ABSTRACT
Triple inhaled therapy for chronic obstructive pulmonary disease (COPD) consists of an inhaled corticosteroid (ICS), a long-acting β2-agonist (LABA) and a long-acting muscarinic antagonist (LAMA) taken in combination. Triple therapy is recommended by the Global initiative for Chronic Obstructive Lung Disease (GOLD) for patients who experience recurrent exacerbations despite treatment with either a dual bronchodilator (preferred initial therapy) or LABA/ICS combination (alternative initial therapy). Although there is evidence for the greater efficacy of triple therapy compared with LABA/ICS and LAMA monotherapy with regards to improved lung function, health status, and exacerbation rate, the efficacy of triple therapy when compared with dual bronchodilation (LABA/LAMA) is as yet unknown. As ICS use is associated with an increased risk of developing pneumonia, it is important to assess the risk/benefit ratio of triple therapy on an individual basis, and identify patients most likely to benefit. The role of elevated blood eosinophils as a biomarker for the identification of candidates for ICS treatment is currently debated, and further prospective evidence is required. This review assesses evidence for the efficacy and safety of triple therapy and postulates on the prospective evidence from ongoing studies. The potential for treating patients who experience further exacerbations on dual bronchodilation according to phenotype is also considered, as well as withdrawal of ICS from triple therapy in patients who are unlikely to benefit.
Introduction
Chronic obstructive pulmonary disease (COPD) is a significant cause of morbidity and mortality worldwide, with its burden increasing due to an ageing population and continued exposure to risk factors (Citation1). Bronchodilators, which are effective and well tolerated in patients with stable disease, are the cornerstone of the pharmacological management of COPD (Citation1).
In the past, inhaled corticosteroids (ICS) in combination with long-acting β2-agonists (LABA) were recommended for the treatment of patients at high risk of exacerbation, following evidence for their efficacy in preventing COPD exacerbations and hospitalizations, and improving quality of life (Citation2–5). However, effective long-acting muscarinic antagonists (LAMAs) such as tiotropium emerged, demonstrating similar benefits to LABA/ICS regarding exacerbation prevention (Citation6). In addition, evidence began to accumulate suggesting an association between ICS-containing regimens and increased pneumonia risk (Citation7–9). These developments, coupled with the emergence of effective dual bronchodilator therapies (LABA/LAMA), led to revisions in the Global initiative for chronic Obstructive Lung Disease (GOLD) recommendations for the management of patients with COPD (Citation1,Citation10). Patients with COPD commonly receive a LABA/ICS combined with a LAMA (Citation11,Citation12); however, clearer guidance is required regarding when treatment escalation is necessary and in which patients this should be considered.
GOLD currently recommends triple therapy (ICS plus LABA plus LAMA) for high-risk patients with severe symptoms (GOLD Group D) who develop further exacerbations on dual bronchodilation (preferred initial therapy) or LABA/ICS (alternative initial therapy) (Citation1). This recommendation is based on studies that applied former GOLD recommendations for the identification of ‘high-risk’ patients (severe-to-very severe airflow limitation or an exacerbation history) (Citation13). In GOLD 2017, only exacerbation history is taken into account when assessing patients for exacerbation risk and data using the GOLD 2017 classification system are limited. There are recommendations to restrict triple therapy use further, to only patients who are likely to respond to ICS (such as those with asthma-COPD overlap or elevated blood eosinophils) (Citation14,Citation15).
There is a clear need for better evidence upon which to base treatment recommendations. In this review, we discuss published studies that examine the efficacy of triple therapy in COPD management. We also discuss evidence for the withdrawal of ICS from triple therapy, as a tool for identifying appropriate patients for triple therapy treatment. Our intention is to clarify what we know with certainty and highlight those areas where further evidence is needed to inform the management of patients with COPD.
Evidence for triple therapy: efficacy and safety
Several studies have demonstrated the superiority of triple therapy over LABA/ICS and LAMA monotherapy in patients with COPD. We will present these studies in chronological order, and separate them into those published pre-2016 () (Citation16–23) and those published from 2016 onwards (), (Citation24–29) as newer studies utilize more modern and potentially more potent fixed-dose combinations (Citation30–33).
Table 1. Patient disposition/enrollment criteria and key results from initial studies (pre-2016) demonstrating the efficacy of triple therapy combinations of LABA/ICS and LAMA administered using different inhaler devices.
Table 2. Patient disposition/enrollment criteria and key results from modern recent studies (2016 onwards), and studies yet to be published, demonstrating the efficacy of fixed-dose triple therapy combinations.
Initial data (pre-2016), with triple therapy combinations
All triple therapy regimens discussed within this section consist of combinations of LABA/ICS and LAMA administered using different inhaler devices.
The OPTIMAL study investigated the comparative efficacy of triple therapy with salmeterol/fluticasone propionate combination (SFC) 25/250 µg two puffs twice daily (BID) plus tiotropium 18 µg once daily (QD) versus tiotropium 18 µg QD monotherapy (Citation16). While the study also contained a tiotropium 18 µg QD plus salmeterol 25 µg two puffs BID arm, it was not designed or powered to compare the differences between the triple therapy and tiotropium plus salmeterol groups. There were no significant differences between triple therapy and tiotropium monotherapy with regards to the rate of exacerbations or time-to-first exacerbation (of any severity), or Transition Dyspnea Index (TDI) total score over 52 weeks () (Citation16). However, triple therapy significantly improved lung function (increase in forced expiratory volume in 1 second [FEV1]: 27 mL vs. 86 mL, respectively; p = 0.049) and health status (change in St. George's Respiratory Questionnaire [SGRQ] total score: –4.5 units vs. –8.6 units, respectively; p = 0.01) compared with tiotropium monotherapy (Citation16). An important limitation of this study was the high rate of discontinuation amongst patients receiving tiotropium (43%) or tiotropium plus salmeterol (47%), resulting in the under powering and potential bias of results in the intention-to-treat population (Citation16,Citation34). Furthermore, the mean change in pre-bronchodilator FEV1 within treatment arms was relatively low compared with other studies.
In the CLIMB (Evaluation of Efficacy and Safety of Symbicort as add-on Treatment to Spiriva in Patients with Severe COPD) study, triple therapy with budesonide/formoterol (BUD/FOR) 320/9 µg BID plus tiotropium 18 µg QD significantly improved lung function (65 mL increase in pre-dose FEV1, and 123 and 131 mL increase in post-dose FEV1 at 5 and 60 min, respectively; all p < 0.001) versus tiotropium 18 µg QD monotherapy (Citation18). BUD/FOR plus tiotropium also significantly improved health status (mean difference in SGRQ total score: –2.3 units; p = 0.023) and symptoms (mean differences in Global Chest Symptoms scores: p < 0.05; mean differences in COPD symptoms rating scale scores: p < 0.001) compared with tiotropium monotherapy. Further, BUD/FOR plus tiotropium significantly reduced the rate of moderate and severe exacerbations (defined as worsening of COPD leading to treatment with systemic corticosteroids [oral or parenteral] and/or hospitalization/emergency room visits) (rate ratio 0.38; 95% confidence interval [CI]: 0.25–0.57; p < 0.001) compared with tiotropium monotherapy (Citation18). However, interpretation of the CLIMB study is made more difficult by its relatively short duration (3 months). The study indicates an initial benefit of triple therapy on exacerbations (i.e. the intervention worked), but it does not indicate whether this effect is sustained beyond the brief study period, which may not allow for the achievement of maximal responses to treatment. Furthermore, in studies of this kind, seasonality may influence the observed changes in exacerbation rate, as the incidence of exacerbations is higher in winter compared with other seasons (Citation35). One positive feature of this trial was the absence of differential withdrawal between treatment arms, possibly due to the short study duration. Differential dropout rates between study arms can result in a loss of statistical power to detect differences in exacerbation rates (Citation36).
Some support for these observations came from Hanania et al., who found that treatment with SFC 50/250 µg BID plus tiotropium 18 µg QD significantly improved lung function (least squares mean [LSM] difference in trough FEV1: 115 mL; p < 0.001) and rescue medication use (LSM difference: −0.6 puffs/day; p = 0.01) compared with tiotropium 18 µg QD monotherapy over 24 weeks in patients with moderate-to-severe COPD. No significant between-group differences were observed in dyspnea, health status or exacerbation rate () (Citation20).
In the GLISTEN study, SFC 50/500 µg BID plus glycopyrronium bromide 50 µg QD significantly improved lung function (increase in trough FEV1: 101 mL; p < 0.001), health status (LSM treatment difference in SGRQ total score −2.15 units; p = 0.02) and rescue medication use (between-group mean difference −0.72 puffs/day; p < 0.001) compared with SFC 50/500 µg BID over 12 weeks in patients with moderate-to-severe COPD. Furthermore, improvements in lung function, health status and rescue medication use observed with SFC plus glycopyrronium versus SFC were similar to those seen with SFC 50/500 µg BID plus tiotropium 18 µg QD versus SFC () (Citation23).
Additionally, a retrospective cohort study using a National Health Service database of UK patients with COPD supports the idea that the addition of tiotropium to LABA/ICS may confer benefits in reducing all-cause mortality (hazard ratio [HR] 0.65; 95% CI: 0.57–0.75; p < 0.001) and hospital admissions (HR 0.85; 95% CI: 0.73–0.99; p = 0.04) compared with LABA/ICS (Citation22).
Other pre-2016 studies demonstrating the superiority of triple therapy over LABA/ICS and LAMA monotherapy are summarized in (Citation17,Citation19,Citation21). All studies listed within this section compared triple therapy administered via different inhalers (usually LABA/ICS plus LAMA) with LABA/ICS or LAMA monotherapy (the dual bronchodilator arm of the OPTIMAL study gave each inhaler separately). Moreover, data from several of these studies were not collected for long enough or in an appropriate population to provide a conclusive answer about the benefit of triple therapy on exacerbations.
More recent data (2016 onwards) with fixed-dose triple therapy combinations
All triple therapy regimens discussed in this section consist of fixed-dose combinations of ICS, LABA, and LAMA administered using a single inhaler device.
In the TRILOGY trial, triple therapy with beclometasone dipropionate/formoterol fumarate/glycopyrronium (BDP/FOR/GLY) 100/6/12.5 µg two puffs BID significantly improved pre-dose FEV1 (adjusted mean difference: 81 mL; p < 0.001) and 2-hour post-dose FEV1 (adjusted mean difference: 117 mL; p < 0.001; two of three co-primary endpoints) compared with BDP/FOR 100/6 µg two puffs BID after 26 weeks; however, there were no significant improvements in TDI total score (third co-primary endpoint) after 26 weeks () (Citation24). The incidence of pneumonia was similar between the two treatment groups (23 and 18 cases in the BDP/FOR/GLY and BDP/FOR groups, respectively; 3% for both).
In the TRINITY trial, triple therapy with BDP/FOR/GLY 100/6/12.5 µg two puffs BID significantly reduced the rate of moderate or severe exacerbations (rate ratio 0.80; 95% CI: 0.69–0.92; p = 0.0025) and improved lung function (mean difference in pre-dose FEV1: 61 mL; p < 0.0001) compared with tiotropium 18 µg QD monotherapy in symptomatic COPD patients over 52 weeks (Citation25). The incidence of pneumonia was similar between the BDP/FOR/GLY group (28 cases; 3%), the tiotropium group (19 cases; 2%) and the BDP/FOR 100/6 µg two puffs BID plus tiotropium 18 µg QD group (12 cases; 2%) (Citation25).
The interpretation of the TRILOGY and TRINITY studies is somewhat limited by the low incidence of exacerbations in the study population: the mean number of reported exacerbations in the previous 12 months was 1.2 and only approximately 20% of patients had ≥2 exacerbations, or ≥1 hospitalization in that time frame (Citation24,Citation25,Citation37). Furthermore, the rate of moderate and severe exacerbations in the studies were low; for instance, in TRINITY, the combined rate of moderate and severe exacerbations per treatment arm were 0.46 (BDP/FOR/GLY), 0.57 (tiotropium), and 0.45 (BDP/FOR plus tiotropium) exacerbations per year (Citation25). As these patients may not be considered as ‘frequent exacerbators’ at high risk of future exacerbations, it is difficult to draw conclusions on the results of this study in a higher risk population.
The FULFIL (lung FUnction and quality of LiFe assessment in COPD with closed trIpLe therapy) study demonstrated that treatment with fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) 100/62.5/25 µg QD significantly improved lung function compared with BUD/FOR 400/12 µg BID at all time-points over the 24-week treatment period in the intention-to-treat population (between-treatment difference in trough FEV1 at Week 24: 171 mL; p < 0.001) and in the 52-week extension population (between-treatment difference in trough FEV1 at Week 52: 179 mL; p < 0.001) (Citation26). FF/UMEC/VI significantly improved health status compared with BUD/FOR at Week 24 (between-treatment difference in SGRQ total score: –2.2 units; p < 0.001), but the treatment difference did not reach statistical significance at Week 52 (between-treatment difference in SGRQ total score: –2.7 units; p = 0.065). A greater proportion of patients in the FF/UMEC/VI group than the BUD/FOR group experienced a clinically meaningful improvement (≥4 unit decrease from baseline) in SGRQ total score at Week 24 (50% vs. 41% of patients, respectively; odds ratio 1.41; p < 0.001). Furthermore, FF/UMEC/VI significantly reduced the annual rate of moderate and severe exacerbations compared with BUD/FOR based on data up to 24 weeks (35% reduction in rate; p = 0.002). After 24 weeks, the incidence of pneumonia was almost three-fold higher in the FF/UMEC/VI group (20 cases; 2.2%) than the BUD/FOR group (7 cases; 0.8%: p-value not provided); in the 52-week extension population, the incidence of pneumonia was 1.9% and 1.8% (both 4 cases) in the FF/UMEC/VI and BUD/FOR groups, respectively (Citation26).
Studies to date have compared triple therapy with LABA/ICS or LAMA monotherapy. Triple therapy led to improvements in lung function, health status, rescue medication use and exacerbation rate compared with LABA/ICS and LAMA monotherapy, with no significant difference observed in the rate of adverse events between treatment groups. Generally, the incidences of pneumonia were low across the discussed studies, with a slightly higher number of pneumonia cases seen with triple therapy (3%) versus LAMA monotherapy (2%) in TRINITY (Citation24–26). However, the more relevant and informative comparison is triple therapy versus LABA/LAMA. Any benefit seen with triple therapy may be driven mainly by dual bronchodilation; the benefit of adding ICS to LABA plus LAMA is as yet unknown (Citation38,Citation39).
Expected data
All triple therapy regimens discussed within this section consist of fixed-dose combinations of ICS, LABA, and LAMA administered using a single inhaler device, and are yet to report results.
The IMPACT (InforMing the PAthway of COPD Treatment) study will evaluate the efficacy of triple therapy with FF/UMEC/VI 100/62.5/25 µg QD compared with UMEC/VI 62.5/25 µg QD and FF/VI 100/25 µg QD in patients with severe-to-very severe COPD. IMPACT has broad inclusion criteria and enrolled approximately 10,000 patients with a post-bronchodilator FEV1 <50% predicted and a documented history of ≥1 moderate or severe COPD exacerbation in the previous 12 months; patients with a post-bronchodilator FEV1 50–80% predicted and a documented history of ≥2 moderate exacerbations in the previous 12 months; or patients with documented history of ≥1 severe COPD exacerbation in the previous 12 months (Citation27,Citation40). The primary outcome of IMPACT is the rate of moderate and severe exacerbations over 52 weeks of treatment. The rate and time to first moderate-or-severe exacerbation will be analyzed in a subset of patients with a blood eosinophil count ≥150 cells/µL. IMPACT is expected to complete in July 2017.
TRIBUTE will evaluate the efficacy of triple therapy with BDP/FOR/GLY 100/6/12.5 µg BID compared with indacaterol/glycopyrronium (IND/GLY) 85/43 µg QD in approximately 1,500 patients with severe-to-very severe COPD and a history of ≥1 exacerbation in the previous 12 months (Citation28). The primary outcome of TRIBUTE is the rate of moderate and severe COPD exacerbations over 52 weeks of treatment. TRIBUTE is expected to complete in July 2017.
ETHOS will evaluate the efficacy of triple therapy with BUD/FOR/GLY 320/9.6/14.4 µg compared with FOR/GLY 9.6/14.4 µg and BUD/FOR 320/9.6 µg in approximately 8,000 patients with moderate-to-very severe COPD (dosing frequency of treatment regimens not currently specified but likely to be twice daily). Recruited patients had a post-bronchodilator FEV1 <65% predicted and a history of exacerbations (Citation29). The primary outcome of ETHOS is the rate of moderate or severe COPD exacerbations over 52 weeks of treatment. ETHOS is expected to complete in December 2018.
In summary, further evidence is required for the efficacy of triple therapy compared with LABA/LAMA, which will be provided by a number of upcoming studies.
Methodological considerations – run-in treatment
Several of the trials described above incorporated a run-in period prior to randomization to study treatment in the study design, to bridge the gap between discontinuation of usual medication and commencement of study treatment. It has been suggested that run-in treatment can introduce bias in favor of the medication/class used in the run-in period, since patients responding to that treatment are more likely to reach randomization than those who do not respond or who worsen following withdrawal of other treatments (Citation41). Several of the triple therapy trials discussed here had a run-in period of open-label LAMA treatment ( and ), requiring patients using ICS or LABA/ICS before entering the trial to discontinue that treatment. In clinical practice it is recommended that ICS are withdrawn in a controlled manner with adequate bronchodilation in place (as discussed below) and it is possible that ICS withdrawal could have increased risk of exacerbations in previously treated patients during the run-in and study treatment periods. Few trials have included analyses to address this potential bias, although in the CLIMB study, which incorporated a 2-week run-in period of open-label tiotropium, no effect of ICS withdrawal on exacerbation rates in the trial was observed (Citation18). Studies using LABA/ICS (Citation24) or LABA/LAMA (Citation28) in the run-in may provide information on potential clinical benefits of stepping up to triple therapy in a way that more closely reflects likely clinical practice (Citation24).
Stepping down from triple therapy to dual bronchodilation
Despite treatment with triple therapy, patients may still experience frequent exacerbations. GOLD recommends stepping down treatment from triple therapy to dual bronchodilation if no benefit of ICS is seen. However, GOLD does not provide guidance regarding how to withdraw ICS and switch treatments in clinical practice, and acknowledges that there is a lack of data in this area.
There is evidence that ICS may be withdrawn from LABA/ICS in patients at low risk of exacerbation, provided they are in receipt of an effective long-acting bronchodilator (Citation42). The INSTEAD (Indacaterol: Switching Non-exacerbating Patients with Moderate COPD From Salmeterol/Fluticasone to Indacaterol) study investigated the effect of switching from SFC 50/500 µg BID to indacaterol monotherapy 150 µg QD in patients with no exacerbations in the previous year, who were receiving treatment with SFC 50/500 µg BID for ≥3 months prior to screening (Citation42). There were no significant differences in terms of lung function, dyspnea, health status, rescue medication use, or exacerbation risk between switching to indacaterol monotherapy or continuing treatment with SFC.
In patients with more severe COPD, evidence suggests that ICS may be withdrawn from triple therapy provided adequate dual bronchodilator therapy is in place. (WISDOM Withdrawal of inhaled glucoorticoids and exacerbations of COPD) investigated the impact of stepwise ICS withdrawal on the risk of moderate or severe COPD exacerbations compared with ICS continuation in patients with severe-to-very severe COPD and ≥1 exacerbation in the previous year (Citation39). All patients received triple therapy with SFC 50/500 µg BID plus tiotropium 18 µg QD during the 6-week run-in period, following which the fluticasone dose was reduced incrementally in the ICS-withdrawal arm over the subsequent 12 weeks. The risk of moderate or severe exacerbations was similar among patients in whom ICS was withdrawn and those who continued ICS therapy. There was a statistically significant difference in FEV1 after ICS withdrawal in favor of continued ICS treatment, which began in the first week after complete ICS withdrawal and persisted for a further 2 weeks (Citation39). FEV1 decline stabilized between Week 18 and study end at Week 52 (Citation39,Citation43). During the stabilization phase, FEV1 slopes were similar between patients who continued ICS treatment and those who withdrew ICS treatment (Citation43).
Further evidence is required for the outcomes of withdrawing ICS from triple therapy. SUNSET will assess the efficacy and safety of SFC 50/500 µg BID plus tiotropium 18 µg QD versus IND/GLY 110/50 µg QD in COPD patients with moderate-to-severe airflow limitation and ≤1 moderate or severe exacerbation in the previous year who had been treated with triple therapy for ≥6 months prior to the study (Citation44). The primary outcome of SUNSET is the mean change from baseline in post-dose trough FEV1 following 26 weeks of treatment. SUNSET is expected to complete in August 2017.
Currently, GOLD does not provide any recommendations regarding the method of ICS withdrawal in patients with COPD, and there are no head-to-head studies directly comparing abrupt ICS cessation versus stepwise withdrawal. However, a stepwise withdrawal of ICS, such as that conducted in WISDOM, may minimize the potential risk of rebound steroid effects, which are common following abrupt cessation of chronic systemic corticosteroid therapy (Citation45). However, in INSTEAD, in which ICS was withdrawn immediately in patients at low risk of exacerbation, there were no differences in lung function, symptoms, health status and exacerbations between ICS withdrawal and continuation (Citation42).
In light of the available evidence and GOLD recommendations for stepping down from triple therapy, an algorithm is required for the withdrawal of ICS that considers withdrawal method, optimal bronchodilation following withdrawal, risk factors and patient follow-up. presents a flow-chart for ICS withdrawal proposed by Kaplan et al (Citation46); however, the recommendation that an increase in blood eosinophils during stepwise withdrawal supports the re-introduction of ICS is not supported by data. More recently, the International Primary Care Respiratory Group have proposed an algorithm to identify the minority of patients who might benefit from ICS treatment, and provide guidance on how to withdraw ICS in COPD patients in whom it is not needed (Citation47). In practice, many clinicians are reluctant to stop therapy once instituted, even though the patient is stable, as exacerbations are not easily predicted and the next exacerbation may be attributed to treatment change. Whether or not a culture of stepping treatment up and down in patients with COPD can be instituted, with results that reflect those from the studies available, is an unanswered question.
Figure 1. A proposed algorithm for ICS withdrawal adapted from Kaplan A, et al. (Citation46). ACO, asthma-COPD overlap; COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist.
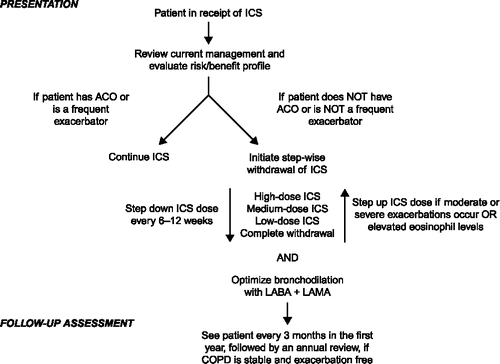
Potential alternatives to triple therapy with LABA/LAMA/ICS
Patients who experience further exacerbations on dual bronchodilation may benefit from additional treatment with a phosphodiesterase-4 (PDE4)-inhibitor or a macrolide instead of ICS; however, few studies have addressed these combinations. GOLD also recommend treatment with mucolytics, such as carbocysteine and N-acetylcysteine, for patients not receiving ICS and who are still experiencing exacerbations, while the European Respiratory Society/American Thoracic Society (ERS/ATS) guidelines for the management of exacerbations provide guidance on the use of oral steroids and antibiotics in ambulatory patients, and on the initiation of pulmonary rehabilitation post-discharge (Citation1,Citation48).
PDE4-inhibitors
When combined with LABA or LAMA, roflumilast significantly improved lung function compared with either agent alone (Citation49). Other studies investigating the use of roflumilast combined with LABA demonstrated significant improvements in lung function and quality of life versus roflumilast monotherapy (Citation50). In spite of this evidence, GOLD currently recommends roflumilast only as an additional treatment in Group D patients with post-bronchodilator FEV1 <50% predicted and chronic bronchitis who continue to experience exacerbations on triple therapy (Citation1).
REACT (Roflumilast and Exacerbations in patients receiving Appropriate Combination Therapy) investigated the effect of adding roflumilast to triple therapy on exacerbation rate compared with triple therapy alone in patients with severe COPD, chronic bronchitis and a risk of frequent exacerbations, who were receiving LABA/ICS with or without tiotropium for at least 12 months prior to the baseline visit (Citation51). The rate of moderate or severe exacerbations was numerically lower with LABA/ICS plus roflumilast versus LABA/ICS alone following Poisson regression analysis, and was significantly lower following negative binomial regression analysis. Furthermore, LABA/ICS plus roflumilast significantly reduced the rate of severe exacerbations, and exacerbations requiring hospital admission compared with LABA/ICS alone (Citation51). In a post-hoc analysis of RE(2)SPOND (Roflumilast Effect on Exacerbations in Patients on Dual [LABA/ICS] Therapy), roflumilast plus LABA/ICS significantly reduced the rate of moderate or severe exacerbations versus placebo in patients with severe-to-very severe COPD and chronic bronchitis, a history of >3 exacerbations and/or ≥1 hospitalizations in the prior year (Citation52). A further post-hoc analysis of REACT (51) and RE(2)SPOND (Citation52) demonstrated that the rate of moderate or severe exacerbations and severe exacerbations leading to hospitalization and/or death (observed with LABA/ICS ± LAMA plus roflumilast versus LABA/ICS ± LAMA) were considerably reduced in patients with ≥1 COPD-related hospitalization in the previous year versus the overall population (Citation53).
Macrolides
When added to usual care, azithromycin significantly delayed the median time to first exacerbation and significantly reduced the frequency of exacerbations compared with usual care alone among patients at risk of exacerbations in a 1-year randomized controlled trial (Citation54). Similar findings were reported in COLUMBUS (COPD: influence of Macrolides on exacerbation frequency in patients): the addition of azithromycin to usual care significantly reduced exacerbation rate and significantly increased the time-to-first exacerbation compared with usual care alone in patients with COPD who had received treatment for ≥3 exacerbations in the previous year despite maximal bronchodilation therapy (Citation55). Similarly, the addition of erythromycin therapy to usual care in patients with moderate-to-severe COPD was associated with a significant reduction in exacerbations over 12 months compared with usual care alone (Citation56).
Further alternatives
The ERS/ATS guidelines advise that the use of antibiotics in patients with exacerbations reduces the risk of treatment failure and prolongs the time to the next exacerbation (Citation48). The guidelines recommend the use of oral corticosteroids in ambulatory patients experiencing a COPD exacerbation, to improve lung function and result in fewer hospitalizations (Citation48). It is also stated that initiation of pulmonary rehabilitation, within 3 weeks of discharge following a COPD exacerbation, reduces hospital admissions and improves patient quality of life (Citation48). GOLD suggests that treatment with mucolytics, such as carbocysteine and N-acetylcysteine, may reduce exacerbations and somewhat improve health status in patients not receiving ICS (Citation1).
Identification of patients who are appropriate for triple therapy
Long-term ICS use is associated with an increased risk of pneumonia and other potential adverse events (Citation7–9,Citation57,Citation58) therefore careful identification of patients who may benefit most from triple therapy is required, with consideration for the risk/benefit balance. Patients at risk of exacerbation differ in terms of phenotype and response to treatment. For example, patterns of biomarkers can be used to identify predefined exacerbation phenotypes (Citation59). INSPIRE demonstrated that patients receiving LABA/ICS suffered less frequently from exacerbations requiring treatment with systemic corticosteroids than patients receiving LAMA monotherapy (Citation6), and this has also been demonstrated versus LABA monotherapy (Citation60).
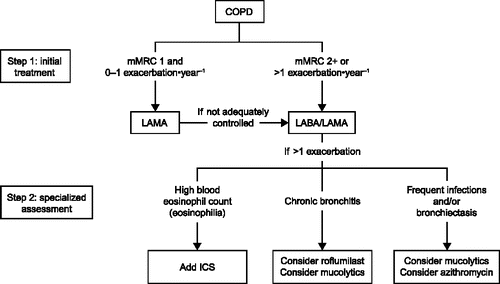
Local guidelines limit triple therapy to certain populations and phenotypes. For example, the Spanish COPD guidelines (GesEPOC) recommend triple therapy in high-risk patients with asthma-COPD overlap (Citation61). Triple therapy may also be an option for patients with exacerbations and emphysema, or exacerbations and chronic bronchitis (Citation61). Miravitlles and Anzueto propose an algorithm for the management of patients who continue to experience exacerbations despite treatment with a dual bronchodilator, and recommend additional treatment according to phenotype or treatable traits () (Citation14). In the proposed algorithm, patients recommended to receive triple therapy are those with elevated blood eosinophils (Citation14).
Figure 2. A two-step treatment algorithm for the treatment of COPD from Miravitlles and Anzueto. Eur Respir J 2017 (14). Reproduced with permission from the ©ERS 2017. European Respiratory Journal Feb, 2017, 49 (2) 1602200; DOI: 10.1183/13993003.02200–2016, At the Step 2: in patients with >1 exacerbation on LABA/LAMA, comorbidities and compliance to treatment should be assessed in order to determine additional treatment according to individual patient phenotype. COPD, chronic obstructive pulmonary disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council dyspnea scale.
Blood eosinophils as a biomarker for ICS use
Post-hoc analyses indicate that patients with elevated blood eosinophils gained a greater benefit from LABA/ICS versus long-acting bronchodilator monotherapy and placebo than patients with lower blood eosinophils (Citation62–64). A post-hoc analysis of two COPD trials (Citation65,Citation66) examined the effect of blood eosinophil levels on exacerbation risk in patients receiving LABA or LABA/ICS (Citation67). The analysis indicated a smaller reduction in exacerbation risk in patients with a high blood eosinophil count who received LABA monotherapy, compared with patients who received LABA/ICS (Citation67). Furthermore, pre-specified subgroup analyses of TRINITY demonstrated that the reductions in the rates of moderate or severe exacerbations with triple therapy versus tiotropium observed in patients with ≥2% blood eosinophils were considerably greater than those observed in the <2% subgroup (Citation25).
A post-hoc analysis of WISDOM demonstrated that in patients with ≥4% and ≥5% blood eosinophils, the rate of moderate or severe exacerbations was higher following ICS withdrawal versus ICS continuation (Citation68). In further post-hoc investigations, WISDOM data were stratified according to blood eosinophil levels in combination with prior exacerbations (Citation69). The analysis found that ICS withdrawal was only associated with an increased exacerbation rate versus ICS continuation in patients with both ≥2 exacerbations in the previous year and elevated blood eosinophils (≥300 cells/µL). Although a significant effect was seen in patients with ≥300 cells/µL blood eosinophils, this was more pronounced in patients with ≥400 cells/µL blood eosinophils (Citation69). However, it should be noted that the numbers of patients with high blood eosinophils decreased as the threshold increased, and that this was a post-hoc analysis of a subgroup, therefore caution is required when interpreting the results.
Additionally, a prospective analysis of FLAME demonstrated that LAMA/LABA was superior to LABA/ICS in the prevention of COPD exacerbations regardless of baseline eosinophil level (Citation70,Citation71). There was a trend between increasing blood eosinophil count and increased response to LABA/ICS treatment (Citation71). However, LABA/ICS was not significantly superior to LABA/LAMA at any cut off (Citation71). The potency of the dual bronchodilator used may have ‘overpowered’ the effect of SFC even in patients with high blood eosinophil counts (Citation72). In addition, a post-hoc analysis of ISOLDE demonstrated that ICS significantly reduced the rate of exacerbations versus placebo in patients with <2% blood eosinophils at baseline compared with patients with ≥2% blood eosinophils at baseline (Citation73).
One potential drawback of blood eosinophils as a biomarker for ICS efficacy is the variability of blood eosinophils over time (Citation25,Citation62,Citation73,Citation74). In their analysis, Pascoe et al. found that 33% of patients with blood eosinophils <2% at screening had blood eosinophils ≥2% at Week 52 (Citation62). Singh et al. also identified an “intermittent” subgroup of patients (49%) with variable eosinophil levels that oscillated above and below 2% (Citation74). Furthermore, Oshagbemi, et al. demonstrated that the stability of blood eosinophils was higher in COPD patients with baseline eosinophil count <340 cells/µL versus those with ≥340 cells/µL (Citation75).
Further prospective research is required to validate the use of blood eosinophils to predict relative ICS efficacy, and if successful, to determine the most appropriate blood eosinophil threshold for ICS use. To date, only the FLAME study has prospectively investigated the role of blood eosinophils in stratifying treatment response. This hypothesis will be explicitly tested as a pre-specified secondary endpoint in IMPACT (Citation27); although, ‘real world’ studies will be needed to determine whether blood eosinophil-directed treatment choices are useful for individual patients.
Based on what we already know, blood eosinophils (in conjunction with exacerbation history) might also help to identify patients in whom ICS withdrawal may lead to an increased exacerbation rate (Citation69).
Reducing exposure to ICS and associated risks
In patients who require ICS, there is a need for further insight into how to reduce the risks associated with long-term ICS use. GOLD suggest that the long-term safety (>3 years) of ICS in patients with COPD is unclear and requires further investigation (Citation1). The seasonality of exacerbations is one potential area for further research; there is a higher incidence of exacerbations in winter compared with other seasons (Citation35). The exact causes of this seasonality are unknown, but are thought to include increased exposure to viral infections and increased host susceptibility, possibly caused by reduced vitamin D levels or increased airway inflammation (Citation76).
Conclusions
The evidence currently available for the efficacy of triple therapy is in comparison with LABA/ICS and LAMA monotherapy. However, the publication of several studies that examine the efficacy and safety of triple therapy versus LABA/LAMA, a more relevant comparison, is imminent. Patients who are in receipt of triple therapy unnecessarily face the risks associated with ICS use, without the benefits, as well as increased treatment costs.
Furthermore, patients may continue to experience exacerbations on triple therapy. In these patients, GOLD recommend additional therapy on top of triple therapy, or withdrawal of the ICS component (Citation1). WISDOM provides evidence for the withdrawal of ICS from triple therapy, without increased risk of exacerbation (Citation39). However, further evidence is required, and further guidance is needed as to how and when ICS should be withdrawn and appropriate follow-up with patients (Citation46,Citation47).
Individualized treatments and patient phenotyping are recognized as important areas for further research; the determination of predictive markers could aid clinical decision making by identifying patients most likely to benefit from ICS treatment (Citation14,Citation61). Blood eosinophils may help to identify patients who would benefit from the addition of ICS to dual bronchodilation, although further evidence from prospective studies is required for this comparison. In patients who require ICS, steps may be taken to reduce long-term exposure to ICS.
In light of the evidence to date, we believe that patients on LABA/LAMA who still experience exacerbations and have elevated blood eosinophils may benefit from triple therapy; however, a clear threshold for elevated blood eosinophils is yet to be defined. Personalized therapy based on patient phenotype may help address unmet needs in COPD and help to optimize patient outcomes; however, we acknowledge that gaps in knowledge remain with regards to triple therapy and its relative efficacy versus dual bronchodilation, answers which will hopefully be provided by ongoing clinical trials.
Declaration of Interest
The authors were assisted in the preparation of the manuscript by Emily Fisher and Hannah Birchby, professional medical writers at CircleScience, an Ashfield Company, part of UDG Healthcare plc (Tytherington, UK). Medical writing support was funded by Novartis Pharma AG (Basel, Switzerland).
Peter M.A. Calverley has received speaker fees from Boehringer Ingelheim, AstraZeneca and Recipharm and consulting fees from Boehringer Ingelheim, GSK, Recipharm and Zambon.
Marc Miravitlles has received speaker fees from Boehringer Ingelheim, AstraZeneca, Chiesi, GlaxoSmithKline, Menarini, Teva, Grifols and Novartis, and consulting fees from Boehringer Ingelheim, GlaxoSmithKline, Gebro Pharma, CLS Behring, Cipla, MediImmune, Mereo Biopharma, Teva, Novartis and Grifols.
Helgo Magnussen has received speaker fees from Boehringer Ingelheim, Astra Zenaca, Menarini and Novartis, and consulting fees from Boehringer Ingelheim, Astra Zeneca, Novartis and GSK.
Jadwiga A. Wedzicha has not received any speaker or consulting fees since January 2015. She has received research grants from GSK, Vifor Pharma, Johnson and Johnson, and Takeda.
Acknowledgments
The authors were assisted in the preparation of the manuscript by Emily Fisher and Hannah Birchby, professional medical writers at CircleScience, an Ashfield Company, part of UDG Healthcare plc (Tytherington, UK).
References
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Arch Bronconeumol. 2017;53(3):128–149.
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555.
- Szafranski W, Cukier A, Ramirez A, Menga G, Sansores R, Nahabedian S, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(1):74–81.
- Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22(6):912–919.
- Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789.
- Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26.
- Crim C, Calverley PM, Anderson JA, Celli B, Ferguson GT, Jenkins C, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34(3):641–647.
- Yawn BP, Li Y, Tian H, Zhang J, Arcona S, Kahler KH. Inhaled corticosteroid use in patients with chronic obstructive pulmonary disease and the risk of pneumonia: a retrospective claims data analysis. Int J Chron Obstruct Pulmon Dis. 2013;8:295–304.
- Suissa S, Patenaude V, Lapi F, Ernst P. Inhaled corticosteroids in COPD and the risk of serious pneumonia. Thorax 2013;68(11):1029–1036.
- Wilkie M, Finch S, Schembri S. Inhaled corticosteroids for chronic obstructive pulmonary disease – The shifting treatment paradigm. COPD 2015;12(5):582–590.
- Vestbo J, Vogelmeier C, Small M, Higgins V. Understanding the GOLD 2011 Strategy as applied to a real-world COPD population. Respir Med. 2014;108(5):729–736.
- Price D, West D, Brusselle G, Gruffydd-Jones K, Jones R, Miravitlles M, et al. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–905.
- Vestbo J, Hurd SS, Agusti AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365.
- Miravitlles M, Anzueto A. A new two-step algorithm for the treatment of COPD. Eur Respir J 2017;49(2): pii: 1602200. doi: 10.1183/13993003.02200-2016.
- Cooper CB, Barjaktarevic I. A new algorithm for the management of COPD. Lancet Respir Med. 2015;3(4):266–268.
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146(8):545–555.
- Singh D, Brooks J, Hagan G, Cahn A, O'Connor BJ. Superiority of "triple" therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax 2008;63(7):592–598.
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(8):741–750.
- Hoshino M, Ohtawa J. Effects of adding salmeterol/fluticasone propionate to tiotropium on airway dimensions in patients with chronic obstructive pulmonary disease. Respirology 2011;16(1):95–101.
- Hanania NA, Crater GD, Morris AN, Emmett AH, O'Dell DM, Niewoehner DE. Benefits of adding fluticasone propionate/salmeterol to tiotropium in moderate to severe COPD. Respir Med. 2012;106(1):91–101.
- Jung KS, Park HY, Park SY, Kim SK, Kim YK, Shim JJ, et al. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respir Med. 2012;106(3):382–389.
- Short PM, Williamson PA, Elder DH, Lipworth SI, Schembri S, Lipworth BJ. The impact of tiotropium on mortality and exacerbations when added to inhaled corticosteroids and long-acting b-agonist therapy in COPD. Chest 2012;141(1):81–86.
- Frith PA, Thompson PJ, Ratnavadivel R, Chang CL, Bremner P, Day P, et al. Glycopyrronium once-daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study-a randomised controlled trial. Thorax 2015;70(6):519–527.
- Singh D, Papi A, Corradi M, Pavlisova I, Montagna I, Francisco C, et al. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting beta2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet 2016;388(10048):963–973.
- Vestbo J, Papi A, Corradi M, Blazhko V, Montagna I, Francisco C, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet 2017;389(10082):1919–1929.
- Lipson DA, Barnacle H, Birk R, Brealey N, Locantore N, Lomas DA, et al. FULFIL trial: once-daily triple therapy in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(4):438–446.
- Pascoe SJ, Lipson DA, Locantore N, Barnacle H, Brealey N, Mohindra R, et al. A phase III randomised controlled trial of single-dose triple therapy in COPD: the IMPACT protocol. Eur Respir J. 2016;48(2):320–330.
- ClinicalTrials.gov. NCT02579850. (TRIBUTE) 2-arm Parallel Group Study of Fixed Combination of CHF 5993 vs Ultibro® in COPD Patients. Updated September 30, 2016. [internet]. [Last accessed February 9, 2017]. Available at https://clinicaltrials.gov/ct2/show/NCT02579850.
- ClinicalTrials.gov. NCT02465567. (ETHOS) Study to Assess the Efficacy and Safety of PT010 Relative to PT003 and PT009 in Subjects With Moderate to Very Severe COPD. Updated December 22, 2015. [internet]. [Last accessed March 8, 2017]. Available at https://clinicaltrials.gov/ct2/show/NCT02465567.
- Donohue JF, Betts KA, Du EX, Altman P, Goyal P, Keininger DL, et al. Comparative efficacy of long-acting beta2-agonists as monotherapy for chronic obstructive pulmonary disease: a network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:367–381.
- Ismaila AS, Huisman EL, Punekar YS, Karabis A. Comparative efficacy of long-acting muscarinic antagonist monotherapies in COPD: a systematic review and network meta-analysis. Int J Chron Obstruct Pulmon Dis. 2015;10:2495–2517.
- Dransfield MT, Feldman G, Korenblat P, LaForce CF, Locantore N, Pistolesi M, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (100/25 mcg) versus twice-daily fluticasone propionate/salmeterol (250/50 mcg) in COPD patients. Respir Med. 2014;108(8):1171–1179.
- Larsson K, Janson C, Lisspers K, Jorgensen L, Stratelis G, Telg G, et al. Combination of budesonide/formoterol more effective than fluticasone/salmeterol in preventing exacerbations in chronic obstructive pulmonary disease: the PATHOS study. J Intern Med. 2013;273(6):584–594.
- Suissa S, Ernst P, Vandemheen KL, Aaron SD. Methodological issues in therapeutic trials of COPD. Eur Respir J. 2008;31(5):927–933.
- Jenkins CR, Celli B, Anderson JA, Ferguson GT, Jones PW, Vestbo J, et al. Seasonality and determinants of moderate and severe COPD exacerbations in the TORCH study. Eur Respir J. 2012;39(1):38–45.
- Calverley PM, Eriksson G, Jenkins CR, Anzueto AR, Make BJ, Persson A, et al. Early efficacy of budesonide/formoterol in patients with moderate-to-very-severe COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:13–25.
- Fabbri LM, Roversi S, Beghe B. Triple therapy for symptomatic patients with COPD. Lancet 2017;389(10082):1864–1865.
- Calverley PM. COPD therapy: if two is good, is three better? Lancet 2016;388(10048):937–938.
- Magnussen H, Disse B, Rodriguez-Roisin R, Kirsten A, Watz H, Tetzlaff K, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294.
- ClinicalTrials.gov. NCT02164513. A study comparing the efficacy, safety and tolerability of fixed dose combination (FDC) of FF/UMEC/VI with the FDC of FF/VI and UMEC/VI; administered once-daily via a dry powder inhaler (DPI) in subjects with chronic obstructive pulmonary disease (COPD). Updated November 21, 2016. [internet]. [Last accessed November 28, 2016]. Available at: http://clinicaltrials.gov/ct2/show/NCT02164513.
- Suissa S. Run-in bias in randomised trials: the case of COPD medications. Eur Respir J. 2017;49(6): pii: 1700361. doi: 10.1183/13993003.00361-2017.
- Rossi A, van der Molen T, del Olmo R, Papi A, Wehbe L, Quinn M, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548–1556.
- Magnussen H, Tetzlaff K, Bateman ED, Watz H, Kirsten AM, Wouters EF, et al. Lung function changes over time following withdrawal of inhaled corticosteroids in patients with severe COPD. Eur Respir J. 2016;47(2):651–654.
- ClinicalTrials.gov. NCT02603393 (SUNSET). Evaluation of the efficacy and safety of QVA149 (110/50 µg o.d.) vs tiotropium (18 µg o.d.) + salmeterol/fluticasone propionate FDC (50/500 µg b.i.d.) in patients with moderate to severe COPD. Updated January 21, 2016. [internet]. [Last accessed April 26, 2016]. Available at: https://clinicaltrials.gov/ct2/show/NCT02603393?term=nct02603393&rank=1.
- Suissa S, Barnes PJ. Inhaled corticosteroids in COPD: the case against. Eur Respir J. 2009;34(1):13–16.
- Kaplan AG. Applying the wisdom of stepping down inhaled corticosteroids in patients with COPD: a proposed algorithm for clinical practice. Int J Chron Obstruct Pulmon Dis. 2015;10:2535–2548.
- Kaplan A, Roman-Rodriguez M, Price D, Tsiligianni I. Evaluation of appropriateness of inhaled corticosteroid (ICS) therapy in COPD and guidance on ICS withdrawal. IPCRG Desktop Helper 2017; No. 6. [internet] [Last accessed August18, 2017]. Available at: http://www.theipcrg.org/display/TreatP/Desktop+helper+6%3A+Evaluation+of+appropriateness+of+inhaled+corticosteroid+%28ICS%29+therapy+in+COPD+and+guidance+on+ICS+withdrawal
- Wedzicha JA, Miravitlles M, Hurst JR, Calverley PM, Albert RK, Anzueto A, et al. Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J. 2017;49(3):1600791. doi: 10.1183/13993003.00791-2016.
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet 2009;374(9691):695–703.
- Wedzicha JA, Calverley PM, Rabe KF. Roflumilast: a review of its use in the treatment of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:81–90.
- Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015;385(9971):857–866.
- Martinez FJ, Rabe KF, Sethi S, Pizzichini E, McIvor A, Anzueto A, et al. Effect of roflumilast and inhaled corticosteroid/long-acting beta2-agonist on chronic obstructive pulmonary disease exacerbations (RE2SPOND). A randomized clinical trial. Am J Respir Crit Care Med. 2016;194(5):559–567.
- Martinez FJ, Rabe KF, Calverley P, Fabbri LM, Sethi S, Pizzichini E, et al. Effect of roflumilast on exacerbations in patients with severe COPD and a history of hospitalization receiving inhaled combination therapy: A pooled analysis of two randomized phase 4 studies. C49 COPD: TREATMENT. Am Thorac Soc Int Conf Abstr: Am Thorac Soc. 2017;195:A5724.
- Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr., Criner GJ, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698.
- Uzun S, Djamin RS, Kluytmans JA, Mulder PG, van't Veer NE, Ermens AA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2(5):361–368.
- Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178(11):1139–1147.
- Festic E, Bansal V, Gupta E, Scanlon PD. Association of inhaled corticosteroids with incident pneumonia and mortality in COPD patients; systematic review and meta-analysis. COPD 2015;13(3):312–326.
- Price D, Yawn B, Brusselle G, Rossi A. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim Care Respir J. 2013;22(1):92–100.
- Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671.
- Martinez FJ, Vestbo J, Anderson JA, Brook RD, Celli BR, Cowans NJ, et al. Effect of fluticasone furoate and vilanterol on exacerbations of chronic obstructive pulmonary disease in patients with moderate airflow obstruction. Am J Respir Crit Care Med. 2016;195(7):881–888.
- Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, et al. Spanish COPD Guidelines (GesEPOC): pharmacological treatment of stable COPD. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2012;48(7):247–257.
- Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3(6):435–442.
- Pavord ID, Lettis S, Locantore N, Pascoe S, Jones PW, Wedzicha JA, et al. Blood eosinophils and inhaled corticosteroid/long-acting beta-2 agonist efficacy in COPD. Thorax 2016;71(2):118–125.
- Siddiqui SH, Guasconi A, Vestbo J, Jones P, Agusti A, Paggiaro P, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):523–525.
- Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–223.
- Singh D, Kampschulte J, Wedzicha JA, Jones PW, Cohuet G, Corradi M, et al. A trial of beclomethasone/formoterol in COPD using EXACT-PRO to measure exacerbations. Eur Respir J. 2013;41(1):12–17.
- Siddiqui SH, Pavord I, Barnes N, Lettis S, Guasconi A, Petruzzelli S. Blood eosinophils (EOS) are a biomarker of COPD exacerbation reduction with inhaled corticosteroids (ICS): an across-trials model-based approach. Eur Respir J. 2016;48(Suppl 60):OA1763.
- Watz H, Tetzlaff K, Wouters EF, Kirsten A, Magnussen H, Rodriguez-Roisin R, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390–398.
- Calverley PM, Tetzlaff K, Vogelmeier C, Fabbri LM, Magnussen H, Wouters EF, et al. Eosinophilia, frequent exacerbations, and steroid response in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(9):1219–1221.
- Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234.
- Roche N, Chapman KR, Vogelmeier CF, Herth FJ, Thach C, Fogel R, et al. Blood eosinophils and response to maintenance COPD treatment: data from the FLAME trial. Am J Respir Crit Care Med. 2017;195(9):1189–1197.
- Calverley PMA. A light in the darkness? The FLAME trial, blood eosinophils, and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(9):1125–1127.
- Barnes NC, Sharma R, Lettis S, Calverley PM. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J. 2016;47(5):1374–1382.
- Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–1700.
- Oshagbemi OA, Burden AM, Braeken DC, Henskens Y, Wouters EF, Driessen JH, et al. Stability of blood eosinophils in COPD and controls and the impact of gender, age, smoking and baseline counts. Am J Respir Crit Care Med. 2017;195(10):1402–1404.
- Donaldson GC, Wedzicha JA. The causes and consequences of seasonal variation in COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2014;9:1101–1110.
- Global initiative for chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. Updated 2009. [internet] [Last accessed October 29, 2014]. Available at: www.goldcopd.com.
