ABSTRACT
This randomised, open-label, cross-over, placebo-containing inhaler study assessed patient preference indicators for ELLIPTA and HandiHaler dry powder inhalers in patients with COPD (NCT02786927; GSK identifier: 204983). The primary objective of this study was to assess patient preference between ELLIPTA and HandiHaler based on the number of steps needed to use the inhaler. Eligible patients ≥40 years of age with COPD were randomised 1:1 to receive their current COPD medication plus a placebo-containing ELLIPTA or HandiHaler inhaler once daily for 7 ± 2 days (treatment period 1); this was followed by a 7 ± 2-day placebo treatment with the alternative inhaler. A 5-item questionnaire assessed inhaler-related patient preferences. A total of 212 patients (mean age, 65.1 years) were enrolled at 22 US sites; 73% had a COPD duration ≥5 years. Median (range) exposure was 8 (Citation5, Citation13) days for ELLIPTA and 8 (Citation1, 16) days for HandiHaler. Significantly more patients preferred ELLIPTA to HandiHaler in terms of the number of steps to use and all secondary attributes (size, comfort of the mouthpiece, remaining doses, and ease of use of the two inhalers; all p < 0.001). Similar results were observed irrespective of the order of inhaler use. Eighteen patients (8%) reported at least one AE and two (<1%) patients reported four non-fatal SAEs; none were related to the study treatment. Patient attitude toward a particular inhaler and their experiences in using it can affect adherence to therapy, which can in turn strongly influence effectiveness of inhaled medications. This study uses a robust methodology to assess patient preference.
Introduction
Inhaled bronchodilator therapies form the mainstay of treatment for many patients with chronic obstructive pulmonary disease (COPD) Citation(1). Inhalation devices include nebulizers, metered-dose inhalers (MDIs) and single-dose and multi-dose dry powder inhalers (DPIs). Superiority of one inhaler/formulation has not been established in randomised controlled trials, and the choice of inhaler depends on access, cost, prescriber and, importantly, patient ability in technique and preferences Citation(1). Patient attitudes to and experiences with inhalers can impact adherence to treatment which may, in turn, influence outcomes Citation(2–5). The choice of inhaler is therefore an important consideration in the management of COPD Citation(2–6).
The ELLIPTA DPI is approved for administration of a range of therapies, including the long-acting muscarinic antagonist (LAMA) umeclidinium bromide for the long-term maintenance treatment of COPD Citation(7). ELLIPTA is owned by or licensed to the GSK group of companies. The HandiHaler DPI is approved for administration of therapies including the LAMA tiotropium for the long-term maintenance treatment of COPD Citation(8).
Studies of patient preference regarding inhalers are generally carried out based on their use when administering particular medications. This may confound patient assessment of the inhaler itself. Here, we present the results of a study designed to robustly assess several inhaler-specific aspects of patient preference with the inhalers ELLIPTA and HandiHaler. The study objective was to assess the patient preference for the inhalers only and not to evaluate the effectiveness of treatment, thus placebo-containing inhalers were used to minimise treatment bias when assessing parameters important to patients.
Materials and methods
Study design
This randomised, open-label, cross-over, placebo-containing inhaler study was performed to assess patient preference indicators for ELLIPTA and HandiHaler in patients with COPD (NCT02786927; GSK identifier: 204983). Eligible patients who had not previously used either inhaler during the 6 months prior to enrolment were randomised 1:1 to receive placebo using either an ELLIPTA or HandiHaler once-daily for 7 ± 2 days (treatment period 1) as an add-on to their COPD medication. Patients subsequently received a 7 ± 2-day placebo treatment with the alternative inhaler (treatment period 2) and then completed a 5-item questionnaire to assess their preference in terms of inhaler attributes and dosing regimens.
Preference questions were answered once by each patient, at the end of treatment period 2 or at early withdrawal visit (if the patient administered at least one dose from each study inhaler). Two inhaler preference questionnaires were used: both asked the same questions but differed in the order in which the inhalers were considered. Patients were assigned to one version of the inhaler preference questionnaire (Version 1 or Version Citation(2) during randomisation.
Patients continued their current COPD medication as prescribed throughout the study. The ELLIPTA placebo DPI provided a total of 30 doses, and the HandiHaler placebo DPI a total of 15 doses. Compliance with study inhaler use was assessed at the beginning and end of each treatment period based on the dose counter reading (ELLIPTA) or number of remaining placebo capsules (HandiHaler).
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) Good Clinical Practice (GCP), and the applicable regulatory requirements.
Patients
Eligible patients were ≥40 years of age with American Thoracic Society (ATS)/European Respiratory Society (ERS)-defined COPD for at least 6 months and a post albuterol forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio <0.70 and FEV1 ≤70% predicted in the previous 2 years, and were current or former smokers (>10 pack-years). Patients with a current diagnosis of asthma or poorly controlled COPD were excluded.
Current COPD therapy was maintenance therapy with ≥1 long-acting bronchodilator (LAMA, LABA or inhaled corticosteroid (ICS)/LABA combination) for ≥4 weeks prior to screening.
All patients provided written informed consent.
Questionnaire development
Questions were developed using item solicitation and validation methods applicable to the generation of inhaler preference items. Items were based on prior qualitative research studies of ELLIPTA and HandiHaler Citation(9–11), however, final questions and wording were refined through 2 rounds of cognitive interviews with 15 patients with COPD assessing both ELLIPTA and HandiHaler. Inclusion and exclusion criteria were used to select participants that were similar to the intended clinical trial population. During each interview round, participants were asked to provide feedback on their interpretation and understanding of the instructions, questions, and response options, as well as on the relevance and importance of the concept captured by each of the items.
The final measure was comprised of five items; four assessed inhaler characteristics (size, comfort of the mouthpiece, remaining doses and the number of steps for use) and one assessed ease of use of the two inhalers. The qualitative research undertaken provided evidence that the final items adequately and appropriately assessed patient preference related to the most important inhaler attributes from the patient perspective, thus supporting the measure's content validity. Furthermore, confirmation was obtained that the wording of the instructions, the questions, and their response options were well understood by adults with COPD.
Endpoints and assessments
The primary objective of this study was to assess patient preference between ELLIPTA and HandiHaler based on the number of steps needed to use the inhaler. Secondary objectives included assessment of patient preference between ELLIPTA and HandiHaler based on how easy it was to see how many doses remained and the size of the inhaler. Patient preference between ELLIPTA and HandiHaler based on the comfort of the mouthpiece and overall patient preference between ELLIPTA and HandiHaler were also assessed. Adverse events (AEs), serious adverse events (SAEs), and COPD exacerbations were monitored.
Sample size and statistical analysis
A sample size of 200 patients provided 90% power to detect a preference for ELLIPTA (assuming 48% preference for ELLIPTA, 28% for HandiHaler and 24% no preference). Significance tests were performed at a 2-sided 5% alpha level, using a hierarchical procedure (primary → secondary → other). Patient populations considered were intent-to-treat (ITT), modified ITT (mITT) and per protocol (PP). The ITT population was used to evaluate baseline demographic and clinical characteristics, as well as safety data. The mITT and PP populations were used in analyses of inhaler preference endpoints. Preference data were analysed using a Cochran–Mantel–Haenszel test adjusted for study inhaler use sequence and preference questionnaire version.
Results
Study population and exposure
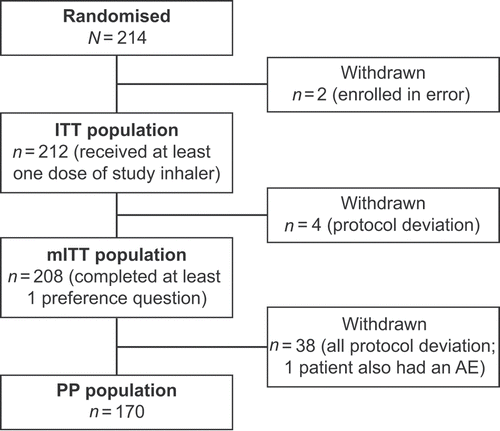
A total of 214 patients were enrolled in the study at 22 sites in the US including two enrolled in error and consequently withdrawn; the remaining 212 patients were randomised to study intervention, received at least one inhaler dose and were included in the ITT population (). Almost all (n = 207 [98%]) patients completed the study. Of the five (2%) patients who withdrew, four were due to a protocol deviation as the primary reason, while one withdrew due to an AE.
Among the ITT population, 208 patients completed at least 1 question from the questionnaire and comprise the mITT population. Subsequent protocol deviations were experienced by a further 42 (20%) patients; 170 patients were included in the PP population.
Baseline demographics (ITT population) are presented in . Most patients were white (89%) with a mean age of 65.1 years and a mean body mass index of 29.0 kg/m2 (slightly overweight). The majority of patients (73%) had a COPD duration of ≥5 years.
Table 1. Patient baseline demographics and disease characteristics (ITT population).
Median (range) exposure was 8 Citation(5–13) days for ELLIPTA and 8 (1–16) days for HandiHaler, most patients (92%) used each inhaler for at least 7 days; median (range) compliance rates were 100% (63–186) and 100% (30–278), respectively.
Patient preference
Overall, a significantly greater proportion of patients preferred ELLIPTA to HandiHaler in terms of the primary preference attribute (number of steps to use) and all secondary attributes (all p < 0.001; ). In the mITT population, preference for ELLIPTA was greatest for the number of steps taken to use the inhaler (73%), followed by overall preference (71%) and the ability to see how many doses were remaining (70%). By comparison, preference for the HandiHaler was reported by 20% of patients for the number of steps taken to use the inhaler, 21% for overall preference and 18% for the ability to see how many doses were remaining; the remaining patients reported no preference. Preference outcomes for the PP population were consistent with those in the mITT population (). Similar results were observed irrespective of the order of inhaler use (ELLIPTA → HandiHaler or HandiHaler → ELLIPTA).
Figure 2. Overall* preferences for each inhaler-specific attribute of ELLIPTA and HandiHaler mITT, modified intent-to-treat; PP, per-protocol.*Based on pooled data from both inhaler use sequences; ***p < 0.001.
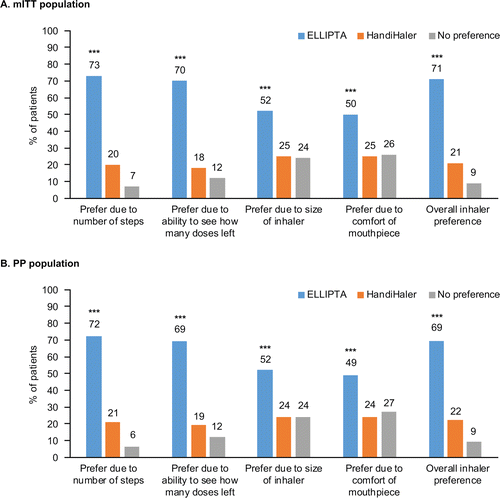
Analysis combining patients who reported a preference for the HandiHaler and those expressing no preference, versus those who reported preference for ELLIPTA, showed no change in the statistical significance of the primary endpoint: 151 patients (73%) preferred the ELLIPTA inhaler, based on the number of steps needed to use the inhaler, compared with 57 patients (27%) who preferred the HandiHaler or had no preference (p < 0.001). This was also true for the preference attributes “how easy it was to tell how many doses were left” (ELLIPTA, 146 patients [70%] vs. HandiHaler/no preference, 62 patients [30%], p < 0.001) and “overall inhaler preference” (ELLIPTA, 147 patients [71%] vs. HandiHaler/no preference, 61 subjects [29%], p < 0.001).
Safety
Eighteen patients (8%) reported at least one AE and two (<1%) patients reported four non-fatal SAEs; none were considered related to the study treatment. AEs reported in more than one patient were dyspnoea (n = 3 [1%]), arthralgia (n = 2, [<1%]) and back pain (n = 2, [<1%]).
SAEs were COPD exacerbation (in one patient, leading to study withdrawal) and acute kidney injury, Escherichia urinary tract infection and sepsis (in the other patient). COPD exacerbations occurred in four patients; three while using ELLIPTA, none of which required hospitalisation, and one while using HandiHaler, which required hospitalisation and led to study withdrawal. No deaths occurred during the study.
Discussion
The primary objective of this study was to evaluate whether more subjects with COPD prefer the ELLIPTA inhaler over the HandiHaler inhaler based on the number of activation and treatment steps needed to take the appropriate dose. The study met the primary endpoint and demonstrated statistically significant patient preference for ELLIPTA compared with HandiHaler based on the number of steps needed to use the inhaler (p < 0.001).
The study also evaluated patient preference based on how easy it was to tell how many doses were left in the inhaler, the size of the inhaler, comfort of the mouthpiece and overall inhaler preference. A statistically significant preference for the ELLIPTA inhaler over the HandiHaler inhaler was shown for all four of these endpoints (p < 0.001).
These outcomes were consistent regardless of the sequence in which the inhalers were used, the preference questionnaire version used, or the investigator site, demonstrating that these factors had no statistically significant impact on the outcome of the study. In addition, when patients who expressed a preference for the ELLIPTA inhaler were compared with those who preferred the HandiHaler inhaler or who stated “no preference,” the preference for the ELLIPTA inhaler continued to be statistically significant for the primary endpoint (preference based on the number of steps needed to use the inhaler) and two other attributes (ability to tell how many doses were left and overall preference). Preference for the remaining two attributes (size of the inhaler and comfort of the mouthpiece) was similar irrespective of inhaler preference.
Patient attitudes toward a particular inhaler and their experiences in using it can influence adherence to therapy Citation(2–5). Alongside the inhaler characteristics and the physician's familiarity with inhalers and their skill in understanding the patient's needs and preferences, the patient's knowledge, attitudes, and preference around particular inhalers is a key factor to consider. Citation(2) The effectiveness of inhaled medications is strongly influenced by adherence to these medications Citation(9) and decreased medication delivery associated with incorrect inhaler technique has been demonstrated to lead to poor efficacy Citation(12). Studies show that 36–49% of patients with COPD have at least 1 critical inhaler error (i.e., those likely to result in the inhalation of significantly reduced, minimal or no medication) with currently used inhalers Citation(12, 13), and consideration of patient preferences around inhalers has the potential to significantly influence treatment adherence and, consequently, effectiveness.
The ELLIPTA DPI is a single-step breath-activated inhaler featuring a cover that is opened by the patient to both reveal the mouthpiece and automatically load a single dose of medication. It contains 30 doses Citation(7). By comparison, the HandiHaler DPI uses individually-wrapped medication capsules (in packages of 30 or 90)(8) which are manually inserted into the inhaler. GOLD strategy document recommend that patients should receive inhaler skills training; Citation(1) however, this may not always occur. In a single visit crossover study, critical errors made by self-taught patients with COPD after reading the patient information leaflet only occurred in 17/118 (14%) patients for ELLIPTA compared with 57/118 (48%) for HandiHaler (p < 0.001) Citation(10). Most patients (57–70%) made no errors using ELLIPTA and did not require investigator instruction, compared with 38% of HandiHaler users.
When prescribing an inhaled medicine, consideration of patient preference for a particular medication or inhaler may be associated with improved adherence to treatment and thus affect long-term treatment outcome Citation(4). Treatment outcome is influenced by both medication and inhaler use. A limitation of the present study is that it does not directly assess clinical outcomes: no inferences about clinical efficacy should be made. Another limitation of this study is that information on current or prior inhaler use were not collected; furthermore, eligibility criteria only required patients to be inhaler-naïve for ELLIPTA and HandiHaler in the preceding 6 months; information on inhaler use prior to this period was not collected.
This study utilised a placebo over 7 days in each of the ELLIPTA and HandiHaler inhalers, allowing patients to continue with their routine medication while on study. This robust design enables a true assessment of patient preference for a particular inhaler without the confounding influence of medication. Preference items used in the questionnaire were developed in conjunction with a patient panel that was exposed to both the ELLIPTA and HandiHaler inhalers to ensure relevance and understanding.
Conclusions
Among the many factors that influence the use of inhalers, patient preference is a significant factor that needs to be considered. This study used a robust methodology to assess patient preference, finding that significantly more patients with COPD preferred the ELLIPTA inhaler compared with the HandiHaler inhaler for all preference questions (number of steps, ability to tell how many doses were left, size of the inhaler, comfort of the mouthpiece and overall preference).
Conflicts of interest
KAC, PP, AFP, RHS, and RKS are employees of GlaxoSmithKline and hold stocks/shares in the company. GF has no conflicts of interest to declare.
Author contributions
KAC, PP, AFP, and RKS contributed to the study conception or design, and data analysis or interpretation. RHS contributed to the study conception or design. GF was involved in data acquisition. All authors were involved in the preparation and review of the manuscript and approved the final version to be submitted.
Acknowledgments
Editorial assistance in the preparation of this article (in the form of writing assistance, including development of the initial draft based on author direction, assembling tables and figures, collating and incorporating authors comments, grammatical editing and referencing) was provided by Rachel Edwards, PhD, of Fishawack Indicia Ltd., UK and was funded by GSK.
Additional information
Funding
References
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease [Internet]. 2017. Available from: http://goldcopd.org/
- Hodder R, Price D. Patient preferences for inhaler devices in chronic obstructive pulmonary disease: experience with Respimat Soft Mist inhaler. Int. J. Chron. Obstruct. Pulmon. Dis. [Internet]. 2009[cited 2017 Jun 7];4:381–390. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19888356. doi:10.2147/COPD.S3391. PMID:19888356.
- Bateman ED, Rabe KF, Calverley PMA, Goehring UM, Brose M, Bredenbröker D, Fabbri LM. Roflumilast with long-acting β2-agonists for COPD: influence of exacerbation history. Eur. Respir. J. [Internet]. 2011 Sep [cited 2014 Sep 17];38(3):553–560. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21737553. doi:10.1183/09031936.00178710. PMID:21737553.
- Anderson LA, Gadalla S, Morton LM, Landgren O, Pfeiffer R, Warren JL, Berndt SI, Ricker W, Parsons R, Engels EA. Population-based study of autoimmune conditions and the risk of specific lymphoid malignancies. Int. J. Cancer [Internet]. 2009;125(2):398–405. Available from: http://www3.interscience.wiley.com/cgi-bin/fulltext/121655446/PDFSTART. doi:10.1002/ijc.24287. PMID:19365835.
- Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir. Med. [Internet]. 2013 Oct[cited 2017 Jun 7];107(10):1481–1490. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0954611113001340. doi:10.1016/j.rmed.2013.04.005. PMID:23643487.
- Fink JB, Colice GL, Hodder R. Inhaler devices for patients with COPD. COPD [Internet]. 2013 Aug 28 [cited 2017 Jun 7];10(4):523–535. Available from: http://www.tandfonline.com/doi/full/10.3109/15412555.2012.761960. doi:10.3109/15412555.2012.761960. PMID:23537191.
- GlaxoSmithKline. ANORO ELLIPTA prescribing information [Internet]. 2017. Available from: www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Anoro_Ellipta/pdf/ANORO-ELLIPTA-PI-MG.PDF
- Boehringer-Ingelheim. SPIRIVA HANDIHALER prescribing information [Internet]. 2016. Available from: http://docs.boehringer-ingelheim.com/PrescribingInformation/PIs/Spiriva/Spiriva.pdf
- Riley JH, Tabberer M, Richard N, Donald A, Church A, Harris SS. Correct usage, ease of use, and preference of two dry powder inhalers in patients with COPD: analysis of five phase III, randomized trials. Int J Chron Obstruct Pulmon Dis [Internet]. 2016 Aug [cited 2017 Jul 5];11:1873–80. Available from: https://www.dovepress.com/correct-usage-ease-of-use-and-preference-of-two-dry-powder-inhalers-in-peer-reviewed-article-COPD. doi:10.2147/COPD.S109121. PMID:27578968.
- van der Palen J, Thomas M, Chrystyn H, Sharma RK, van der Valk PD, Goosens M, Wilkinson T, Stonham C, Chauhan AJ, Imber V, et al. A randomised open-label cross-over study of inhaler errors, preference and time to achieve correct inhaler use in patients with COPD or asthma: Comparison of ELLIPTA with other inhaler devices. NPJ Prim care Respir Med [Internet]. 2016 Nov 24 [cited 2017 Jun 7];26(1):16079. Available from: http://www.nature.com/articles/npjpcrm201679. doi:10.1038/npjpcrm.2016.79. PMID:27883002.
- Melani AS, Canessa P, Coloretti I, DeAngelis G, DeTullio R, Del Donno M, Giacobbe R, Scarlato I, Serafini A, Barbato N, et al. Inhaler mishandling is very common in patients with chronic airflow obstruction and long-term home nebuliser use. Respir Med [Internet]. 2012 May [cited 2017 Jun 7];106(5):668–676. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22277996. doi:10.1016/j.rmed.2011.11.016. PMID:22277996.
- Melani AS. Inhalatory therapy training: a priority challenge for the physician. Acta Biomed [Internet]. 2007 Dec [cited 2017 Jun 7];78(3):233–245. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18330086
- Anderson P. Patient preference for and satisfaction with inhaler devices. Eur Respir Rev [Internet]. 2005 [cited 2017 Jun 7];14:109–116. Available from: http://err.ersjournals.com/content/errev/14/96/109.full.pdf. doi:10.1183/09059180.05.00009606.