-square tests for categorical data, or Mann–Whitney U-tests and Kruscall Wallis tests for numerical data, since the distribution was skewed. Correlations were performed using Spearman correlation coefficient. All p-values <0.05 were considered statistically significant. Data were analysed using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL, USA) and graphs have been created using GraphPad Prism 6 (GraphPad Software, Inc La Jolla CA USA).
Results
Demographic and functional characteristics of the study participants are shown in . Patients with significant emphysema, as defined by the presence of emphysematous lesions in ≥15% of the pulmonary parenchyma, were older and less frequently current smokers, while they had lower BMI and more impaired lung function.
Table 1. Demographic, functional, and inflammatory characteristics of the study participants according to the presence of emphysematous lesions in the lung parenchyma.
Blood and sputum eosinophils according to the presence of emphysema
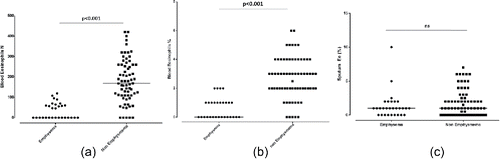
Patients with significant emphysema had significantly lower blood eosinophil counts compared to patients without emphysema [median (IQR) 34.6 (0.0, 63.0) vs. 169.0 (110.0, 260.0) cells/µL, p < 0.001 for the absolute number and 0.6 (0.0, 1.0) vs. 3.0 (2.0, 4.0) %, p < 0.001 for the percentage of blood eosinophils in patients with and without emphysema, respectively]. Interestingly, no difference was observed in sputum eosinophils in patients with and without emphysema. () ().
Figure 1. Eosinophil counts in patients with and without significant emphysema (a) absolute counts of blood eosinophils, (b) blood eosinophils expressed as the percentage of white cell count (c) sputum eosinophils expressed as the percentage of sputum cells.
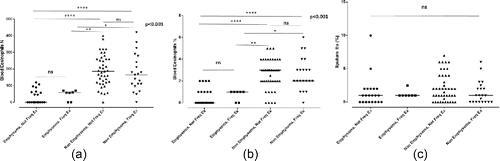
When patients were divided according to the history of exacerbations in the previous year to frequent (≥2 exacerbations) and non-frequent (0–1 exacerbations), patients with emphysema continued to present lower levels of eosinophils regardless of the exacerbation history; this was evident for blood (both absolute count and percentage) but not for sputum eosinophils (). There was no significant difference between frequent and non-frequent exacerbators in neither sputum nor blood eosinophils (p = 0.419, p = 0.596, and p = 0.685 for sputum eosinophils, absolute count and percentage of blood eosinophils, respectively).
Figure 2. Eosinophil counts in patients with and without significant emphysema according to exacerbation history; (a) absolute counts of blood eosinophils, (b) blood eosinophils expressed as the percentage of white cell count (c) sputum eosinophils expressed as the percentage of sputum cells.
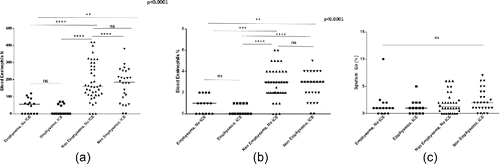
When patients were further divided in subgroups according to the treatment with ICS, patients with emphysema continued to present lower levels of eosinophils regardless of the use of ICS; this was evident for blood (both absolute count and percentage) but not for sputum eosinophils ().
Figure 3. Eosinophil counts in patients with and without significant emphysema according to the use of inhaled corticosteroids (ICS); (a) absolute counts of blood eosinophils, (b) blood eosinophils expressed as the percentage of white cell count (c) sputum eosinophils expressed as the percentage of sputum cells.
Correlations of eosinophils with emphysema score and other parameters
Blood eosinophils presented significant negative correlations to the score of emphysema in HRCT: r = −0.735, p<0.001 and r = −0.739, p<0.001, for the absolute number and the percentage of blood eosinophils, respectively. Blood eosinophils presented additionally weak correlations with lung function parameters and DLco (). In contrast, sputum eosinophils did not present a significant correlation with the score of emphysema or DLco. The absence of correlation between sputum eosinophils and the score of emphysema was present both in patients not receiving and receiving ICS (r = 0.050, p = 0.711 and r = −0.163, p = 0.314, respectively).
Table 2. Correlations of sputum and blood eosinophils.
Difference in blood eosinophils according to the use of ICS
There was no significant difference in blood eosinophils (expressed as absolute number or as percentage between patients receiving and not receiving ICS [median (IQR), 83.0 (0.0, 209.5) vs. 126.5 (64.0, 245.5), p = 0.153 for the absolute number; and 1.5 (0.0, 3.0) vs. 2.0 (1.0, 3.0), p = 0.153 for the percentage of blood eosinophils]. No difference was observed also in % sputum eosinophils between patients receiving and not receiving ICS [1.0 (1.0, 2.8) vs. 1.0 (0.0, 2.0), p = 0.118].
Correlations between blood and sputum eosinophils
There were no significant correlations between sputum eosinophil counts and blood eosinophils expressed either as absolute numbers or as percentage (r = 0.095, p = 0.352 and r = 0.084, p = 0.409 for the absolute number and the percentage, respectively). The absence of significant correlation was irrespective of the use of ICS (data not shown). In receiver operating characteristics (ROC) analysis, blood eosinophils could not predict sputum eosinophilia as defined by ≥3% eosinophils (area under the curve [AUC] 0.586 for absolute blood eosinophil counts and 0.570 for % blood eosinophils). Only in patients not receiving ICS, blood eosinophils presented a modest performance for the prediction of sputum eosinophilia (data not shown).
Discussion
In the present cross-sectional study, we have shown that patients with significant emphysema in HRCT present lower levels of blood but not sputum eosinophils. The differences in blood eosinophils were present both in frequent and non-frequent exacerbators and irrespective of the use of ICS. We observed significant correlations between blood eosinophils and the score of emphysema in HRCT that were not present for sputum eosinophils. No correlations between blood and sputum eosinophils were observed.
In our study, we have observed that patients with significant emphysematous lesions were characterized by more severe airway obstruction expressed by FEV1 and FEV1/FVC ratio. This observation has been reported in previous studies, Citation(20, 23), which have actually shown that the presence of emphysema is related to more severe COPD. Nevertheless, the association between airway eosinophilic inflammation and disease severity in COPD remains unclear, since some studies reported negative correlations between airway eosinophilic inflammation and FEV1 Citation(24, 25), while others have reported no relationship between eosinophilia and COPD severity Citation(26). In one previous study, including patients from the ECLIPSE cohort it has been reported that the increase in the emphysematous lesions progression was enhanced in subjects with persistent blood eosinophil counts <2% Citation(16). This observation is in accordance to our findings showing that emphysematous patients are characterized by lower levels of blood eosinophils and leads to the hypothesis that the presence of emphysema is less likely to allow for significant Th2-type inflammation in the lungs that would be sufficient to lead to peripheral blood eosinophilia.
Previous studies have shown that eosinophils are present in the airways of patients with COPD Citation(6) and that the number of blood eosinophils can be probably used as a predictor of airway eosinophilia Citation(10). In our study, no correlation was found between sputum and blood eosinophils. This discordance between local and systemic inflammation leads to the hypothesis that the presence of blood eosinophilia might not directly reflect airways inflammation. Furthermore, we can hypothesize, that the presence of emphysema is less likely to allow for significant Th2-type inflammation in the lungs, which would be related to peripheral blood eosinophilia, but seems to be rather related to Th1-predominant inflammation. This hypothesis is in accordance to the observation that in the lung parenchyma of former smokers with emphysema there was an increased relative abundance of Th1 and Th17 cells compared with the lung parenchyma of former smokers without emphysema Citation(27).
In a single study in patients with COPD, a management strategy that aimed to minimize eosinophilic airway inflammation was associated with a reduction in severe AECOPD Citation(28). Furthermore, increased blood eosinophil counts were found to be associated with a modest increase in the risk for the development of AECOPD Citation(17, 29), with one study suggesting that elevated blood eosinophil counts may predict COPD exacerbation risk only in ex-smokers Citation(29). Our study further advances our knowledge on this topic, as we have shown that a history of frequent exacerbations did not affect the levels of blood eosinophils in patients with and without significant emphysema, leading us to the hypothesis that the presence of emphysema may be a more significant negative predictor of blood eosinophilia compared to the history of exacerbations.
Our study has some limitations. First, we have used an observational method for the quantification of emphysema in HRCT, instead of dedicated CT software. However, although this method presents excellent correlation with densitometry quantitation Citation(19) and can be performed in clinical practice, our findings need to be confirmed by other similar investigations with more objective measures. Second, exacerbation history was based to the patients' history and medical files in the preceding year, rather than in a prospective follow up after blood and sputum eosinophilia was measured during recruitment to the study. This does not allow us to evaluate possible changes in blood or sputum eosinophilia over time and its possible relation to the development of an AECOPD in our study cohort. Furthermore, there is a question on whether the use of ICS might affect the results, especially regarding sputum eosinophils. Our study was a cross-sectional and cannot clearly demonstrate a causal relationship between inhaled steroids and either blood or/and sputum eosinophils. Longitudinal controlled studies are needed for that purpose. However, the use of that kind of medication did not seem to affect the difference in blood eosinophils between patients with and without emphysema. This is also supported by prospectively collected data from the large randomized controlled FLAME trial, which have shown that the addition of ICS leads to minimal changes in blood eosinophils after 26 and 52 weeks of treatment Citation(30). Finally, we have not performed measurements of static lung volumes using body plethysmography, which would possibly allow us to have more accurate measurements regarding air-trapping and hyperinflation in the different group of patients.
In conclusion, in our study we have shown that patients with significant emphysema in HRCT present lower levels of blood eosinophils and these levers correlate significantly to the score of emphysema in HRCT. Furthermore, the differences between patients with and without significant emphysema were present irrespective of the history of frequent exacerbations, which leads to the conclusion that the presence of emphysema is less likely to be related to significant Th2-type inflammation in the lungs that would be associated to peripheral blood eosinophilia. Blood eosinophils may not represent a clinically relevant biomarker in the presence of emphysema.
Abbreviations
| BMI | = | Body Mass Index |
| COPD | = | Chronic Obstructive Pulmonary Disease |
| DLCO | = | Diffusing Lung Capacity for Carbon Monoxide |
| FEF25–75 | = | Forced Expiratory Flow in the middle 50% of FVC |
| FEV1 | = | Forced Exhaled Volume in one second |
| FVC | = | Forced Exhaled Vital Capacity |
| GOLD | = | Global Initiative for Chronic Obstructive Lung Disease |
| HRCT | = | High Resolution Computed Tomography |
| ICS | = | Inhaled Corticosteroids |
| IQR | = | Interquartile Range |
| LABA | = | Long Acting Beta Agonists |
| LAMA | = | Long Acting Muscarining Antagonists |
| VA | = | Alveolar Volume |
Declaration of interest
Konstantinos Kostikas is a Novartis employee. All other authors declare that they have no conflict of interest related to the present manuscript. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
PB, SL, NK, and SP designed the study; AIP, KK, and SL wrote the manuscript; AIP, KK, and SL performed the data analysis; AP, LA, EP, and GH collected the data; AM has performed the emphysema scoring in the HRCT. SL and PB are the paper guarantors. All authors have read and approved the final version of the manuscript.
References
- Global Strategy for the Diagnosis. Management and prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016. http://www.goldcopd.org. Accessed on September 1st 2016.
- Rennard SI. Looking at the patient-approaching the problem of COPD. N Engl J Med. 2004;350(10):965–6.
- Celli BR. Roger s. Mitchell lecture. Chronic obstructive pulmonary disease phenotypes and their clinical relevance. Proc Am Thorac Soc. 2006;3(6):461–5.
- Wouters EF. Approaches to improving health status in chronic obstructive pulmonary disease: one or several? Proc Am Thorac Soc 2006;3(3):262–9.
- Moermans C, Heinen V, Nguyen M, Henket M, Sele J, Manise M, Corhay JL, Louis R. Local and systemic cellular inflammation and cytokine release in chronic obstructive pulmonary disease. Cytokine 2011;56(2):298–304.
- Brightling CE, Monteiro W, Ward R, Parker D, Morgan MD, Wardlaw AJ, Pavord ID. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2000;356(9240):1480–5.
- D'Silva L, Hassan N, Wang HY, Kjarsgaard M, Efthimiadis A, Hargreave FE, Nair P. Heterogeneity of bronchitis in airway diseases in tertiary care clinical practice. Can Respir J. 2011;18(3):144–8.
- Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(1):39–47.
- Brightling CE, McKenna S, Hargadon B, Birring S, Green R, Siva R, Berry M, Parker D, Monteiro W, Pavord ID et al. Sputum eosinophilia and the short term response to inhaled mometasone in chronic obstructive pulmonary disease. Thorax 2005;60(3):193–8.
- Schleich F, Corhay JL, Louis R. Blood eosinophil count to predict bronchial eosinophilic inflammation in COPD. Eur Respir J. 2016;47(5):1562–4.
- Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–42.
- Siddiqui SH, Guasconi A, Vestbo J, Jones P, Agusti A, Paggiaro P, Wedzicha JA, Singh D. Blood eosinophils: A biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):523–5.
- Barnes NC, Sharma R, Lettis S, Calverley PM. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J. 2016;47(5):1374–82.
- Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, Thach C, Fogel R, Patalano F, Vogelmeier CF. Indacaterol-Glycopyrronium versus Salmeterol-Fluticasone for COPD. N Engl J Med. 2016;374(23):2222–34.
- Watz H, Tetzlaff K, Wouters EF, Kirsten A, Magnussen H, Rodriguez-Roisin R, Vogelmeier C, Fabbri LM, Chanez P, Dahl R et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390–8.
- Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–700.
- Vedel-Krogh S, Nielsen SF, Lange P, Vestbo J, Nordestgaard BG: Blood eosinophils and exacerbations in chronic obstructive pulmonary disease. The Copenhagen General Population Study. Am J Respir Crit Care Med. 2016;193(9):965–74.
- Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–35.
- Park KJ, Bergin CJ, Clausen JL. Quantitation of emphysema with three-dimensional CT densitometry: comparison with two-dimensional analysis, visual emphysema scores, and pulmonary function test results. Radiology 1999;211(2):541–7.
- Boschetto P, Quintavalle S, Zeni E, Leprotti S, Potena A, Ballerin L, Papi A, Palladini G, Luisetti M, Annovazzi L et al. Association between markers of emphysema and more severe chronic obstructive pulmonary disease. Thorax 2006;61(12):1037–42.
- Tseliou E, Bakakos P, Kostikas K, Hillas G, Mantzouranis K, Emmanouil P, Simoes D, Alchanatis M, Papiris S, Loukides S. Increased levels of angiopoietins 1 and 2 in sputum supernatant in severe refractory asthma. Allergy 2012;67(3):396–402.
- Efthimiadis A, Spanevello A, Hamid Q, Kelly MM, Linden M, Louis R, Pizzichini MM, Pizzichini E, Ronchi C, Van Overvel F et al. Methods of sputum processing for cell counts, immunocytochemistry and in situ hybridisation. Eur Respir J Suppl. 2002;37:19s–23s.
- Papaioannou AI, Mazioti A, Kiropoulos T, Tsilioni I, Koutsokera A, Tanou K, Nikoulis DJ, Georgoulias P, Zakynthinos E, Gourgoulianis KI et al. Systemic and airway inflammation and the presence of emphysema in patients with COPD. Respir Med. 2010;104(2):275–82.
- Balzano G, Stefanelli F, Iorio C, De Felice A, Melillo EM, Martucci M, Melillo G. Eosinophilic inflammation in stable chronic obstructive pulmonary disease. Relationship with neutrophils and airway function. Am J Respir Crit Care Med. 1999;160(5 Pt 1):1486–92.
- Lams BE, Sousa AR, Rees PJ, Lee TH. Subepithelial immunopathology of the large airways in smokers with and without chronic obstructive pulmonary disease. Eur Respir J. 2000;15(3):512–6.
- Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–53.
- Shan M, Cheng HF, Song LZ, Roberts L, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Storness-Bliss C et al. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med. 2009;1(4):4ra10.
- Siva R, Green RH, Brightling CE, Shelley M, Hargadon B, McKenna S, Monteiro W, Berry M, Parker D, Wardlaw AJ et al. Eosinophilic airway inflammation and exacerbations of COPD: a randomised controlled trial. Eur Respir J. 2007;29(5):906–13.
- Kerkhof M, Sonnappa S, Postma DS, Brusselle G, Agusti A, Anzueto A, Jones R, Papi A, Pavord I, Pizzichini E et al. Blood eosinophil count and exacerbation risk in patients with COPD. Eur Respir J. 2017;50(1).
- Roche N, Chapman KR, Vogelmeier CF, Herth FJF, Thach C, Fogel R, Olsson P, Patalano F, Banerji D, Wedzicha JA. Blood eosinophils and response to maintenance chronic obstructive pulmonary disease treatment. Data from the FLAME Trial. Am J Respir Crit Care Med. 2017;195(9):1189–97.
