ABSTRACT
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of death in the world and its incidence and prevalence is on the rise. It is evident that COPD is linked to cardiovascular disease. In the last years, several studies demonstrated that COPD may also be a risk factor for stroke, another major cause of death worldwide. Taking in consideration that COPD has multiple comorbidities it is hard to say whether COPD is an independent risk factor for stroke or it is due to confounding effect. This review is aimed to discuss current data on COPD and stroke, potential links, therapy, and prevention. Current data suggest that COPD may increase the risk of hemorrhagic stroke. The incidence of other stroke subtypes may also be increased in COPD or may be due to confounding effect. However, COPD patients who have stroke are at risk for pulmonary and extrapulmonary complications. We conclude that more studies are needed to further clarify the links between COPD and stroke. The management of COPD as well as the use of prevention therapy is essential to decrease the risk for stroke and should be at special attention in pulmonary medicine and neurology.
| Abbreviations | ||
| ARDS | = | acute respiratory distress syndrome |
| AF | = | atrial fibrillation |
| CI | = | confidence interval |
| COPD | = | chronic obstructive pulmonary disease |
| FEV1 | = | forced expiratory volume in 1 second |
| FVC | = | forced vital capacity |
| GERD | = | gastroesophageal reflux disease |
| HRs | = | hazard ratios |
| ICSs | = | inhaled corticosteroids |
| LABAs | = | long-acting β-agonists |
| LAMAs | = | long-acting muscarinic antagonists |
| OR | = | odds ratio |
| PEF | = | peak expiratory flow |
| SABAs | = | short-acting β-agonists |
| SAP | = | stroke-associated pneumonia |
Introduction
Chronic obstructive pulmonary disease (COPD), the fourth leading cause of death in the world (Citation1,Citation2), is currently defined by GOLD as a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases (Citation3). Nevertheless, there is growing evidence that COPD represent the expression in the lungs, of a systemic inflammatory syndrome which affects every organ and system in the body (Citation4). This assumption is based on the observation that COPD is linked to multisystemic comorbidities. Approximately 80% of the patients have at least one comorbidity, which greatly impacts their quality of life, physical and mental state, and complicates their management (Citation5).
Stroke is another growing global health problem (Citation1,Citation6). In the last years, there is growing interest toward the links between these two leading causes of death and disability. It has been demonstrated that there is a tight interplay between COPD, acute, and chronic cardiovascular disease (Citation7–9). But it has also been suggested that COPD is significantly more prevalent among patients with stroke and that the co-existence of both is associated with grim outcomes (Citation10). These findings have been consistently observed in studies evaluating all different subtypes of stroke (ischemic, intracerebral, or subarachnoid hemorrhage) (Citation11). There is growing interest towards neuropulmonology which underlines the complex interconnection between the central nervous and respiratory systems and aspires to optimize the management of patients where these pathologies co-exists, especially in the neurocritical care environment (Citation12).
The purpose of this review is to summarize available data on the association between COPD and stroke and to describe their pathophysiological links.
Shared risk factors between COPD and stroke
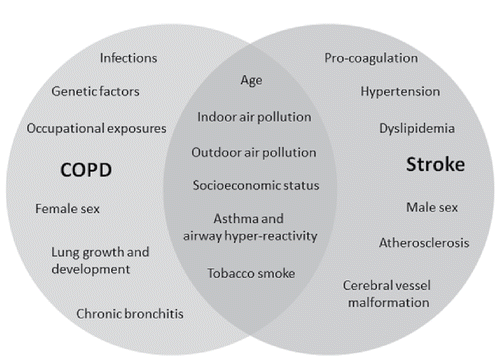
COPD may be one of the risk factors associated with atherothrombotic stroke along with diabetes, hypertension, and transient ischemic attack (Citation13). The major risk factors for stroke (which combined account for 88.1% of population-attributable risks) are history of hypertension, current smoking, waist-to-hip ratio, diet risk score, physical inactivity, diabetes mellitus, alcohol intake, psychosocial stress, cardiac causes, and ratio of apolipoproteins B to A1 (Citation14). These risk factors can also be seen in COPD patients and thus the link between COPD and stroke can be to some degree due to confounding effect () (Citation11).
The prevalence of stroke among patients with versus without COPD was evaluated in a nationwide study from Sweden where COPD patients between 40 and 84 years of age, hospitalized for COPD between 1987 and 2003, were matched with one reference individual who was randomly selected from the general population matched for age, sex, and county of residence were included in the study. Hazard ratios (HRs) for stroke were estimated using Cox regression adjusting for demographics and comorbidities. Incidence of all-cause stroke was significantly increased among COPD patients compared to control individuals (HR 1.24, 95% confidence interval [95% CI]: 1.19–1.28), especially during the first 2 years after COPD diagnosis (HR 1.46, 95% CI: 1.37–1.55) (Citation11). Similar results were found in the Rotterdam Study with a higher risk of both ischemic and hemorrhagic stroke in subjects with COPD (Citation15). Paradoxically the greatest increase in the rate of stroke was found in the youngest age groups compared with the older age groups of COPD (Citation16). Several studies suggest that the risk for all-cause strokes in COPD increases approximately by 20%, but there are data demonstrating that this risk may be even higher (Citation16).
Stroke subtypes and COPD
The nationwide study from Sweden demonstrated that the incidences of ischemic stroke (HR 1.20, 1.15–1.25), intracerebral hemorrhage (HR 1.29, 1.16–1.43), and subarachnoid hemorrhage (HR 1.46, 1.16–1.85) were all increased in COPD patients. Incidence of all stroke subtypes are increased in COPD, especially during the first years after COPD diagnosis (Citation11).
COPD and stroke: Beyond smoking
It is well known that smoking increases the risk of stroke as well as the risk of fatal events (Citation17). The Rotterdam Study demonstrated that after additional adjusting for smoking the risk of stroke among COPD patients versus controls was attenuated with HR, 1.09 (0.91–1.31) for all stroke; HR, 1.13 (0.91–1.42) for ischemic stroke; and HR 1.53 (0.91–2.59) for hemorrhagic stroke (Citation15). These findings suggest that smoking history is only partly responsible for the increased incidence of stroke and, therefore, the presence of COPD may play an independent role in the pathogenesis of stroke, especially of hemorrhagic etiology.
Shared pathophysiological mechanisms
The pathophysiological links between COPD and stroke are still not fully understood and are likely to be interconnected (Citation18). The key factors that promote pathophysiological changes in COPD are systemic inflammation, hypoxia, hypercapnia, and oxidative stress.
Systemic inflammation appears to play a major role in the development and outcomes of stroke. The mechanisms how systemic inflammation impacts stroke include increased neutrophil infiltration of cerebral cortex, disruption of the blood-brain barrier, brain edema, impaired tissue reperfusion, increased platelet activation, microvascular coagulation, and proinflammatory mediators contribution (Citation19–21). It has been suggested that inhibition of the proinflammatory cascades may be a promising therapeutic option in stroke patients which underlines the impact of immune system during brain injury (Citation22). Therefore, COPD, being characterized by chronic, low-grade systemic inflammation, could be a direct predisposing factor for the development of stroke.
Hypoxia is a major feature of COPD, the degree of which increases in the more advance stages of the disease. The role of hypoxia on the pathophysiology of stroke is debatable and recent studies found contradictory results. It seems reasonable to administer oxygen in stroke patients whose brain tissue is hypoperfused and hypoxic, due to the acute stroke (Citation23). Several studies suggested that routine oxygen delivery is associated with a small improvement in neurological recovery and a decrease in the frequency of episodes of desaturation (Citation24–27). On the other hand, recent studies suggested that hypoxia and hypercapnia, or hypercapnia alone, might provide neuroprotective effects (Citation28,Citation29).
Increased oxidative stress and decreased antioxidant enzymes activity may play a role in the pathogenesis of both ischemic stroke and hemorrhagic stroke (Citation30,Citation31). The management of oxidative stress also showed promising results with significant improvement of long-term neurological function (Citation32).
Relationship between FEV1 and stroke risk
Interestingly decreased forced expiratory volume in 1 second (FEV1) is correlated with an increased risk of stroke. An extensive cohort study, with a study population of over 5600 participants demonstrated an inverse association between baseline FEV1 and risk of fatal stroke HR = 1.38 (95% CI: 1.11–1.71) and HR = 1.62 (95% CI: 1.22–2.15) for men and women, respectively (adjusted for age and height). The findings were not explained by smoking history, hypertension, diabetes, atherosclerosis, socioeconomic status, obstructive lung disease, physical inactivity, cholesterol or body mass index, and persisted in subgroups of never-smokers, subgroups without respiratory symptoms and survivors of the first 20 years of follow-up (Citation33).
For each 10% decrease in FEV1 in percentage of predicted, the stroke risk increased by 5% (RR = 1.05, 95% CI: 1.00–1.09, p = 0.03). This represents an approximately 30% higher risk of stroke in the group of people with the lowest lung function as compared to the group with the highest lung function. The RR was 1.11 (95% CI: 1.03–1.19) for each 10% decrease in FEV1 in percentage of expected. This study shows that FEV1 is a predictor of first-time stroke and fatal stroke independent of smoking. The high risk of fatal first-ever stroke in the group of people with low lung function may be of significance in both the design and interpretation of clinical trials (Citation34).
Lower levels of FEV1 are associated with a further increased risk of stroke in those already at high risk, for example, those with ischemic heart disease or hypertension. However, inclusion of FEV1 in a risk score for stroke conferred only a small increase in the absolute risk or the yield of cases in the top fifth of the score distribution during the follow-up period (Citation35).
Impact of stroke on lung function
Complications and disturbances of the respiratory system function are common after stroke (Citation36). Compared to healthy controls patient who suffered from stroke have significantly lower FEV1, forced vital capacity (FVC), peak expiratory flow (PEF) values, and chest excursion. This lung function deterioration may be due to the weakness of respiratory muscles (Citation37). In patients with hemiplegia FEV1 values are lower in the group with right-side hemiplegia. Forced expiratory flow at 25–75% of the pulmonary volume and PEF values were also lower in right hemiplegic group when compared to the control group (p = 0.01 and p = 0.009, respectively) (Citation38). Patients with hemiplegia have a greater degree of hypoxia, hypercapnia and decreased serum bicarbonate level compared to the control group (Citation39). In left-hemiplegic patients diaphragmatic excursion is reduced on the left side and increased on the right side compared to that in control subjects. Left diaphragmatic motion during deep breathing correlates positively with FVC (r = 0.86, p = 0.007) and FEV1 (r = 0.79, p = 0.021) (Citation40). All in all, there is a reduction in diaphragm mobility in patients with COPD, which is associated with a decline in pulmonary function. More specifically, diaphragmatic mobility correlated with airway obstruction, limited ventilatory capacity, and pulmonary hyperinflation (Citation41). Thus, stroke consequences may have a further impact on the lung function of COPD patients who already have severe defects.
Relationship between COPD comorbidities and stroke risk
Well known comorbidities of COPD represent strong predisposing factors for stroke, particularly cardiovascular and metabolic disorders () (Citation4,Citation42–50).
Table 1. COPD comorbidities as risk factors for stroke.
The exact mechanism of vascular dysfunction in COPD is still under investigation, but systemic inflammation, oxidative stress, hypoxia, and sympathetic activation driven by the pathological state of the lung are likely to contribute (Citation51,Citation52). Atherosclerosis frequently coexists with COPD and affects arteries of all calibers starting with aorta and its major branches (Citation53,Citation54). COPD is associated with increased arterial stiffness independently of cigarette smoke exposure and this abnormality is not explained by systemic endothelial dysfunction (Citation55). The severity of emphysema is strongly associated with arterial stiffness and calcification in patients with COPD (Citation53,Citation56). Atherosclerosis, assessed by aortic calcification can also be associated with small pulmonary vascular alteration in COPD (Citation57).
Patients with COPD are at higher risk of cardiovascular complications. COPD is highly prevalent in patients with atrial fibrillation, and is associated with higher rates of cardiovascular death, all-cause death, and the composite outcome of any thromboembolic death (Citation58). Patients with atrial fibrillation (AF) and COPD had ischemic cerebrovascular accident 2.05 (95% CI: 1.203–3.94; p = 0.007) times more frequently compared to those with either COPD or AF. Logistic regression showed AF plus COPD was a stronger predictor of ischemic cerebrovascular accident (p = 0.001) than AF alone (p = 0.07) or COPD alone (p = 0.8, nonsignificant). Odds ratio (OR) of ischemic cerebrovascular accident was 2.85 (CI, 1.57–5.16; p = 0.001) for AF plus COPD versus 1.81 (CI, 0.94–3.47; p = 0.71) for AF only and 1.08 (CI: 0.58–2.10; p = 0.8) for COPD only (Citation59).
Arterial and venous thromboembolism is a frequent complication in COPD patients (Citation60). Fibrin clots in COPD patients have an altered network that is resistant to lysis and patients are in a constant prothrombogenic status due to the proinflammatory state (Citation61–63). They also have significantly increased platelet count, along with a reduced mean platelet volume when compared to healthy controls (Citation64). The mean platelet volume also tends to decrease during acute exacerbation of COPD and increases during the stable phase (Citation65). Thus, COPD comorbidities may also contribute to the increased risk for stroke.
Exacerbation of COPD and stroke
After an acute severe exacerbation, subjects with COPD had a 6.66-fold (2.42–18.20) increased risk of stroke (Citation15). COPD exacerbation are also associated with poststroke mortality (OR, 1.34, 95% CI: 1.20–1.52), epilepsy (OR, 1.43; 95% CI: 1.22–1.67), and pneumonia (OR, 1.50; 95% CI: 1.39–1.62) (Citation66). Another study demonstrates that the risk of stroke (1.26-fold [95% CI: 1.0–1.6; p = 0.05]) is maintained 1 to 49 days after the exacerbation (Citation67).
On the other hand, a study from the UK compared frequent exacerbators (≥2 exacerbations in the year prior to their stroke) and infrequent exacerbators (≤1 exacerbation in the year prior to their stroke) who had a first stroke (6441 cases) and they were matched on age, sex, and general practice to controls with COPD but without a stroke (19,323 controls). There was no evidence that frequent exacerbators had increased odds of stroke compared to infrequent exacerbators (OR = 0.95, 95% CI: 0.89–1.01). Although in the subgroup analysis investigating stroke subtype, frequent exacerbators had 33% lower odds of hemorrhagic stroke than infrequent exacerbators (OR = 0.67, 95% CI: 0.51–0.88, p = 0.003), no association was found with other stroke types (Citation68).
Impact of COPD on stroke outcomes
The crude and age-adjusted in-hospital mortality rates for stroke patients with COPD and without COPD in the US are 6.33% (95% CI: 6.14–6.53) and 5.99% (95% CI: 4.05–7.94), respectively. On multivariable analyses, COPD is modestly associated with overall stroke mortality (OR 1.06; 95% CI: 1.02–1.08; p = 0.018) but it is strongly associated with higher mortality among patients with intracerebral hemorrhage (OR 1.12; 95% CI: 1.03–1.20; p = 0.005) and ischemic stroke (OR 1.08; 95% CI: 1.03–1.13, p = 0.001). However, no excess mortality was observed among COPD patients when the analysis was restricted to ischemic stroke patients who received recombinant tissue plasminogen factor (Citation10).
Another study demonstrates using logistic regression that COPD is an independent risk factor for the development of seizures after stroke (p < 0.001). The occurrence of seizures was not related to the severity of the COPD or to its treatment and the most plausible explanatory mechanism was frequent nocturnal oxygen desaturation (Citation27). This can be supported by other studies that involved patients with epilepsy and demonstrated that the degree of desaturation is significantly correlated with the seizure duration (p = 0.001). Epilepsy can also cause central apneas in 50% of cases and mixed or obstructive apneas in 9% of cases (Citation69). Furthermore, an overlap between COPD and sleep apnea further complicates the prognosis and management of stroke patients (Citation70).
Impact of stroke on COPD outcomes
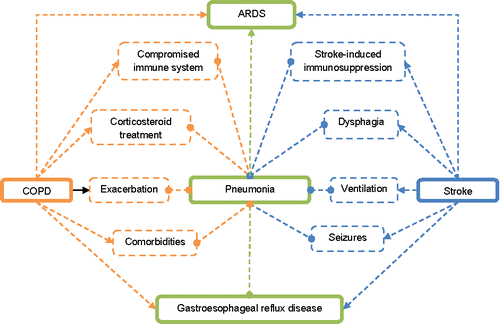
Dysphagia, gastroesophageal reflux disease (GERD), aspiration, and pneumonia are major complications in stroke patients (Citation71,Citation72). GERD is considered to be a risk factor for COPD exacerbations and this can further increase the mortality in this group of patients (Citation73). The use of acid suppressing agents (histamine-2 receptor antagonists and proton pump inhibitors) which are commonly used in GERD are associated with an increased risk of stroke-associated pneumonia (SAP) (Citation74,Citation75). The compromised immune state and the use of corticosteroids already puts COPD patients at higher risk for pneumonia (Citation76). Seizures are another risk factor for pneumonia and stroke patients with COPD have a higher risk for their development (Citation27,Citation77). SAP and stroke-induced immunosuppression are two other major problems in neurocritical care associated with poor outcomes (Citation78). Dysphagia, stroke-induced immunosuppression syndrome and GERD are risk factors for SAP (Citation72,Citation78). A healthcare professional already faces these problems in a COPD patient thus stroke has several complications which overlap with the already existing pathological state (). This is further supported by Park and coworkers which demonstrate stroke patients with COPD had higher risk of aspiration than stroke patients without COPD (Citation79).
Finally smoking is risk factor for acute respiratory distress syndrome (ARDS) (Citation80,Citation81). Lungs as a source of infection and stroke are also major risk factors for ARDS (Citation82,Citation83).
Impact of COPD treatment on stroke
There is currently limited data on whether COPD treatment reduces the risk of stroke (Citation84). CRP levels are raised in COPD patients and reduced in patients with COPD using inhaled corticosteroids (ICS) (Citation85). It seems logical since there are studies which show that local inflammation in COPD occurs earlier than systemic (Citation86). Still other studies demonstrate that ICS reduce lung-specific but not systemic inflammation in COPD (Citation87).
New use of short- (SABA) and long-acting β-agonists (LABA) and muscarinic antagonists (LAMA) in patients with COPD may slightly increase the risk of cardiac arrhythmia which is a potential risk factor for stroke (Citation88,Citation89). The rate of cardiac arrhythmias was elevated with the new use of short-acting (RR, 1.27; 95% CI: 1.03–1.57) and long-acting (RR, 1.47; 95% CI: 1.01–2.15) β-agonists. This should be taken into consideration particularly in patients who receive the treatment for the first time (Citation90). The rate of arrhythmia is also raised with the new use of ipratropium (RR, 2.4; 95% CI: 1.4–4.0). The same study demonstrated that a higher risk for arrhythmias with the use of LABAs (RR, 4.5; 95% CI: 1.4–14.4) but no risk elevation with new use of short-acting β-agonists or methylxanthines (Citation91).
Lin and co-workers, in a nationwide, population-based study with a follow up duration of three years and a matched cohort design demonstrated that among COPD patients, the use of SABA is associated with an increased risk of stroke (adjusted HR = 1.67, 95% CI: 1.45–1.91; p <0.001), and combination treatment with LABA and ICS relates to a risk reduction (adjusted HR 0.75, 95% CI: 0.60–0.94; p = 0.014) (Citation92). Inhaled ipratropium bromide was also associated with stroke risk in COPD patients and this effect depended on the duration of treatment and combination with SABA or theophylline (Citation93). Tiotropium use in COPD showed proischemic and proarrhythmic effects (Citation94). However other studies demonstrated no increase in mortality (Citation95), stroke, and transient ischemic attack risk with the use of tiotropium Handihaler (Citation96).
Oxygen therapy may be another beneficial intervention in COPD patients with stroke. Routine controlled oxygen supplementation started within 24 h of hospital admission with acute stroke may lead to a small improvement in neurological recovery (Citation24,Citation25). It also decreases the number of nocturnal desaturations which may be one of the risk factor for seizures in stroke patients (Citation26,Citation27). But there are also data suggesting that hypoxia and hypercapnia, or hypercapnia alone, provides neuroprotective effects (Citation28,Citation29).
The data on the use of noninvasive ventilation are limited, but show an improvement in cerebral hemodynamics in COPD patients and thus may be useful in stroke patients (Citation97,Citation98).
Finally major studies that evaluated COPD treatment such as TORCH (Citation99), SUMMIT (Citation100), UPLIFT (Citation101), FLAME (Citation100), TRILOGY (Citation102), did not find an increased risk of stroke.
Prevention of stroke in COPD patients
Since stroke is an acute, burdensome, and preventable condition several preventive options may be useful in COPD patients. Hematological changes often occur in patients with COPD. Thrombocytosis is of particular interest for stroke prevention. Administration of antiplatelet treatment correlated with a reduction in 1-year mortality in COPD patients (Citation103). The absolute risk of death due to circulatory disease is raised among COPD patients receiving long-term oxygen and antiplatelet therapy can be of potential use in this case (Citation104,Citation105).
Statins appear to have beneficial effects in COPD, as it has been suggested that they may reduce the risk of exacerbation by up to 30% and the risk of mortality by 21–78% (Citation106,Citation107). However, data are still conflicting and while a recent meta-analysis confirmed that statins are associated with a significant reduction of the risk for a myocardial infection, they failed to demonstrate any benefit for stroke prevention (Citation108).
Future points
It is important to clarify whether COPD increases the risk of all stroke types and to elaborate safe and effective methods for stroke prevention. More studies are required to evaluate whether oxygen therapy or permissive hypoxia and/or hypercapnia may be used in stroke patients with COPD. More studies that show the effectiveness of preventive therapy may also help us to understand what drugs should be added to the current management of COPD.
Moreover, basic research is required to explore the inflammatory interplay between the two diseases.
Conclusions
COPD is a complex disease with several key pathophysiological mechanisms that impact the whole body. Taking this into consideration there are enough data to suspect that it may also cause stroke. Current data suggests that COPD may increase the risk of hemorrhagic stroke. The incidence of other stroke subtypes may also be increased in COPD or may be due to confounding effect. The management of COPD as well as the use of prevention therapy is essential to decrease the risk for stroke and improve its outcomes and both respiratory clinicians and neurologists should be aware.
Declaration of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the manuscript.
References
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID:23245604.
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: global burden of disease study. Lancet. 1997;349(9064):1498–504. doi:10.1016/S0140-6736(96)07492-2. PMID:9167458.
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557–82. doi:10.1164/rccm.201701-0218PP. PMID:28128970.
- Corlateanu A, Covantev S, Mathioudakis AG, Botnaru V, Siafakas N. Prevalence and burden of comorbidities in chronic obstructive pulmonary disease. Respir Investig. 2016;54(6):387–96. doi:10.1016/j.resinv.2016.07.001. PMID:27886849.
- Fumagalli G, Fabiani F, Forte S, Napolitano M, Balzano G, Bonini M, De Simone G, Fuschillo S, Pentassuglia A, Pasqua F, et al. INDACO project: COPD and link between comorbidities, lung function and inhalation therapy. Multidiscip Respir Med. 2015;10(1):4. doi:10.1186/2049-6958-10-4. PMID:25973198.
- Feigin VL, Krishnamurthi R, Parmar P, Norrving B, Mensah GA, Bennett DA, Barker-Collo S, Moran A, Sacco RL, Truelsen T, et al. Update on the global burden of ischaemic and haemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology. 2015;45(3):161–76. doi:10.1159/000441085. PMID:26505981.
- de Lucas-Ramos P, Izquierdo-Alonso JL, Moro JMR-G, Frances JF, Lozano PV, Bellón-Cano JM, group Cs. Chronic obstructive pulmonary disease as a cardiovascular risk factor: results of a case–control study (CONSISTE study). Int J Chron Obstruct Pulmon Dis. 2012;7:679–86. PMID:23055717.
- Rothnie KJ, Yan R, Smeeth L, Quint JK. Risk of myocardial infarction (MI) and death following MI in people with chronic obstructive pulmonary disease (COPD): a systematic review and meta-analysis. BMJ Open. 2015;5(9):e007824. doi:10.1136/bmjopen-2015-007824. PMID:26362660.
- Curkendall SM, DeLuise C, Jones JK, Lanes S, Stang MR, Goehring E, Jr., She D. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann Epidemiol. 2006;16(1):63–70. doi:10.1016/j.annepidem.2005.04.008. PMID:16039877.
- Lekoubou A, Ovbiagele B. Prevalance and influence of chronic obstructive pulmonary disease on stroke outcomes in hospitalized stroke patients. eNeurologicalSci. 2017;6:21–4. doi:10.1016/j.ensci.2016.11.007. PMID:28018982.
- Soderholm M, Inghammar M, Hedblad B, Egesten A, Engstrom G. Incidence of stroke and stroke subtypes in chronic obstructive pulmonary disease. Eur J Epidemiol. 2016;31(2):159–68. doi:10.1007/s10654-015-0113-7. PMID:26711630.
- Balofsky A, George J, Papadakos P. Neuropulmonology. Handb Clin Neurol. 2017;140:33–48. doi:10.1016/B978-0-444-63600-3.00003-9. PMID:28187807.
- Arboix A, Morcillo C, Garcia-Eroles L, Oliveres M, Massons J, Targa C. Different vascular risk factor profiles in ischemic stroke subtypes: a study from the “Sagrat cor hospital of barcelona stroke registry.” Acta Neurol Scand. 2000;102(4):264–70. doi:10.1034/j.1600-0404.2000.102004264.x. PMID:11071113.
- O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao-Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376(9735):112–23. doi:10.1016/S0140-6736(10)60834-3. PMID:20561675.
- Portegies ML, Lahousse L, Joos GF, Hofman A, Koudstaal PJ, Stricker BH, Brusselle GG, Ikram MA. Chronic obstructive pulmonary disease and the risk of stroke. the rotterdam study. Am J Respir Crit Care Med. 2016;193(3):251–8. doi:10.1164/rccm.201505-0962OC. PMID:26414484.
- Feary JR, Rodrigues LC, Smith CJ, Hubbard RB, Gibson JE. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: a comprehensive analysis using data from primary care. Thorax. 2010;65(11):956–62. doi:10.1136/thx.2009.128082. PMID:20871122.
- Lund Haheim L, Holme I, Hjermann I, Tonstad S. Risk-factor profile for the incidence of subarachnoid and intracerebral haemorrhage, cerebral infarction, and unspecified stroke during 21 years' follow-up in men. Scand J Public Health. 2006;34(6):589–97. doi:10.1080/14034940600731523. PMID:17132592.
- Austin V, Crack Peter J, Bozinovski S, Miller Alyson A, Vlahos R. COPD and stroke: are systemic inflammation and oxidative stress the missing links? Clin Sci. 2016;130(13):1039–50. doi:10.1042/CS20160043. PMID:27215677.
- Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother. 2015;15(5):523–31. doi:10.1586/14737175.2015.1035712. PMID:25865856.
- Denes A, Ferenczi S, Kovacs KJ. Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood- brain barrier damage and brain oedema independently of infarct size. J Neuroinflammation. 2011;8:164. doi:10.1186/1742-2094-8-164. PMID:22114895.
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28(38):9451–62. doi:10.1523/JNEUROSCI.2674-08.2008. PMID:18799677.
- Kleinig TJ, Vink R. Suppression of inflammation in ischemic and hemorrhagic stroke: therapeutic options. Curr Opin Neurol. 2009;22(3):294–301. doi:10.1097/WCO.0b013e32832b4db3. PMID:19434798.
- Ferdinand P, Roffe C. Hypoxia after stroke: a review of experimental and clinical evidence. Exp Transl Stroke Med. 2016;8:9. doi:10.1186/s13231-016-0023-0. PMID:27980710.
- Roffe C, Ali K, Warusevitane A, Sills S, Pountain S, Allen M, Hodsoll J, Lally F, Jones P, Crome P. The SOS pilot study: a RCT of routine oxygen supplementation early after acute stroke–effect on recovery of neurological function at one week. PLoS One. 2011;6(5):e19113. doi:10.1371/journal.pone.0019113. PMID:21625533.
- Ali K, Warusevitane A, Lally F, Sim J, Sills S, Pountain S, Nevatte T, Allen M, Roffe C. The stroke oxygen pilot study: a randomized controlled trial of the effects of routine oxygen supplementation early after acute stroke–effect on key outcomes at six months. PLoS One. 2014;8(6):e59274. doi:10.1371/journal.pone.0059274. PMID:23755093.
- Roffe C, Sills S, Pountain SJ, Allen M. A randomized controlled trial of the effect of fixed-dose routine nocturnal oxygen supplementation on oxygen saturation in patients with acute stroke. J Stroke Cerebrovasc Dis. 2010;19(1):29–35. doi:10.1016/j.jstrokecerebrovasdis.2009.02.008. PMID:20123224.
- De Reuck J, Proot P, Van Maele G. Chronic obstructive pulmonary disease as a risk factor for stroke-related seizures. Eur J Neurol. 2007;14(9):989–92. doi:10.1111/j.1468-1331.2007.01829.x. PMID:17718690.
- Tregub P, Kulikov V, Motin Y, Bespalov A, Osipov I. Combined exposure to hypercapnia and hypoxia provides its maximum neuroprotective effect during focal ischemic injury in the brain. J Stroke Cerebrovasc Dis. 2015;24(2):381–7. doi:10.1016/j.jstrokecerebrovasdis.2014.09.003. PMID:25498739.
- Westermaier T, Stetter C, Kunze E, Willner N, Holzmeier J, Kilgenstein C, Lee JY, Ernestus RI, Roewer N, Muellenbach RM. Controlled transient hypercapnia: a novel approach for the treatment of delayed cerebral ischemia after subarachnoid hemorrhage? J Neurosurg. 2014;121(5):1056–62. doi:10.3171/2014.7.JNS132611. PMID:25148012.
- Icme F, Erel O, Avci A, Satar S, Gulen M, Acehan S. The relation between oxidative stress parameters, ischemic stroke, and hemorrhagic stroke. Turk J Med Sci. 2015;45(4):947–53. doi:10.3906/sag-1402-96. PMID:26422872.
- Milanlioglu A, Aslan M, Ozkol H, Cilingir V, Nuri Aydin M, Karadas S. Serum antioxidant enzymes activities and oxidative stress levels in patients with acute ischemic stroke: influence on neurological status and outcome. Wien Klin Wochenschr. 2016;128(5-6):169–74. doi:10.1007/s00508-015-0742-6. PMID:25854910.
- Kleinschnitz C, Grund H, Wingler K, Armitage ME, Jones E, Mittal M, Barit D, Schwarz T, Geis C, Kraft P, et al. Post-stroke inhibition of induced NADPH oxidase type 4 prevents oxidative stress and neurodegeneration. PLoS Biol. 2010;8(9). doi:10.1371/journal.pbio.1000479. PMID:20877715.
- Gulsvik AK, Gulsvik A, Skovlund E, Thelle DS, Mowe M, Humerfelt S, Wyller TB. The association between lung function and fatal stroke in a community followed for 4 decades. J Epidemiol Community Health. 2012;66(11):1030–6. doi:10.1136/jech-2011-200312. PMID:22493479.
- Truelsen T, Prescott E, Lange P, Schnohr P, Boysen G. Lung function and risk of fatal and non-fatal stroke: the copenhagen city heart study. Int J Epidemiol. 2001;30(1):145–51. doi:10.1093/ije/30.1.145. PMID:11171876.
- Wannamethee SG, Shaper AG, Ebrahim S. Respiratory function and risk of stroke. Stroke. 1995;26(11):2004–10. doi:10.1161/01.STR.26.11.2004. PMID:7482639.
- Rochester CL, Mohsenin V. Respiratory complications of stroke. Semin Respir Crit Care Med. 2002;23(3):248–60. doi:10.1055/s-2002-33033. PMID:16088617.
- Ezeugwu VE, Olaogun M, Mbada CE, Adedoyin R. Comparative lung function performance of stroke survivors and age-matched and sex-matched controls. Physiother Res Int. 2013;18(4):212–9. doi:10.1002/pri.1547. PMID:23359511.
- de Almeida ICL, Clementino ACCR, Rocha EHT, Brandão DC, Dornelas de Andrade A. Effects of hemiplegy on pulmonary function and diaphragmatic dome displacement. Respiratory Physiology & Neurobiology. 2011;178(2):196–201. doi:10.1016/j.resp.2011.05.017.
- Khedr EM, El Shinawy O, Khedr T, Abdel AAY, Awad EM. Assessment of corticodiaphragmatic pathway and pulmonary function in acute ischemic stroke patients. Eur J Neurol. 2000;7(5):509–16. doi:10.1046/j.1468-1331.2000.00104.x. PMID:11054135.
- Jung KJ, Park JY, Hwang DW, Kim JH, Kim JH. Ultrasonographic diaphragmatic motion analysis and its correlation with pulmonary function in hemiplegic stroke patients. Ann Rehabil Med. 2014;38(1):29–37. doi:10.5535/arm.2014.38.1.29. PMID:24639923.
- Kang HW, Kim TO, Lee BR, Yu JY, Chi SY, Ban HJ, Oh IJ, Kim KS, Kwon YS, Kim YI, et al. Influence of diaphragmatic mobility on hypercapnia in patients with chronic obstructive pulmonary disease. J Korean Med Sci. 2011;26(9):1209–13. doi:10.3346/jkms.2011.26.9.1209. PMID:21935278.
- Battaglia S, Basile M, Scichilone N, Bellia V. Prevalence of co-morbidities and severity of COPD. COPD. 2015;12(4):390–4. doi:10.3109/15412555.2014.974734. PMID:25415502.
- Higashimoto Y, Yamagata T, Maeda K, Honda N, Sano A, Nishiyama O, Sano H, Iwanaga T, Chiba Y, Fukuda K, et al. Influence of comorbidities on the efficacy of pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Geriatr Gerontol Int. 2016;16(8):934–41. doi:10.1111/ggi.12575. PMID:26246006.
- Mahishale V, Mahishale A, Patil B, Sindhuri A, Eti A. Screening for diabetes mellitus in patients with chronic obstructive pulmonary disease in tertiary care hospital in India. Niger Med J. 2015;56(2):122–5. doi:10.4103/0300-1652.150699. PMID:25838628.
- Hayashi T, Fukamizu S, Hojo R, Komiyama K, Tanabe Y, Tejima T, Nishizaki M, Hiraoka M, Ako J, Momomura S, et al. Prevalence and electrophysiological characteristics of typical atrial flutter in patients with atrial fibrillation and chronic obstructive pulmonary disease. Europace. 2013;15(12):1777–83. doi:10.1093/europace/eut158. PMID:23787904.
- Konecny T, Park JY, Somers KR, Konecny D, Orban M, Soucek F, Parker KO, Scanlon PD, Asirvatham SJ, Brady PA, et al. Relation of chronic obstructive pulmonary disease to atrial and ventricular arrhythmias. Am J Cardiol. 2014;114(2):272–7. doi:10.1016/j.amjcard.2014.04.030. PMID:24878126.
- Divo M, Cote C, de Torres JP, Casanova C, Marin JM, Pinto-Plata V, Zulueta J, Cabrera C, Zagaceta J, Hunninghake G, et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155–61. doi:10.1164/rccm.201201-0034OC. PMID:22561964.
- Smith MC, Wrobel JP. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2014;9:871–88. doi:10.2147/COPD.S49621. PMID:25210449.
- Lam KB, Jordan RE, Jiang CQ, Thomas GN, Miller MR, Zhang WS, Lam TH, Cheng KK, Adab P. Airflow obstruction and metabolic syndrome: the guangzhou biobank cohort study. Eur Respir J. 2010;35(2):317–23. doi:10.1183/09031936.00024709. PMID:19574332.
- Park SK, Larson JL. The relationship between physical activity and metabolic syndrome in people with chronic obstructive pulmonary disease. J Cardiovasc Nurs. 2014;29(6):499–507. doi:10.1097/JCN.0000000000000096. PMID:24165700.
- Clarenbach CF, Thurnheer R, Kohler M. Vascular dysfunction in chronic obstructive pulmonary disease: current evidence and perspectives. Expert Rev Respir Med. 2012;6(1):37–43. doi:10.1586/ers.11.82. PMID:22283577.
- Fimognari FL, Scarlata S, Conte ME, Incalzi RA. Mechanisms of atherothrombosis in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2008;3(1):89–96. PMID:18488431.
- Dransfield MT, Huang F, Nath H, Singh SP, Bailey WC, Washko GR. CT emphysema predicts thoracic aortic calcification in smokers with and without COPD. COPD. 2010;7(6):404–10. doi:10.3109/15412555.2010.528085. PMID:21166628.
- Lahousse L, van den Bouwhuijsen QJ, Loth DW, Joos GF, Hofman A, Witteman JC, van der Lugt A, Brusselle GG, Stricker BH. Chronic obstructive pulmonary disease and lipid core carotid artery plaques in the elderly: the rotterdam study. Am J Respir Crit Care Med. 2013;187(1):58–64. doi:10.1164/rccm.201206-1046OC. PMID:23144329.
- Maclay JD, McAllister DA, Mills NL, Paterson FP, Ludlam CA, Drost EM, Newby DE, Macnee W. Vascular dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(6):513–20. doi:10.1164/rccm.200903-0414OC. PMID:19542477.
- McAllister DA, Maclay JD, Mills NL, Mair G, Miller J, Anderson D, Newby DE, Murchison JT, Macnee W. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(12):1208–14. doi:10.1164/rccm.200707-1080OC. PMID:17885263.
- Matsuoka S, Yamashiro T, Diaz A, Estepar RS, Ross JC, Silverman EK, Kobayashi Y, Dransfield MT, Bartholmai BJ, Hatabu H, et al. The relationship between small pulmonary vascular alteration and aortic atherosclerosis in chronic obstructive pulmonary disease: quantitative CT analysis. Acad Radiol. 2011;18(1):40–6. doi:10.1016/j.acra.2010.08.013. PMID:20947389.
- Proietti M, Laroche C, Drozd M, Vijgen J, Cozma DC, Drozdz J, Maggioni AP, Boriani G, Lip GY. Impact of chronic obstructive pulmonary disease on prognosis in atrial fibrillation: a report from the EURObservational research programme pilot survey on atrial fibrillation (EORP-AF) general registry. Am Heart J. 2016;181:83–91. doi:10.1016/j.ahj.2016.08.011. PMID:27823697.
- Nadeem R, Sharieff A, Tanna S, Sidhu H, Molnar J, Nadeem A. Potential augmentation of the risk of ischemic cerebrovascular accident by chronic obstructive pulmonary disease in patients with atrial fibrillation. J Stroke Cerebrovasc Dis. 2015;24(8):1893–6. doi:10.1016/j.jstrokecerebrovasdis.2015.04.034. PMID:26142261.
- Mejza F, Lamprecht B, Nizankowska-Mogilnicka E, Undas A. Arterial and venous thromboembolism in chronic obstructive pulmonary disease: from pathogenic mechanisms to prevention and treatment. Pneumonol Alergol Pol. 2015;83(6):485–94. doi:10.5603/PiAP.2015.0078. PMID:26559802.
- Undas A, Kaczmarek P, Sladek K, Stepien E, Skucha W, Rzeszutko M, Gorkiewicz-Kot I, Tracz W. Fibrin clot properties are altered in patients with chronic obstructive pulmonary disease: beneficial effects of simvastatin treatment. Thromb Haemost. 2009;102(6):1176–82. PMID:19967149.
- Vaidyula VR, Criner GJ, Grabianowski C, Rao AK. Circulating tissue factor procoagulant activity is elevated in stable moderate to severe chronic obstructive pulmonary disease. Thromb Res. 2009;124(3):259–61. doi:10.1016/j.thromres.2008.12.030. PMID:19162305.
- Ashitani J, Mukae H, Arimura Y, Matsukura S. Elevated plasma procoagulant and fibrinolytic markers in patients with chronic obstructive pulmonary disease. Intern Med. 2002;41(3):181–5. doi:10.2169/internalmedicine.41.181. PMID:11929177.
- Biljak VR, Pancirov D, Cepelak I, Popovic-Grle S, Stjepanovic G, Grubisic TZ. Platelet count, mean platelet volume and smoking status in stable chronic obstructive pulmonary disease. Platelets. 2011;22(6):466–70. doi:10.3109/09537104.2011.573887. PMID:21506665.
- Wang RT, Li JY, Cao ZG, Li Y. Mean platelet volume is decreased during an acute exacerbation of chronic obstructive pulmonary disease. Respirology. 2013;18(8):1244–8. doi:10.1111/resp.12143. PMID:23786593.
- Lin C-S, Shih C-C, Yeh C-C, Hu C-J, Chung C-L, Chen T-L, Liao C-C. Risk of stroke and post-stroke adverse events in patients with exacerbations of chronic obstructive pulmonary disease. PLoS ONE. 2017;12(1):e0169429. doi:10.1371/journal.pone.0169429. PMID:28060955.
- Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137(5):1091–7. doi:10.1378/chest.09-2029. PMID:20022970.
- Windsor C, Herrett E, Smeeth L, Quint JK. No association between exacerbation frequency and stroke in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:217–25. PMID:26893553.
- Bateman LM, Li C-S, Seyal M. Ictal hypoxemia in localization-related epilepsy: analysis of incidence, severity and risk factors. Brain. 2008;131(12):3239–45. doi:10.1093/brain/awn277. PMID:18952672.
- Corlateanu A, Botnaru V, Mathioudakis AG, Sircu V, Siafakas N. Overlap syndrome of chronic obstructive pulmonary disease and obstructive sleep apnea: a two-faced janus. Curr Respir Med Rev. 2015;11(4):308–13. doi:10.2174/1573398X11666150915213406.
- Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756–63. doi:10.1161/01.STR.0000190056.76543.eb. PMID:16269630.
- Satou Y, Oguro H, Murakami Y, Onoda K, Mitaki S, Hamada C, Mizuhara R, Yamaguchi S. Gastroesophageal reflux during enteral feeding in stroke patients: a 24-hour esophageal pH-monitoring study. J Stroke Cerebrovasc Dis. 2013;22(3):185–9. doi:10.1016/j.jstrokecerebrovasdis.2011.07.008. PMID:21852155.
- Liang B, Wang M, Yi Q, Feng Y. Association of gastroesophageal reflux disease risk with exacerbations of chronic obstructive pulmonary disease. Dis Esophagus. 2013;26(6):557–60. doi:10.1111/dote.12014. PMID:23301861.
- Ho SW, Hsieh MJ, Yang SF, Yeh YT, Wang YH, Yeh CB. Risk of stroke-associated pneumonia with acid-suppressive drugs: a population-based cohort study. Medicine (Baltimore). 2015;94(29):e1227. doi:10.1097/MD.0000000000001227. PMID:26200649.
- Arai N, Nakamizo T, Ihara H, Koide T, Nakamura A, Tabuse M, Miyazaki H. Histamine H2-blocker and proton pump inhibitor use and the risk of pneumonia in acute stroke: a retrospective analysis on susceptible patients. PLoS One. 2017;12(1):e0169300. doi:10.1371/journal.pone.0169300. PMID:28085910.
- Cascini S, Kirchmayer U, Belleudi V, Bauleo L, Pistelli R, Di Martino M, Formoso G, Davoli M, Agabiti N. Inhaled corticosteroid use in chronic obstructive pulmonary disease and risk of pneumonia: a nested case-control population-based study in Lazio (Italy)-the OUTPUL study. COPD. 2017;14(3):311–7. doi:10.1080/15412555.2016.1254172. PMID:28406337.
- Huang CW, Saposnik G, Fang J, Steven DA, Burneo JG. Influence of seizures on stroke outcomes: a large multicenter study. Neurology. 2014;82(9):768–76. doi:10.1212/WNL.0000000000000166. PMID:24489133.
- Hoffmann S, Harms H, Ulm L, Nabavi DG, Mackert BM, Schmehl I, Jungehulsing GJ, Montaner J, Bustamante A, Hermans M, et al. Stroke-induced immunodepression and dysphagia independently predict stroke-associated pneumonia – The PREDICT study. J Cereb Blood Flow Metab. 2017;37(12):3671–82.
- Park GW, Kim SK, Lee CH, Kim CR, Jeong HJ, Kim DK. Effect of chronic obstructive pulmonary disease on swallowing function in stroke patients. Ann Rehabil Med. 2015;39(2):218–25. doi:10.5535/arm.2015.39.2.218. PMID:25932418.
- Hsieh SJ, Zhuo H, Benowitz NL, Thompson BT, Liu KD, Matthay MA, Calfee CS. Prevalence and impact of active and passive cigarette smoking in acute respiratory distress syndrome. Crit Care Med. 2014;42(9):2058–68. doi:10.1097/CCM.0000000000000418. PMID:24942512.
- Calfee CS, Matthay MA, Kangelaris KN, Siew ED, Janz DR, Bernard GR, May AK, Jacob P, Havel C, Benowitz NL, et al. Cigarette smoke exposure and the acute respiratory distress syndrome. Crit Care Med. 2015;43(9):1790–7. doi:10.1097/CCM.0000000000001089. PMID:26010690.
- Seethala RR, Hou PC, Aisiku IP, Frendl G, Park PK, Mikkelsen ME, Chang SY, Gajic O, Sevransky J. Early risk factors and the role of fluid administration in developing acute respiratory distress syndrome in septic patients. Ann Intensive Care. 2017;7(1):11. doi:10.1186/s13613-017-0233-1. PMID:28116595.
- Zhao JN, Liu Y, Li HC. Aspiration-related acute respiratory distress syndrome in acute stroke patient. PLoS One. 2015;10(3):e0118682. doi:10.1371/journal.pone.0118682. PMID:25790377.
- Corlateanu A, Montanari G, Mathioudakis AG, Botnaru V, Siafakas N. Management of Stable COPD: An Update. Curr Respir Med Rev. 2013;9(6):352–9. doi:10.2174/1573398X10666140222001646.
- Pinto-Plata VM, Mullerova H, Toso JF, Feudjo-Tepie M, Soriano JB, Vessey RS, Celli BR. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax. 2006;61(1):23–8. doi:10.1136/thx.2005.042200. PMID:16143583.
- He Z, Chen Y, Chen P, Wu G, Cai S. Local inflammation occurs before systemic inflammation in patients with COPD. Respirology. 2010;15(3):478–84. doi:10.1111/j.1440-1843.2010.01709.x. PMID:20210891.
- Sin DD, Man SF, Marciniuk DD, Ford G, FitzGerald M, Wong E, York E, Mainra RR, Ramesh W, Melenka LS, et al. The effects of fluticasone with or without salmeterol on systemic biomarkers of inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(11):1207–14. doi:10.1164/rccm.200709-1356OC. PMID:18310480.
- Mathioudakis AG, Chatzimavridou-Grigoriadou V, Evangelopoulou E, Mathioudakis GA, Siafakas NM. Comparative mortality risk of tiotropium administered via handihaler or respimat in COPD patients: are they equivalent? Pulm Pharmacol Ther. 2014;28(2):91–7. doi:10.1016/j.pupt.2014.04.009. PMID:24846455.
- Mathioudakis AG, Mastoris I, Chatzimavridou-Grigoriadou V, Mathioudakis GA. The risk of tachyarrhythmias in patients with moderate-to-severe chronic kidney disease receiving tiotropium bromide. Int J Cardiol. 197:105–6. doi:10.1016/j.ijcard.2015.06.051. PMID:26142194.
- Wilchesky M, Ernst P, Brophy JM, Platt RW, Suissa S. Bronchodilator use and the risk of arrhythmia in COPD: part 2: reassessment in the larger quebec cohort. Chest. 2012;142(2):305–11. doi:10.1378/chest.11-1597. PMID:22871756.
- Wilchesky M, Ernst P, Brophy JM, Platt RW, Suissa S. Bronchodilator use and the risk of arrhythmia in COPD: part 1: saskatchewan cohort study. Chest. 2012;142(2):298–304. doi:10.1378/chest.10-2499. PMID:22871755.
- Lin H-W, Chung C-L, Lin YS, Yu C-M, Lee C-N, Bien M-Y. Inhaled pharmacotherapy and stroke risk in patients with chronic obstructive pulmonary disease: a nationwide population based study using two-stage approach. PLoS ONE. 2015;10(7):e0130102. doi:10.1371/journal.pone.0130102. PMID:26158649.
- Wang MT, Tsai CL, Lo YW, Liou JT, Lee WJ, Lai IC. Risk of stroke associated with inhaled ipratropium bromide in chronic obstructive pulmonary disease: a population-based nested case-control study. Int J Cardiol. 2012;158(2):279–84. doi:10.1016/j.ijcard.2012.02.012. PMID:22386700.
- Singh S, Loke YK, Enright P, Furberg CD. Republished: pro-arrhythmic and pro-ischaemic effects of inhaled anticholinergic medications. Postgrad Med J. 2014;90(1062):205–7. doi:10.1136/postgradmedj-2011-201275rep. PMID:24643260.
- Mathioudakis AG, Kanavidis P, Chatzimavridou-Grigoriadou V, Gialmanidis IP, Amanetopoulou SG, Christopoulou E, Evangelopoulou E, Mathioudakis GA. Tiotropium HandiHaler improves the survival of patients with COPD: a systematic review and meta-analysis. J Aerosol Med Pulm Drug Deliv. 2013;27(1):43–50. doi:10.1089/jamp.2012.1012. PMID:23521168.
- Verhamme KM, Afonso AS, van Noord C, Haag MD, Koudstaal PJ, Brusselle GG, Sturkenboom MC. Tiotropium Handihaler and the risk of cardio- or cerebrovascular events and mortality in patients with COPD. Pulm Pharmacol Ther. 2012;25(1):19–26. doi:10.1016/j.pupt.2011.10.004. PMID:22051450.
- Oliveira MF, Rodrigues MK, Treptow E, Cunha TM, Ferreira EM, Neder JA. Effects of oxygen supplementation on cerebral oxygenation during exercise in chronic obstructive pulmonary disease patients not entitled to long-term oxygen therapy. Clin Physiol Funct Imaging. 2012;32(1):52–8. doi:10.1111/j.1475-097X.2011.01054.x. PMID:22152079.
- Rodrigues MK, Oliveira MF, Soares A, Treptow E, Neder JA. Additive effects of non-invasive ventilation to hyperoxia on cerebral oxygenation in COPD patients with exercise-related O2 desaturation. Clin Physiol Funct Imaging. 2013;33(4):274–81. doi:10.1111/cpf.12024. PMID:23692616.
- Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–89. doi:10.1056/NEJMoa063070. PMID:17314337.
- Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, Thach C, Fogel R, Patalano F, Vogelmeier CF. Indacaterol–glycopyrronium versus salmeterol–fluticasone for COPD. N Engl J Med. 2016;374(23):2222–34. doi:10.1056/NEJMoa1516385. PMID:27181606.
- Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–54. doi:10.1056/NEJMoa0805800. PMID:18836213.
- Singh D, Papi A, Corradi M, Pavlišová I, Montagna I, Francisco C, Cohuet G, Vezzoli S, Scuri M, Vestbo J. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting b-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. The Lancet. 2016;388(10048):963–73. doi:10.1016/S0140-6736(16)31354-X.
- Harrison MT, Short P, Williamson PA, Singanayagam A, Chalmers JD, Schembri S. Thrombocytosis is associated with increased short and long term mortality after exacerbation of chronic obstructive pulmonary disease: a role for antiplatelet therapy? Thorax. 2014;69(7):609–15. doi:10.1136/thoraxjnl-2013-203996. PMID:24743560.
- Ekstrom MP, Wagner P, Strom KE. Trends in cause-specific mortality in oxygen-dependent chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183(8):1032–6. doi:10.1164/rccm.201010-1704OC. PMID:21216882.
- Ekstrom MP, Hermansson AB, Strom KE. Effects of cardiovascular drugs on mortality in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):715–20. doi:10.1164/rccm.201208-1565OC. PMID:23328521.
- Wang MT, Lo YW, Tsai CL, Chang LC, Malone DC, Chu CL, Liou JT. Statin use and risk of COPD exacerbation requiring hospitalization. Am J Med. 2013;126(7):598–606 e2. doi:10.1016/j.amjmed.2013.01.036. PMID:23684060.
- Lahousse L, Loth DW, Joos GF, Hofman A, Leufkens HG, Brusselle GG, Stricker BH. Statins, systemic inflammation and risk of death in COPD: the Rotterdam study. Pulm Pharmacol Ther. 2013;26(2):212–7. doi:10.1016/j.pupt.2012.10.008. PMID:23142156.
- Cao C, Wu Y, Xu Z, Lv D, Zhang C, Lai T, Li W, Shen H. The effect of statins on chronic obstructive pulmonary disease exacerbation and mortality: a systematic review and meta-analysis of observational research. Sci Rep. 2015;5:16461. doi:10.1038/srep16461. PMID:26553965.