ABSTRACT
Hyperinflation, gas trapping and their responses to long-acting bronchodilator are clinically important in COPD. The forced oscillation technique (FOT) measures of respiratory system resistance and reactance are sensitive markers of bronchodilator response in COPD. The relationships between changes in resistance and reactance, and changes in hyperinflation and gas trapping, following long-acting bronchodilator (LA-BD) have not been studied.
15 subjects with mild-moderate COPD underwent FOT, spirometry then body plethysmography, before and 2 hours after a single 150 microg dose of the LA-BD indacaterol. Hyperinflation was quantified as the inspiratory capacity to total lung capacity ratio (IC/TLC), and gas trapping as residual volume to TLC ratio (RV/TLC).
At baseline, FOT parameters were moderately correlated with IC/TLC (|r| 0.53–0.73, p < 0.05). At 2 hours post-LA-BD, there were moderate correlations between change in FOT and change in RV/TLC (|r| 0.60–0.82, p < 0.05). Baseline FOT parameters also correlated with the subsequent post-LA-BD change in both IC/TLC (|r| 0.54–0.62, p < 0.05) and RV/TLC (|r| 0.57–0.76, p < 0.05).
FOT impedance reflects hyperinflation and gas trapping in COPD, and the potential for long-acting bronchodilator responsiveness. These results provide us with further insight into the physiological mechanisms of action of long-acting bronchodilator treatment, and may be clinically useful for predicting treatment responses.
| Abbreviations | ||
| AX | = | Area under the reactance frequency spectrum curve from 5 Hz to resonant frequency |
| BD | = | Bronchodilator |
| COPD | = | Chronic obstructive pulmonary disease |
| DLCO | = | Diffusing capacity for carbon monoxide |
| EFL | = | Expiratory flow limitation |
| FEV1 | = | Forced expiratory volume in 1 second |
| FOT | = | Forced oscillation technique |
| FRC | = | Functional residual capacity |
| FVC | = | Forced vital capacity |
| IC | = | Inspiratory capacity |
| LA-BD | = | Long-acting bronchodilator |
| R5 | = | Resistance at 5 Hz |
| R5in | = | Inspiratory phase resistance at 5 Hz |
| R19 | = | Resistance at 19 Hz |
| R5-R19 | = | Resistance at 5 Hz minus resistance at 19 Hz, a measure of the frequency dependence of resistance |
| RV | = | Residual volume |
| TLC | = | Total lung capacity |
| X5 | = | Reactance at 5 Hz |
| X5in | = | Inspiratory phase reactance at 5 Hz |
| X5in-ex | = | Inspiratory phase minus expiratory phase of X5, a measure of expiratory flow limitation |
1. Introduction
Chronic obstructive pulmonary disease (COPD) is characterised by progressive airway narrowing and loss of lung elastic recoil, resulting in airflow obstruction, gas trapping and hyperinflation. Gas trapping, measured as an increase in residual volume (RV) relative to the total lung capacity (RV/TLC), is one of the earliest physiological manifestations of COPD (Citation1). Increased RV without a commensurate increase in TLC reduces the vital capacity (VC) which, in turn, reduces the forced expiratory volume in 1 second (FEV1) (Citation2). Increased RV/TLC contributes to hyperinflation, which is a strong determinant of exercise limitation and the sensation of exertional dyspnoea (Citation3,Citation4). For these reasons, identifying gas trapping and hyperinflation, reducing them pharmacologically, and quantifying their responses, is desirable from a clinical perspective.
In COPD, treatment with long-acting bronchodilator (LA-BD) improves symptom control (Citation5) and exercise performance (Citation6). However, the FEV1 response to bronchodilator (BD) relates poorly to the observed clinical improvements following LA-BD therapy (Citation7,Citation8). This suggests that the change in FEV1 alone does not adequately describe the complex physiological responses to LA-BD in COPD.
The forced oscillation technique (FOT) is used to measure respiratory system impedance by applying low-amplitude, oscillatory pressure waves at the airway opening during resting tidal breathing, and describes the resultant relationship between pressure and airflow. Respiratory system impedance has two main components: resistance (R), the component of impedance which is in-phase with airflow, and which is a measure of airway calibre; and reactance (X), the out-of-phase component, which reflects the elastic and inertive properties of the respiratory system. X at low frequencies is particularly sensitive to the expiratory flow limitation (EFL) (Citation9), airway closure and ventilation heterogeneity that are typically seen in COPD (Citation10). Short-acting BD administration decreases R and the magnitude of X (i.e. X at low frequencies becomes less negative) (Citation11), which most likely represents improvements in each of these aforementioned processes. Therefore, changes in FOT measurements following BD may be useful indicators of physiological changes in the lungs not necessarily reflected by the change in spirometry, and thus provide information that is complementary to that of spirometry. The changes in R and X in response to short-acting BD (Citation12–14) and to LA-BD (Citation15,Citation16) show weak or inconsistent correlations with FEV1. However, it is unknown how changes in FOT parameters relate to physiological changes in lung mechanics such as gas trapping and hyperinflation in COPD.
We hypothesised that FOT parameters reflect abnormalities in respiratory system mechanics due to gas trapping and hyperinflation in patients with COPD. Hence, the administration of LA-BD should lead to concurrent improvements in gas trapping, hyperinflation and FOT parameters. We therefore determined the relationships between changes in FOT parameters, and gas trapping and hyperinflation, after a single dose of the LA-BD indacaterol.
2. Methods
2.1. Study protocol
The study was approved by Sydney Local Health District Human Research Ethics Committee – CRGH (CH62/6/2014-142). Subjects underwent a screening visit where FOT and standard lung function tests were performed to confirm eligibility. On a subsequent visit, subjects underwent pre-bronchodilator measurements in the order of FOT, spirometry, then body plethysmography. A single 150 microg inhaled dose of the long-acting beta-agonist indacaterol (Novartis Pharmaceuticals, Basel, Switzerland) was then administered. FOT followed by spirometry were measured at pre-defined time points over 2 hours (5, 10, 15, 30, 45, 60, 90 and 120 minutes) to confirm maximal FOT and spirometry responses. Finally, body plethysmography was repeated at 2 hours.
2.2. Subjects
After obtaining written informed consent and permission for their data to be used in the study, we recruited 15 subjects with mild-moderate COPD (GOLD stages 1–3, FEV1 > 40% predicted, FEV1/forced vital capacity [FVC] ratio < lower limit of normal) and no history of any other significant lung or cardiac disease. We excluded subjects who had experienced an acute exacerbation within the previous 6 weeks.
2.3. Standard lung function testing
Spirometry, body plethysmography and single-breath diffusing capacity for carbon monoxide (DLCO) were performed using Vmax Encore v21-2B software on a V62J Autobox (CareFusion, Yorba Linda, CA), with the following minimum withholding periods: LA-BD, 14 days; short-acting BD, 8 hours, inhaled corticosteroid, 12 hours. Measurements were performed according to American Thoracic Society/European Respiratory Society quality criteria (Citation17,Citation18). Predicted values were determined according to published reference equations: Global Lung Initiative (GLI) (Citation19) for spirometry; Quanjer et al (Citation20) for plethysmographic lung volumes; Thompson et al (Citation21) for DLCO.
2.4. Forced oscillation technique
FOT measurements were performed using the tremoFlo C-100 Oscillometry system (v. 1.0.36.35, Thorasys Thoracic Medical Systems Inc, Montreal). The default perturbation was used, i.e., a multifrequency pseudorandom noise signal in the range 5–37 Hz, with a mean pressure wave amplitude of <2 cmH2O peak-to-peak, and sampling frequency of 1.024 kHz. A second-order bandpass filter of bandwidth 2 Hz was used to extract the frequency components, and impedance was calculated in 1-s windows, with an overlap of 95%. R(f) and X(f) at each frequency f were derived on a breath-by-breath basis. A calibration check was performed at all frequencies prior to each test session, using the manufacturer-supplied test load of known resistance and reactance.
Subjects were seated at rest, with no forced respiratory manoeuvres (e.g., spirometry) for at least 3 minutes prior to each test. They were instructed to “breathe normally” whilst sitting upright, with head in a neutral position, wearing a nose clip, and supporting their own cheeks. After a period of breathing stabilisation, confirmed by real-time monitoring of the volume–time and flow–time curves, pressure oscillations were applied for 60 seconds. Baseline measurements were performed in triplicate. To track the impedance time course following bronchodilator administration, the remainder were performed as single measurements only. For all measurements, the operator monitored the subject for signs of leak, and the volume-time curve for large breaths, coughs or swallows. Any measurements containing these artefacts were terminated and excluded, and the measurement repeated. Poor coherence, conventionally used as a quality control measure (Citation22), was flagged but not used as an exclusion criterion given the known poor coherence and the lack of consensus on the threshold of acceptability in COPD (Citation23).
After completion of testing, each measurement was assessed offline for quality using the tremoFlo software (v. 1.0.36.35). Breaths containing negative and outlier (>5 SDs) resistances at at least 30% of the frequencies were automatically excluded by the software. Individual breaths containing artefacts, including irregular flow or volume profiles, were further excluded by the operator. Measurements with fewer than 3 acceptable breaths were excluded.
The following indices were used in this analysis: mean resistance and reactance at 5 Hz (R5 and X5, respectively); mean resistance and reactance at 5 Hz of the inspiratory phase (R5in and X5in, respectively); mean resistance at 19 Hz (R19); R5 minus resistance at 19 Hz (R5-R19); mean inspiratory minus expiratory reactance at 5 Hz (X5in-ex, an index of EFL) (Citation9); and area under the reactance curve from 5 Hz to resonant frequency (AX). Predicted values for R5, X5 and AX were calculated from Oostveen et al (Citation11).
2.5. Statistical analyses
Changes in spirometry, lung volumes and FOT indices following LA-BD were calculated as post-BD minus pre-BD values; thus, improved airway function results in a negative ΔR and positive ΔX. Decreases in hyperinflation and gas trapping result in a positive ΔIC/TLC and negative ΔRV/TLC. In order to control for known co-variates, Z-scores were calculated from published reference equations. However, there are no known predicted values for IC/TLC ratio. In order to adjust for presumed covariates of age and sex, we performed multiple linear regression using age and sex as independent variables, on the pre-BD values. The unstandardised residuals from this model were used in subsequent analysis as “adjusted” values. A similar adjustment process was performed for the FOT parameters R5in, R19, R5-R19, X5in and Xin-ex, for which no population reference equations exist, using sex, age, height and weight as independent variables. The sizes of adjustments were minimal, and details of the adjustment models are provided in the Online Supplement.
The majority of results were found to be non-normally distributed based on visual inspection of the frequency histograms, and by Shapiro–Wilk test for normality. Hence non-parametric tests were used for consistency of analysis and reporting. The pre- to post-LA-BD changes were examined by Wilcoxon matched-pairs signed rank test. Correlations between variables were examined by Spearman rank correlation. All data were analysed using SPSS Statistics v24 (IBM Corporation, Armonk, NY). Graphs were generated using Prism v6 (GraphPad Software Inc, San Diego, CA).
3. Results
3.1. Baseline demographics
15 subjects with COPD of mild-moderate severity (mean ± SD FEV1 64 ± 17% predicted; GOLD stages 1, 2 and 3 comprising n = 5, 9 and 1, respectively) were recruited; 5 were current smokers. There was minimal gas trapping (mean ± SD RV/TLC 104 ± 23% predicted) and mild hyperinflation (mean ± SD functional residual capacity [FRC] 120 ± 26% predicted) (), with a wide range of abnormalities. The mean Z-score for R5 was within the limits of normal yet the mean X5 Z-score was significantly more negative.
Table 1. Subject demographics.
3.2. Pre- to post-LA-BD changes
The time courses of FOT and spirometry responses confirmed that complete bronchodilator responses had occurred, and is detailed in the Online Supplement. shows the changes in lung function 2 hours after administration of LA-BD. Compared to pre-BD results, there were significant increases in FEV1, FVC and inspiratory capacity (IC), and a concomitant decrease in FRC, but no significant changes in RV or TLC. IC/TLC increased significantly, whilst there was a non-significant trend towards reduction in RV/TLC. All FOT parameters significantly improved following LA-BD, except for R19 and X5in-ex.
Table 2. Spirometry, lung volumes and forced oscillation technique (FOT) measurements pre- and 2 hrs-post long-acting bronchodilator.
3.3. Baseline relationships between impedance, gas trapping and hyperinflation
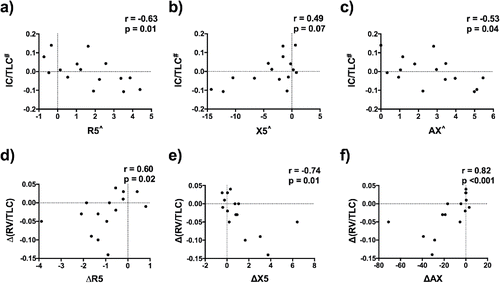
At baseline there were moderate correlations between adjusted FOT parameters and adjusted IC/TLC ratio (; ). The strongest correlations related to the adjusted inspiratory phase parameters R5in (r = −0.73, p = 0.029) and X5in (r = 0.68, p = 0.007), as well as adjusted R5-R19 (r = −0.71, p = 0.003). There was a significant correlation between adjusted X5in-ex and adjusted RV/TLC ratio (r = 0.54, p = 0.045).
Table 3. Relationships between forced oscillation technique (FOT), hyperinflation and gas trapping pre-long-acting bronchodilator.
3.4. Relationships between changes in impedance, and changes in gas trapping and hyperinflation
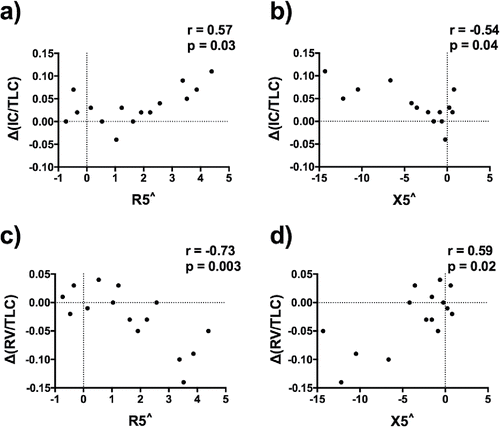
The changes in X5in and AX were the only FOT parameters that significantly correlated with change in IC/TLC ratio (r = 0.57, p = 0.041 and r = −0.54, p = 0.038, respectively) at 2 hours post-LA-BD. However, changes in R5, R5in, R5-R19, X5, X5in, AX, and X5in-ex were moderately correlated with change in RV/TLC ratio (; ).
Table 4. Relationship between change in forced oscillation technique (FOT) and change in hyperinflation and gas trapping from baseline to 2 hrs post-long-acting bronchodilator.
3.5. Baseline FOT relates to post-LA-BD improvements in gas trapping and hyperinflation
Baseline adjusted FOT parameters correlated with the subsequent change at 2 hours post-LA-BD in both IC/TLC and RV/TLC (; ).
Table 5. Relationship between baseline forced oscillation technique (FOT), and subsequent change in hyperinflation and gas trapping 2 hrs post-long-acting bronchodilator.
3.6. Reactance relates to communicating lung volume
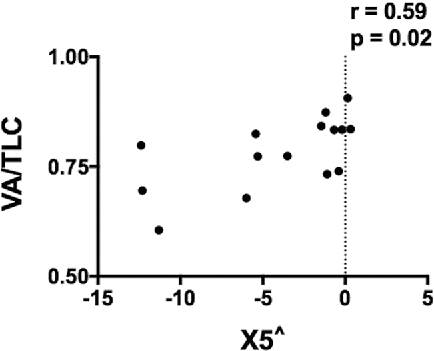
Given the known dependence of X5 on lung recruitment and derecruitment (Citation24), we explored the potential contribution of non-communicating lung volume to X5. We examined the ratio of single-breath alveolar volume (VA, measured during the DLCO test) to plethysmographic TLC (VA/TLC) at the screening visit. Adjusted X5 was significantly correlated with VA/TLC ratio (r = 0.59, p = 0.02) but not with adjusted VA or TLC (r = 0.37, p = 0.2 and r = −0.41, p = 0.1 respectively; ).
4. Discussion
In the present study, we have demonstrated a relationship between FOT parameters, hyperinflation and gas trapping measurements in subjects with COPD. Changes in gas trapping following a LA-BD are accompanied by changes in FOT. Furthermore, FOT parameters measured at baseline are related to the subsequent improvement in both hyperinflation and gas trapping following LA-BD. To our knowledge, this is the first study directly examining the relationship between FOT, hyperinflation and gas trapping, and their responses to LA-BD, in COPD.
4.1. Assessing BD response in COPD
The changes in gas trapping and hyperinflation following BD in COPD are arguably more relevant than the change in FEV1 in determining therapeutic effect. The release of trapped gas measured by a reduction in RV shows a greater magnitude of change, and a greater proportion of responders, than FEV1 across a range of severities (Citation25). These so-called volume responders may be missed if measuring the FEV1 response alone (Citation26) and this may account for the poor correlation between FEV1 response and clinical outcomes (Citation8). In contrast, the improvement in hyperinflation and gas trapping following BD has been shown to correlate with the subsequent improvement in symptoms and exercise performance (Citation6,Citation27). The ability to predict the release of trapped gas and a reduction in hyperinflation in response to LA-BD may therefore help to better target the prescription of these medications for individual patients. In our study, lung volume change in response to a single dose of long-acting BD was demonstrated by a significant increase in FVC, FRC and IC/TLC ratio. Somewhat surprisingly, there was no overall significant change in RV or RV/TLC. This may relate to differences in forced and slow VC, and the variability of thoracic gas volume measurements used for the estimation of RV and TLC (Citation28).
There are a number of studies demonstrating improvements in FOT following BD in COPD (Citation12–16,Citation29). The majority of studies have investigated the immediate (10–15 min) response to short-acting BD. However, LA-BD are the cornerstone treatment in COPD. In our study, we measured the response to indacaterol, a high-potency LA-BD with rapid onset of action (Citation30). We chose a 2 hr time period to allow for a sufficient volume response. Our results show not only that FOT measurements improve concomitantly with the release of gas trapping, but also that FOT measurements at baseline may predict LA-BD-induced improvements in gas trapping and hyperinflation. This is particularly attractive from a clinical perspective since FOT is measured during normal tidal breathing yet relates to measurements collected using body plethysmography and during maximal expiration. The latter is cumbersome, and requires significant cooperation and coordination on the part of the patient. FOT may therefore be an appropriate alternative lung function modality for use in the management of COPD since these patients are often older and may find plethysmography difficult to perform. It has also been shown that the BD response measured by FOT predicts the post-BD improvement in symptoms during exercise better than the response measured by FEV1 or IC (Citation31). This suggests that FOT measurements may be complementary to traditional lung function techniques in predicting therapeutic responses to BD. This needs to be tested in larger studies incorporating clinical outcomes.
4.2. Relationships between impedance, and hyperinflation and gas trapping
We observed an inverse correlation between R and IC/TLC ratio, meaning that increased resistance occurs with greater hyperinflation. We also found a positive correlation between X and IC/TLC, meaning that more negative reactance occurs with greater hyperinflation ( and ). These relationships are as expected, and are likely due to R and X reflecting the severity of impairment of airway function which then leads to hyperinflation.
In COPD, R and X measurements at low frequencies are likely the summation of complex but similar processes in the lung, hence we found concordant correlations with lung volume. Results of computational modelling (Citation32,Citation33) and observational studies (Citation9,Citation34) suggest that X is sensitive to severe airway narrowing, EFL, heterogeneity of airway calibre, and airway closure. In COPD, airway closure is present during tidal breathing (Citation35) and may therefore contribute substantially to our findings. Pressure oscillations are unable to penetrate these closed lung segments; instead, the pressure oscillations are absorbed by the airway walls, with a resulting increase in the apparent stiffness of the respiratory system indicated by a more negative X. Since VA/TLC ratio is decreased in airway obstruction (Citation36) and emphysema (Citation37) due to severe airway narrowing and airway closure, the positive correlation between X5 and VA/TLC ratio is consistent with the concept that the lung volume that can be ‘seen’ or is accessible to the oscillations, rather than the total thoracic gas volume, is the more important determinant of X.
The mechanisms by which LA-BD reduces R and increases X (suggesting an increase in oscillatory or dynamic compliance) are uncertain, but are likely to be complex and include changes to airway calibre, airway wall stiffness and lung parenchymal compliance. In the normal lung, increasing lung volume above FRC increases the calibre of airways due to parenchymal traction, which reduces R38, 39 but (minimally) decreases X (Citation24,Citation40). The latter presumably reflects the lungs moving towards the less-compliant portion of the pressure-volume curve. However, in asthma (Citation24) and COPD (Citation40), increasing lung volume has the opposite effect on X, i.e., increasing lung volume increases X, which is most likely due to lung recruitment (Citation34). This is consistent with our findings, where LA-BD results in lung recruitment via the opening of previously closed airways, which in turn increases X and concomitantly releases hyperinflation. LA-BD could also affect lung tissue compliance, either by direct effects on contractile elements the lung or indirectly via the release of hyperinflation, moving EELV to a more compliant part of the pressure-volume curve. It has been suggested that AX may be more useful than X5, since the former incorporates resonant frequency and hence reflects elastance and respiratory system inertive properties (Citation41). The fact that our results showed similar correlations between these two parameters and lung volumes, suggests that AX may not add any more clinically useful information when assessing BD responses in COPD.
4.3. Limitations
There are several limitations in the present study. First, this was a small, open-label physiological study and for this reason we did not include clinical endpoints such as symptom responses. A semi-quantitative symptom measurement, such as a visual analogue scale, may have been useful in this instance. The physiological relationships described provide clinically relevant information, however these need to be reproduced in larger cohorts incorporating clinical outcomes such as symptom scores or exercise performance. Second, subjects with severe COPD were excluded since it was deemed that withholding LA-BD for 2 weeks prior to testing may increase the risk of an exacerbation or unacceptable increase in symptoms. The two-week LA-BD withholding time was chosen to ensure lung volumes were not influenced by any LA-BD other than the single dose used in our study. While it is possible that appreciable changes in FOT and lung volumes following a single dose of LA-BD could be measured in these patients without a long withholding time, our results cannot confidently be extrapolated to patients with more severe disease. Thirdly, for parameters for which there are no population reference equations, we have attempted to control for presumed covariates using multiple linear regression. However, reference equations from large-scale normative data would give greater confidence that these potential confounders have been adequately controlled for. Finally, we have assessed only the short-term response to a single LA-BD. Different LA-BDs over longer time frames may show different responses, and our results cannot be generalised to all LA-BDs.
5. Conclusion
In this study we have demonstrated that R and X measured by FOT relate to hyperinflation, and that LA-BD-induced changes in R and X are correlated with improvements in hyperinflation and gas trapping. These findings suggest that FOT measurements reflect a complex array of physiological abnormalities in COPD, allowing a more detailed characterisation of patients that is complementary to other clinical measurements such as spirometry. The results provide a physiological insight into the effects of LA-BD on lung mechanics. More importantly, FOT measurements could also predict the volume response to a single dose of LA-BD, which may prove useful in determining clinically significant BD responses in patients with COPD. Our data support the need to incorporate FOT measurements in larger clinical studies in COPD.
Declaration of interests
SM reports personal fees from Novartis Pharmaceuticals, Boehringer Ingelheim and Menarini, outside the submitted work
CH and SW report no conflicts of interest
CSF reports personal fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Menarini, and Mundipharma, outside the submitted work.
CT reports membership of the ATS/ERS Task Force for Technical Standards for Forced Oscillation Technique, and intellectual property arrangements (non-financial) with Thorasys and Restech.
GGK reports grants from National Asthma Foundation, National Health and Medical Research Council, Boehringer Ingelheim, GlaxoSmithKline, Menarini, Mundipharma; fees for consultancy paid to the Woolcock Institute and used for funding the research group from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Novartis and MundiPharma; personal travel support from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Menarini, Mundipharma and Novartis; co-chair of the ATS/ERS Task Force for Technical Standards for Forced Oscillation Technique; intellectual property arrangements (non-financial) with Thorasys and Restech.
Prior presentation
Preliminary data from this study have been presented in abstract form:
| • | Thoracic Society of Australia and New Zealand Annual Scientific Meeting 2016 (Perth, Australia) 5 March 2016. Published as: Milne S, Hammans C, Watson S, Farah CS, Thamrin C, King GG. Forced oscillation technique measurements relate to hyperinflation and lung volume improvements following long-acting bronchodilator in COPD [abstract]. Respirol 2016;21:75–76 | ||||
| • | American Thoracic Society Congress 2016 (San Francisco, CA) 17 May 2016. Published as: Milne S, Hammans C, Watson S, Farah CS, Thamrin C, King GG. Forced Oscillation Technique Measurements Relate to Hyperinflation and Lung Volume Improvements Following Long-Acting Bronchodilator in Chronic Obstructive Pulmonary Disease [abstract]. Am J Respir Crit Care Med 2016;193:A6358 | ||||
Additional information
Funding
References
- Macklem PT. Therapeutic implications of the pathophysiology of COPD. Eur Respir J. 2010;35(3):676–680. doi:10.1183/09031936.00120609. PMID:20190332.
- Ruppel GL. What is the clinical value of lung volumes? Respir Care. 2012;57(1):26–38. doi:10.4187/respcare.01374. PMID:22222123.
- O'Donnell DE, Lam MIU, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(5):1557–1565. doi:10.1164/ajrccm.158.5.9804004. PMID:9817708.
- O'Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. COPD. 2006;3(4):219–232. doi:10.1080/15412550600977478. PMID:17361503.
- Chung VC, Ma PH, Hui DS, Tam WW, Tang JL. Indacaterol for chronic obstructive pulmonary disease: systematic review and meta-analysis. PLoS ONE. 2013;8(8):e70784. doi:10.1371/journal.pone.0070784. PMID:23967106.
- O'Donnell DE, Casaburi R, Vincken W, Puente-Maestu L, Swales J, Lawrence D, Kramer B, Inable study group. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respir Med. 2011;105(7):1030–1036. doi:10.1016/j.rmed.2011.03.014. PMID:21498063.
- Calverley PM, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58(8):659–664. doi:10.1136/thorax.58.8.659. PMID:12885978.
- Colacone A, Dajczman E, Kreisman H, Wolkove N. The relationship between pulmonary function and dyspnea in obstructive lung disease. Chest. 1989;96(6):1247–1251. doi:10.1378/chest.96.6.1247. PMID:2582829.
- Dellacà RL, Santus P, Aliverti A, Stevenson N, Centanni S, Macklem PT, Pedotti A, Calverley PMA. Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J. 2004;23(2):232–240. doi:10.1183/09031936.04.00046804. PMID:14979497.
- Bates JH, Irvin CG, Farre R, Hantos Z. Oscillation mechanics of the respiratory system. Compr Physiol. 2011;1(3):1233–1272. PMID:23733641.
- Oostveen E, Boda K, van der Grinten CPM, James AL, Young S, Nieland H, Hantos Z. Respiratory impedance in healthy subjects: baseline values and bronchodilator response. Eur Respir J. 2013;42(6):1513–1523. doi:10.1183/09031936.00126212. PMID:23598954.
- Kitaguchi Y, Fujimoto K, Komatsu Y, Hanaoka M, Honda T, Kubo K. Additive efficacy of short-acting bronchodilators on dynamic hyperinflation and exercise tolerance in stable COPD patients treated with long-acting bronchodilators. Respir Med. 2013;107(3):394–400. doi:10.1016/j.rmed.2012.11.013. PMID:23245993.
- da Costa GM, Faria AC, Di Mango AM, Lopes AJ, Lopes de Melo P. Respiratory impedance and response to salbutamol in healthy individuals and patients with COPD. Respiration. 2014;88(2):101–111. doi:10.1159/000362691. PMID:24992904.
- Costa GM, Faria AC, Di Mango AM, Lopes AJ, Jansen JM, Melo PL. Bronchodilation in COPD: Beyond FEV1—the effect of albuterol on resistive and reactive properties of the respiratory system. J Bras Pneumol. 2009;35(4):325–333. doi:10.1590/S1806-37132009000400006. PMID:19466270.
- Fujimoto K, Kitaguchi Y, Kanda S, Urushihata K, Hanaoka M, Kubo K. Comparison of efficacy of long-acting bronchodilators in emphysema dominant and emphysema nondominant chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2011;6:219–227. doi:10.2147/COPD.S18461. PMID:21660299.
- Inui N, Matsushima S, Kato S, Yasui H, Kono M, Fujisawa T, Enomoto N, Nakamura Y, Toyoshima M, Suda T. Effects of indacaterol versus tiotropium on respiratory mechanics assessed by the forced oscillation technique in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:1139–1146. doi:10.2147/COPD.S87058. PMID:26124653.
- Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi:10.1183/09031936.05.00035005. PMID:16135736.
- Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright Pv, Van Der Grinten C, Gustafsson P. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805. PMID:16055882.
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, Enright PL, Hankinson JL, Ip MS, Zheng J, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the Global Lung Function 2012 equations. Eur Respir J. 2012;40(6):1324–1343. doi:10.1183/09031936.00080312. PMID:22743675.
- Quanjer PH, Tammeling GJ, Cotes JE, Pedersen OF, Peslin R, Yernault J-C. Lung volumes and forced ventilatory flows. Eur Respir J. 1993;6(Suppl 16):5–40. doi:10.1183/09041950.005s1693. PMID:24576915.
- Thompson BR, Johns DP, Bailey M, Raven J, Walters EH, Abramson MJ. Prediction equations for single breath diffusing capacity (TLCO) in a middle aged caucasian population. Thorax. 2008;63(10):889–893. doi:10.1136/thx.2007.091959. PMID:18390632.
- Oostveen E, MacLeod D, Lorino H, Farre R, Hantos Z, Desager K, Marchal F. ERS Taskforce on Respiratory Impedance Measurements. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22(6):1026–1041. doi:10.1183/09031936.03.00089403. PMID:14680096.
- Wouters EFM, Verschoof AC, Polko AH, Visser BF. Impedance measurements of the respiratory system before and after salbutamol in COPD patients. Respir Med. 1989;83(4):309–313. doi:10.1016/S0954-6111(89)80202-1. PMID:2608952.
- Kelly VJ, Brown NJ, Sands SA, Borg BM, King GG, Thompson BR. Effect of airway smooth muscle tone on airway distensibility measured by the forced oscillation technique in adults with asthma. J Appl Physiol. 2012;112(9):1494–1503. doi:10.1152/japplphysiol.01259.2011. PMID:22362406.
- Deesomchok A, Webb KA, Forkert L, Lam Y-M, Ofir D, Jensen D, O'Donnell DE. Lung hyperinflation and its reversibility in patients with airway obstruction of varying severity. COPD. 2010;7(6):428–437. doi:10.3109/15412555.2010.528087. PMID:21166631.
- Newton MF, O'Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. Chest. 2002;121(4):1042–1050. doi:10.1378/chest.121.4.1042. PMID:11948031.
- O'Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(2):542–549. doi:10.1164/ajrccm.160.2.9901038. PMID:10430726.
- Garcia JG, Hunninghake GW, Nugent KM. Thoracic gas volume measurement. Increased variability in patients with obstructive ventilatory defects. Chest. 1984;85(2):272–275. doi:10.1378/chest.85.2.272.
- Borrill ZL, Houghton CM, Tal‐Singer R, Vessey SR, Faiferman I, Langley SJ, Singh D. The use of plethysmography and oscillometry to compare long‐acting bronchodilators in patients with COPD. Br J Clin Pharmacol. 2008;65(2):244–252. doi:10.1111/j.1365-2125.2007.03013.x. PMID:18251761.
- Battram C, Charlton SJ, Cuenoud B, Dowling MR, Fairhurst RA, Farr D, Fozard JR, Leighton-Davies JR, Lewis CA, McEvoy L, et al. In vitro and in vivo Pharmacological Characterization of 5-[(R)-2-(5,6-Diethyl-indan-2-ylamino)-1-hydroxy-ethyl]-8-hydroxy-1H-quinolin-2-one (Indacaterol), a novel inhaled beta-2 adrenoceptor agonist with a 24-h duration of action. J Pharmacol Exp Ther. 2006;317(2):762–770. doi:10.1124/jpet.105.098251. PMID:16434564.
- Diba C, King GG, Berend N, Salome CM. Improved respiratory system conductance following bronchodilator predicts reduced exertional dyspnoea. Respir Med. 2011;105(9):1345–1351. doi:10.1016/j.rmed.2011.03.013. PMID:21482091.
- Lutchen KR, Gillis H. Relationship between heterogeneous changes in airway morphometry and lung resistance and elastance. J Appl Physiol. 1997;83(4):1192–1201. doi:10.1152/jappl.1997.83.4.1192. PMID:9338428.
- Thorpe CW, Bates JHT. Effect of stochastic heterogeneity on lung impedance during acute bronchoconstriction: a model analysis. J Appl Physiol. 1997;82(5):1616–25. doi:10.1152/jappl.1997.82.5.1616. PMID:9134912.
- Dellacà RL, Olerud MA, Zannin E, Kostic P, Pompilio PP, Hedenstierna G, Pedotti A, Frykholm P. Lung recruitment assessed by total respiratory system input reactance. Intensive Care Med. 2009;35(12):2164. doi:10.1007/s00134-009-1673-3. PMID:19789855.
- Calverley PMA, Koulouris NG. Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology. Eur Respir J. 2005;25(1):186–199. doi:10.1183/09031936.04.00113204. PMID:15640341.
- Weingarten JA, Lederer DJ, Ozturk E, Milite FM, Mooney AM, Thomashow BM, Basner RC. A comparison of single breath and re-breathe diffusing capacity in emphysema patients and controls. Respir Physiol Neurobiol. 2012;183(1):15–19. doi:10.1016/j.resp.2012.05.009. PMID:22633938.
- van der Lee I, van Es HW, Noordmans HJ, van den Bosch JMM, Zanen P. Alveolar volume determined by single-breath helium dilution correlates with the high-resolution computed tomography-derived nonemphysematous lung volume. Respiration. 2006;73(4):468–473. doi:10.1159/000088711. PMID:16205050.
- Fisher AB, DuBois AB, Hyde RW. Evaluation of the forced oscillation technique for the determination of resistance to breathing. J Clin Invest. 1968;47(9):2045. doi:10.1172/JCI105890. PMID:5675425.
- Briscoe WA, Dubois AB. The relationship between airway resistance, airway conductance and lung volume in subjects of different age and body size. J Clin Invest. 1958;37(9):1279. doi:10.1172/JCI103715. PMID:13575526.
- Baldi S, Dellacà R, Govoni L, Torchio R, Aliverti A, Pompilio P, Corda L, Tantucci C, Gulotta C, Brusasco V, et al. Airway distensibility and volume recruitment with lung inflation in COPD. J Appl Physiol. 2010;109(4):1019–1026. doi:10.1152/japplphysiol.00147.2010. PMID:20651219.
- Goldman MD, Saadeh C, Ross D. Clinical applications of forced oscillation to assess peripheral airway function. Respir Physiol Neurobiol. 2005;148(1):179–194. doi:10.1016/j.resp.2005.05.026. PMID:15990365.